-
PDF
- Split View
-
Views
-
Cite
Cite
Takuma Tsuzuki Wada, Kazuhiro Yokota, Sakon Sakai, Machika Soma, Hiroshi Kajiyama, Norihito Tarumoto, Shigefumi Maesaki, Takuya Maeda, Makoto Nagata, Toshihide Mimura, Evaluation of anti-severe acute respiratory syndrome coronavirus 2 antibody levels in coronavirus disease breakthrough infection during immunosuppressive therapy in a patient with connective tissue disease-related interstitial lung disease, Modern Rheumatology Case Reports, Volume 7, Issue 1, January 2023, Pages 288–292, https://doi.org/10.1093/mrcr/rxac052
Close - Share Icon Share
ABSTRACT
Herein, we report the case of a 67-year-old man with severe coronavirus disease (COVID-19) pneumonia and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine breakthrough infection during immunosuppressive therapy for connective tissue disease-related interstitial lung disease (CTD-ILD). The patient received glucocorticoids combined with tacrolimus as maintenance therapy. His serum anti-SARS-CoV-2-immunoglobulin G (IgG) antibody levels were extremely low at the onset of COVID-19 pneumonia, even after the second dose of SARS-CoV-2 mRNA vaccine (BNT162b2). After treatment for COVID-19 pneumonia, the levels of anti-SARS-CoV-2-IgG antibodies increased. These results indicated a lack of the ability to produce neutralising antibodies from immune cells despite the booster vaccination. Therefore, we suggest that advanced-age patients with CTD-ILD receiving immunosuppressive therapy with polypharmacy require consistent personal protection, vaccination of close caregivers, increased awareness, and booster vaccination. Moreover, we recommend that tacrolimus should be withdrawn for a while after vaccination under controlled conditions.
Introduction
Currently, mRNA vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are highly effective in reducing the incidence and severity of coronavirus disease (COVID-19) [1–4]. However, breakthrough infections, SARS-CoV-2 infection more than 2 weeks after a second vaccination of the mRNA vaccine or after a first vaccination of the viral vector vaccine, rarely occur when an individual who has been fully vaccinated against COVID-19 gets infected with SARS-CoV-2 [5–8]. A mechanism of breakthrough infection is decreased serum levels of anti-SARS-CoV-2-IgG antibody in response to vaccination originating from immunocompromised conditions induced by immunosuppressive therapy [9]. However, no reports have evaluated the levels of anti-SARS-CoV-2-IgG antibodies in breakthrough infections in cases undergoing immunosuppressive therapy with polypharmacy for connective tissue disease-related interstitial lung disease (CTD-ILD). Herein, we report a case of severe COVID-19 pneumonia with breakthrough infection, in which changes in anti-SARS-CoV-2-IgG antibody levels were observed. We also present a literature review to highlight the current information on this topic.
Case presentation
A 67-year-old man was admitted to another hospital because of chest trauma 1 year prior to admission to our hospital. At that time, chest computed tomography (CT) incidentally showed reticular shadows with peripheral predominance at the bases of the bilateral lungs. Therefore, the patient was referred to our hospital. Although minimal saturation of percutaneous oxygen (SpO2) was 95% for a 6-min walk, his forced volume capacity was 47.2%. Furthermore, transbronchial lung biopsy revealed interstitial infiltration of inflammatory cells, mainly lymphocytes, and fibrosis with septal expansion. Resultantly, the patient was diagnosed with chronic interstitial lung disease. The patient was positive for anti-aminoacyl-tRNA synthetase antibody (anti-PL-7 antibody) but physical examination revealed no muscular findings. Thereafter, the patient was diagnosed with systemic sclerosis by skin biopsy. Consequently, the patient was diagnosed with CTD-ILD and received 40 mg/day of prednisolone (PSL) 8 months prior to admission. The dosage of PSL was gradually decreased to 26.5 mg/day. However, Gottron papules and mild muscle weakness in the upper and lower limbs appeared 12 weeks prior to admission. The patient was diagnosed with dermatomyositis because of Gottron papules, muscle weakness, 7.7 U/l of serum aldolase level, and 37 mm/h of erythrocyte sedimentation rate. Accordingly, 4 mg/day of tacrolimus (TAC) was added 7 weeks prior to admission.
