-
PDF
- Split View
-
Views
-
Cite
Cite
Ibrahim Durucan, Sabriye Guner, Burcak Kilickiran Avci, Gokcen Unverengil, Melike Melikoglu, Serdal Ugurlu, Post-COVID-19 vaccination inflammatory syndrome: A case report, Modern Rheumatology Case Reports, Volume 7, Issue 1, January 2023, Pages 280–282, https://doi.org/10.1093/mrcr/rxac041
Close - Share Icon Share
ABSTRACT
A previously healthy 24-year-old male patient was referred to our clinic with bilateral lower extremity pain and dark urine, which were developed 2 weeks after receiving the second dose of the BNT162b2 vaccine against severe acute respiratory coronavirus 2. Laboratory tests indicated rhabdomyolysis. Lower extremity magnetic resonance imaging was compatible with myositis. Myositis-related antibodies were negative. Biopsy taken from gastrocnemius muscle revealed muscle necrosis and striking expression of major histocompatibility complex class I antigen. He was successfully treated, and his complaints were resolved. One week later at follow-up, he reported new-onset exertional dyspnoea with palpitations. ST-segment depressions were spotted on electrocardiography. Troponin T was found elevated as 0.595 ng/ml (normal <0.014 ng/ml). Echocardiography showed a hypokinetic left ventricle with an ejection fraction of 40% and pericardial effusion of 2 mm. An appropriate treatment plan was formulated for the diagnosis of myocarditis, eventually, the patient recovered within 10 days. The BNT162b2 messenger ribonucleic acid (mRNA) vaccine was felt to cause the aforementioned condition since no other aetiology could be identified. Although it is known that BNT162b2 may induce myocarditis, myositis concomitant myocarditis appears to be a very rare adverse effect of this vaccine.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected every living member of humankind all around the globe. Hundreds of millions of people were infected and millions of people have deceased due to severe acute respiratory coronavirus 2 (SARS-CoV-2) or related complications so far. Vaccines have played a crucial role in decreasing losses and defeating the pandemic; however, the reports of post-marketing adverse events have raised questions about possible outcomes in susceptible individuals. Scientific publications are needed to better understand the mechanism of interactions causing side effects, which may provide enough insight into how to bring vaccines to perfection. Here, we present a previously healthy 24-year-old male patient who developed myositis with rhabdomyolysis and myocarditis after the second dose of the BNT162b2 mRNA COVID-19 vaccine.
Case presentation
Two weeks after receiving the second dose of the BNT162b2 mRNA COVID-19 vaccine, the 24-year-old patient had experienced a burning sensation in the upper and lower extremity, exacerbating while climbing a ladder. Eventually, the burning sensation turned into constant pain awakening the patient from sleep. The pain was unresponsive to myorelaxants and non-steroidal anti-inflammatory drugs. He also described quite intense pain and swelling at the site of the second vaccination, which lasted 3 days after injection. Neither rash nor erythema was reported. The patient was admitted to another hospital after he had noticed dark urine, and then was referred to our clinic on the 10th day following his admission.
The blood test obtained at his first admission was remarkable with creatine kinase (CK) >22.000 U/l, aspartate aminotransferase (AST) 1160 U/l, alanine aminotransferase (ALT) 619 U/l, lactate dehydrogenase (LDH) 736 U/l, serum creatinine 2.1 mg/dl, and C-reactive protein (CRP) 28 mg/l. In the dipstick test, blood was found positive, but no erythrocytes were seen in microscopy. Polymerase chain reaction for COVID-19 was negative.
On examination, his overall state was fine and his vital signs were stable. Non-pitting pretibial oedema and decreased muscle strength (4/5) in the proximal lower extremity were spotted. He complained of generalised body aches, muscle soreness, and dark urine but did not describe fever or weight loss. He had an active lifestyle but denied any strenuous exercise, trauma, substance use, and previous COVID-19 infection. His past medical and family history were insignificant.
The initial laboratory results are summarised as follows: AST 1423 U/l, ALT 796 U/l, CK 2063 U/l, LDH 1133 U/l, and CRP 76 mg/l. Anti-nuclear antibodies, ENA profile (SSA, SSB, SCL-70, RNP70, SM, u1RNP, centromere B, Jo-1), PM/Scl-100, anti-Jo-1, anti-PL-7, anti-PL-12, anti-Ku (p70/80), anti-Mi-2, anti-MDA-5, anti-NXP-2, and anti-SRP antibodies were all negative. A lower extremity MRI was compatible with myositis (Figure 1).