The patient received the first dose of BNT162b2 mRNA COVID-19 vaccine 44 days prior to admission and the second dose 23 days prior to admission. TAC was continued while the vaccination was administered. Six days prior to admission, the patient developed a dry cough. Four days prior to admission, both his mother-in-law and son living with him were positive for SARS-CoV-2 confirmed by reverse transcriptase polymerase chain reaction (RT-PCR), indicating a familial infection. The patient had a fever of 37°C 2 days prior to admission and presented to our hospital. A RT-PCR test was conducted using his nasopharyngeal swab sample to detect SARS-CoV-2. The test result was positive (threshold cycle value: 17.98), and the patient was diagnosed with COVID-19 and was admitted to our hospital.
The patient had a history of smoking and smoked five cigarettes per day from the age of 18 to 26 years. His history of alcohol consumption involved occasional drinking. There was no history of an underlying disease at risk of aggravation. Other medications used included omeprazole, trimethoprim/sulfamethoxazole, and alendronate sodium hydrate.
On admission, his height was 167 cm, body weight was 60 kg, and body mass index was 21.5. His level of consciousness was alert, body temperature was 36.7°C, blood pressure was 132/95 mmHg, heart rate was 93/min, respiratory rate was 24 breaths/min, and SpO2 was 87% in room air. SpO2 value increased to 95% with the use of a 5 l/min oxygen mask. Chest CT showed heterogeneously distributed diffuse ground-glass opacities in both lungs (Figure 1).

Chest computed tomography. a: One year before admission. b: On admission. c: Hospital day 56.
Blood tests revealed a white blood cell count of 11,700/μl, lymphocyte count of 550/μl, haemoglobin level of 16.3 g/dl, platelet count of 19.2 × 104/μl, serum creatinine (Cr) level of 1.2 mg/dl, estimated glomerular filtration rate (eGFR) of 48.0 ml/min/1.73 m2, lactate dehydrogenase level of 508 U/l, C-reactive protein level of 15.5 mg/dl, ferritin level of 1358 ng/ml, sialylated carbohydrate antigen KL-6 level of 768 U/ml, immunoglobulin G level of 644 mg/dl, interleukin-6 level of 34.5 pg/ml, interferon-λ3 level of 53.1 pg/ml, D-dimer level of 1.5 mg/ml, and TAC trough level of 11.0 ng/ml. Blood gas analysis in room air revealed a pH of 7.44, partial pressure of arterial oxygen of 68.1 mmHg, partial pressure of arterial carbon dioxide of 33.1 mmHg, and bicarbonate level of 22.9 mmol/l. The levels of anti-SARS-CoV-2 antibodies (Access SARS-CoV-2 IgG II Assay Antibody Tests; Beckman Coulter Inc.) were 15.5 arbitrary units [AU]/ml, positive for ≥10 AU/ml and negative for <10 AU/ml. Genome sequencing revealed that the patient was infected with the Delta SARS-CoV-2 variant.