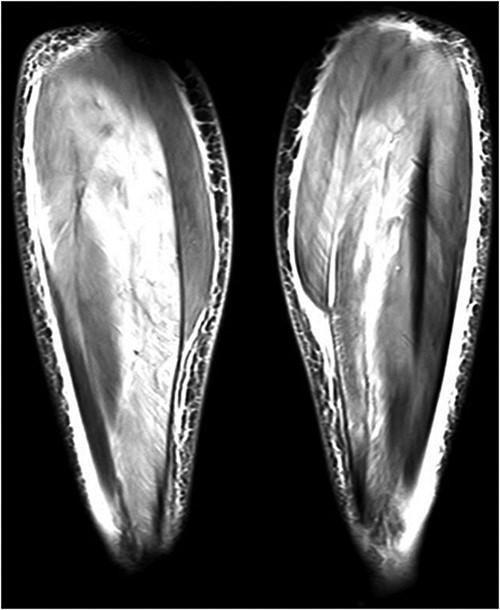
Lower extremity coronal MRI was compatible with myositis, an image showing prominent signal enhancements in posterior crural muscles, effusions, oedema in fasciae, and subcutaneous fat tissue.
Surface electromyography (EMG) demonstrated diminished sensory responses that were felt to occur secondary to oedema. Needle EMG displayed positive spikes, fibrillation, and myotonia in the upper and lower limbs. Low-amplitude motor unit potentials with short wave duration were seen in the deltoid muscle. Biopsy specimen taken from gastrocnemius muscle revealed muscle necrosis and features of muscle fibres phagocyted (Figures 2 and 3). No inflammatory cells were seen in endomysium, but multiple areas stained with major histocompatibility complex class I (MHC-I) marker were reported.
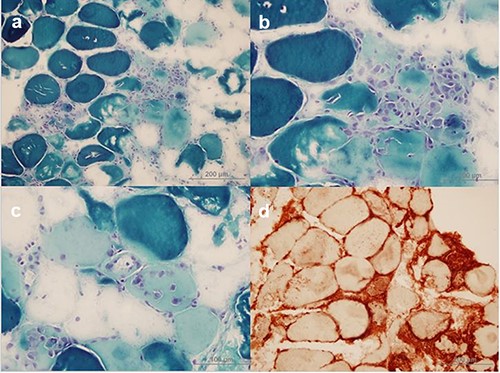
a–c: Necrotic muscle fibres and phagocytic cells near and within necrotic muscle fibres (Modified Gomori Trichrome) d: Notable MHC-I expression can be seen on the surface of all muscle fibres and it is increased in the cytoplasm of regenerating muscle fibres. Mononuclear cells are also stained with MHC-I marker.
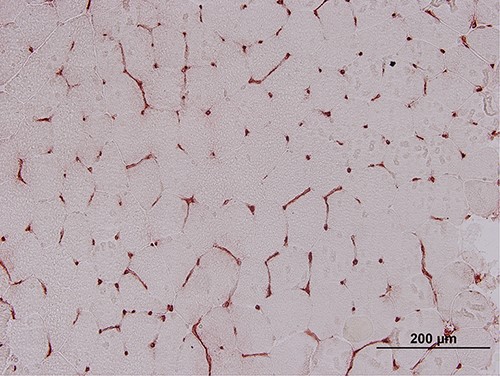
A negative control taken from another individual showing normal MHC-I staining.
The patient was started on pulse steroids of 1 g after biopsy, completed by Day 6 and the treatment was maintained with prednisolone 30 mg per day. On Day 9, it was decided to discharge the patient because his condition was improving as CK and CRP decreased to 800 U/l and 9 mg/l, respectively. Before the development of myocarditis, the patient’s body temperature was 36.7°C. His heart rate was 80 beats/min. Chest radiography was normal and regular sinus rhythm was noted on electrocardiogram (ECG). One week later, he complained of new-onset exertional dyspnoea with palpitations. Sinus rhythm with ST-segment depressions in leads I, II, and aVL refers to a limb lead used on electrocardiography were noted on ECG. His heart rate was 119 beats/min. His body temperature was 36.6°C. Chest X-ray was normal. A prompt echocardiogram displayed global left ventricular (LV) hypokinesia with ejection fraction (EF) of 40%, and hyperechoic pericardium with 2 mm of effusion. Laboratory included Troponin T (TnT) 0.595 ng/ml (normal <0.014 ng/ml), CK 2900 U/l, AST 128 U/l, ALT 259 U/l, LDH 666 U/l, and serum creatinine 0.4 mg/dl. Diagnosis of myocarditis was made based upon radiological and laboratory findings. Metoprolol 100 mg, ramipril 2.5 mg, and spironolactone 25 mg were included in his treatment regimen, which was already containing prednisolone 30 mg, intravenous hydration, and urine alkalinisation. CRP levels did not increase during myocarditis, which ranged from 0.93 to 2.12 mg/l (normal <5 mg/l) in serial blood exams. His parameters subsided within 10 days as TnT 0.313 ng/ml, CK 1200 U/l, AST 60 U/l, and ALT 100 U/l. His muscle strength became normal and dyspnoea resolved. He had no complaints in a 20-day period after hospital discharge.