The clinical course of the patient is shown in (Figure 2). After admission, he was administered intravenous lemdesivir (200 mg on the first day, 100 mg from the second to the fifth day) and baricitinib (2 mg/day) combined with methylprednisolone pulse therapy (1000 mg intravenously per day for 3 days) [10]. TAC treatment was continued at a dose of 4 mg/day. On the second hospital day, his respiratory status worsened and oxygen dosage was increased to 40 l/min along with 80% fractional inspired oxygen (FiO2) with high-flow nasal cannula oxygen therapy. On the third day of hospitalisation, his respiratory status rapidly deteriorated, and the patient was placed on an invasive mechanical ventilator. Additionally, baricitinib was discontinued and tocilizumab (400 mg) was administered intravenously. On the fourth day of hospitalisation, 6.6 mg/day dexamethasone (DEX) was administered intravenously. Subsequently, the respiratory status gradually improved after the hospital day 14. On the hospital day 17, the patient was extubated by himself with 50% FiO2. After self-extubation, the patient’s respiratory condition stabilised. (Figure 2) shows the changes in the serum levels of anti-SARS-CoV-2 antibodies. Antibody levels gradually increased over time after COVID-19 infection. On hospital day 27, the DEX dose was reduced to 6 mg/day, and on hospital day 35, DEX was switched to 30 mg/day of PSL. On hospital day 38, the oxygen dosage was reduced to 1 l/min using a nasal cannula. On hospital day 45, TAC administered was resumed at 3 mg/day, and on hospital day 50, the PSL dose was reduced to 26.5 mg/day. On hospital day 56, chest CT showed improvement in the interstitial shadows (Figure 1). Finally, the patient was transferred to another hospital for rehabilitation on hospital day 63.
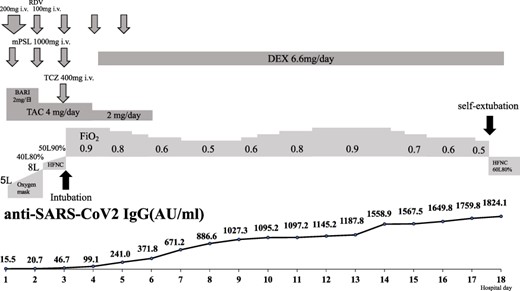
Clinical course and changes in serum levels of anti-severe acute respiratory syndrome coronavirus 2-IgG antibodies.
Discussion
This patient developed a cough on the 17th day after receiving the second dose of BNT162b2 mRNA vaccination and was diagnosed with COVID-19 (SARS-CoV-2 Delta variant) on the 23rd day after the second dose of vaccination. Subsequently, the patient became seriously ill shortly after admission. The patient’s SARS-CoV-2 infection was considered a breakthrough infection because it occurred after the second COVID-19 vaccination. Previously, we reported that Access SARS-CoV-2 IgG II Assay Antibody Tests had a sensitivity of 100% and specificity of 100% for COVID-19 infection, which was used to measure the serum levels of anti-SARS-CoV-2 antibodies in this case [11]. The average antibody levels after vaccination by the same assay system were over 100 AU/ml at the time of the second vaccination and over 1000 AU/ml 7 days after the second vaccination with a cut-off of 10 AU/ml, although the sample size was small [12]. The serum level of anti-SARS-CoV-2 antibodies in our case was 15.5 AU/ml at the time of COVID-19 diagnosis, which was much lower than the average level of anti-SARS-CoV-2 antibodies after the second vaccination. These results suggest a lack of specific antibody production by the immune cells, even after two doses of the vaccination. A possible mechanism for decreased serum levels of anti-SARS-CoV-2-IgG antibodies in response to vaccination is derived from immunosuppressive agents such as glucocorticoids combined with TAC. It has been reported that patients with autoimmune disease who experienced breakthrough infections were associated with inadequate humoral response to SARS-CoV-2 [13]. Since the serum levels of anti-SARS-CoV-2 antibodies in our case gradually increased over time after the treatment for COVID-19, there was a sufficient immune response against SARS-CoV-2 infection, even though he was taking glucocorticoids and TAC, although the anti-SARS-CoV-2 titre did not increase appreciably by the second vaccination. To our knowledge, there have been no reports on the measurement of serum levels of anti-SARS-CoV-2-IgG antibodies during breakthrough infections in patients with CTD-ILD undergoing immunosuppressive therapy. These data would be useful for examining the pathogenesis of breakthrough infections in immunocompromised conditions.
The BNT162b2 mRNA vaccine is most commonly used as a preventative measure against SARS-CoV-2 infection. A multinational, placebo-controlled, observer-blinded, pivotal efficacy trial showed that the BNT162b2 mRNA vaccine was 95% effective in preventing COVID-19 7 days after the second dose [1]. However, the efficacy of the BNT162b2 mRNA vaccine in patients treated with immunosuppressants is not well clarified in that study, because it excluded such patients. Several recent large cohort studies including patients treated with immunosuppressants also showed a high efficacy of the BNT162b2 mRNA vaccine [3, 14–19]. Nevertheless, breakthrough infections of SARS-CoV-2 were diagnosed, even after immunocompromised patients are vaccinated [5, 6, 19]. In a report from Israel, the clinical characteristics of 152 patients with breakthrough infection included hypertension (71%), diabetes (48%), and any type of immunosuppressive therapy (40%) with the highest number of patients taking glucocorticoids (19%) [5]. Moreover, a large U.S. cohort study on immunocompromised patients reported that breakthrough infections were more frequent in organ transplant recipients, those with multiple immunocompromised conditions, antimetabolite users, patients with primary immunodeficiencies and haematologic malignancies, and patients aged ≥65 years [7]. Moreover, regarding immunosuppressive therapy, rituximab and glucocorticoids were the most commonly used agents, with 5 cases each, followed by mycophenolate mofetil (4 cases), methotrexate (3 cases), and TAC (2 cases) in a report of 16 breakthrough infection cases with rheumatic diseases [20].
Among immunosuppressive agents, glucocorticoids, rituximab, mycophenolate mofetil, and abatacept reportedly reduce antibody production from immune cells after BNT162b2 mRNA vaccination [21, 22]. Patients with autoimmune diseases and lung disease taking glucocorticoids were reported to have an increased risk of developing COVID-19 and a worse prognosis, especially when taking over 10 mg/day of PSL equivalent [23, 24]. Consequently, patients with a PSL equivalent of >10 mg/day should be cautioned against breakthrough infections and the severity of COVID-19. Calcineurin inhibitors, such as TAC, reportedly suppress antibody production in renal transplant and haemodialysis patients [25, 26]. Calcineurin inhibitors inhibit the dephosphorylation of nuclear factor of activated T-cells, which is a pivotal family of transcription factors for the immune response, resulting in potent cellular and humoral immunosuppression through the reduced production of inflammatory cytokine production, such as interleukin-2. Therefore, the reduction in anti-SARS-CoV-2-IgG antibody production by the SARS-CoV-2 mRNA vaccine should also be considered for calcineurin inhibitors. However, the Japanese Rheumatoid Arthritis Society Vaccine Survey Report reported that Japanese patients with rheumatic disease treated with calcineurin inhibitor showed better humoral response to SARS-CoV-2 than those who treated with other immunosuppressants [27]. Furthermore, myositis patients with combination therapy of PSL and immunosuppressant were reported to decrease immunogenicity for SARS-CoV-2 [28]. In particular, hypogammaglobinemia might also affect antibody production in this case.
Generally, the American College of Rheumatology (ACR) Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases previously recommended that methotrexate, Janus kinase inhibitors, mycophenolate mofetil, rituximab, and cyclophosphamide should be considered for withdrawal when administering vaccines [29]. Subsequently, the guidance was updated to include calcineurin inhibitors, azathioprine, and belimumab as immunosuppressive agents that should be considered to be withdrawn for 1–2 weeks after vaccination [30]. Since there have been a few reports on the levels of anti-SARS-CoV-2 antibodies and the risk of breakthrough infection during immunosuppressive therapy, it is necessary to accumulate more cases in the future to establish evidence on infection control measures and advisability of medication withdrawal for patients on immunosuppressive therapy. Based on the guidelines and our case, we believe that, especially in the elderly with no autoimmune conditions, it is advisable to follow American College of Rheumatology guidance to withdraw immunosuppressive drugs, such as TAC, after COVID-19 vaccination under controlled conditions. However, for patients taking more than 10 mg/day PSL equivalent glucocorticoids especially complicated with lung disease, more thorough infection control measures should be taken and booster vaccination should be actively considered, given the possibility of decreased serum levels of anti-SARS-CoV-2 antibody production.
Conflict of interest
None declared.
Funding
None declared.
Patient consent
Written informed consent to publish this case report was obtained from the patient.