Discussion
Multiple adverse events of COVID-19 vaccines have been reported in post-marketing settings including myositis and myocarditis [1]. Our patient’s condition distinguishes itself from other COVID-19 vaccination-induced myositis cases and proves its value with respect to the appearance of myositis concomitant myocarditis. One report defined a previously healthy 28-year-old patient who experienced muscle pain and weakness in her thighs, 5 days after receiving the first dose of the Moderna mRNA COVID-19 vaccine [2]. CK level was found elevated at 17,959 U/l, which is a similar finding to our case. MRI and EMG findings were in line with myositis, and myositis-related antibodies were found negative. However, no myocardial involvement was reported, contrasting with our patient. In another paper, the authors described three unrelated cases that developed inflammatory myositis after getting the ChAdOx1 COVID-19 vaccine [3]. Myositis emerged in two patients after the first dose, whereas the other patient developed myositis ensuing the second dose. All of the cases had MRI findings that were compatible with myositis. Biopsy performed only on one patient revealed myositis and vasculitis. Our report differs from these three cases since those were older than 70 years of age, and myocardium was spared in all individuals. Additionally, there are also reports of rhabdomyolysis that occurred after getting BNT162b2; however, the presence of myositis is uncertain in these cases since no diagnostic procedure, such as MRI or muscle biopsy, was performed [4, 5]. In summary, multiple myositis cases that emerged after the COVID-19 vaccination have been reported, but to our knowledge, myositis concomitant myocarditis appears to be an exceptional side effect of BNT162b2.
The patient’s biopsy result was in line with inflammatory myositis as MHC-I expressions were spotted. Aschman et al. [6] reported striking expression of MHC- I antigens in autopsy specimens taken from skeletal muscles of patients with COVID-19, denoting skeletal muscles participated in the response mechanism against SARS-CoV-2. Vojdani et al. [7] revealed that antibodies against the protein structure of SARS-CoV-2 had reactions with human tissue antigens, including skeletal muscles and heart muscle. Thus, we argue that the vaccine possibly induced myositis and myocarditis by cross-interactions between the patient’s tissues and antibodies targeting SARS-CoV-2. Myositis-related antibodies were negative in the patient’s results, conforming to our argument. In autopsy specimens, inflammatory alterations were more prominent in skeletal muscles than cardiac muscle [6], which may explain why our patient’s cardiac complaints appeared at later stages of the onset, the inflammation possibly needed more time to become symptomatic.
Echocardiography and ECG suggested myocarditis as a reason for elevated TnT levels. In contrasted cardiac MRI, performed on the second day of his re-admission, global LV hypokinesia with EF of 48% was present. Other findings suggesting myocarditis such as signal enhancement in T2-weighted times and late gadolinium enhancement were absent. Given the patient mentioned cardiovascular symptoms on the 28th day ensuing the second dose of vaccination and the high dose of corticosteroids he received from the very beginning, we believe that possible MRI findings suggesting myocardial injury may have dissolved.
Interestingly, our patient’s CRP level remained within physiological limits during myocarditis. However, the effects of highly potent corticosteroids on the CRP course should be taken into consideration. Moreover, according to the result of a study including 230 patients who had symptoms of non-valvular, non-ischaemic heart failure and were diagnosed with myocardial inflammation by endomyocardial biopsy, the median CRP level of the patients was found 5.4 mg/l [8]. In the light of this information, it appears that CRP is not expected to rise overtly during myocarditis, and it is comprehensible why our patient’s CRP level remained within the normal range.
Vaccines are our only weapon to end the COVID-19 pandemic. As the vaccination campaign goes on, various side effects will inevitably be seen in susceptible individuals if the number of vaccinated people worldwide is considered. Clinicians should be mindful of post-vaccination adverse events varying from mild to severe. Myositis ought to be investigated in patients presenting with muscle weakness and myalgia developed after vaccination with BNT162b2. Further investigations are needed to establish a causal link between mRNA COVID-19 vaccine and autoimmunity.
Conflict of interest
None declared.
Funding
No funding was received to conduct this study.
Patient consent
Written informed consent was obtained from the patient for publication of this case report.



