-
PDF
- Split View
-
Views
-
Cite
Cite
Yusuke Ohkubo, Shin-ichiro Ohmura, Ryuhei Ishihara, Toshiaki Miyamoto, Possible case of polyarteritis nodosa with epididymitis following COVID-19 vaccination: A case report and review of the literature, Modern Rheumatology Case Reports, Volume 7, Issue 1, January 2023, Pages 172–176, https://doi.org/10.1093/mrcr/rxac085
Close - Share Icon Share
ABSTRACT
The global outbreak of coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus type 2 has prompted the rapid spread and development of vaccines to prevent the spread of the disease. COVID-19 vaccine has demonstrated excellent efficacy in reducing morbidity and severity of the disease, and most adverse reactions are very minor. However, some patients have been reported to develop autoimmune diseases, such as rheumatoid arthritis, myocarditis, Guillain–Barre syndrome, and vasculitis, following COVID-19 vaccination. Herein, we present a case of polyarteritis nodosa with epididymitis, following COVID-19 mRNA vaccination. The patient’s initial symptoms were fever and testicular pain, and magnetic resonance imaging showed epididymitis. He was diagnosed as having polyarteritis nodosa with epididymitis and was treated with high-dose prednisolone, with a good clinical outcome.
Introduction
The new coronavirus disease (COVID-19) has spread worldwide, sometimes causing severe conditions and leading to poor outcomes. COVID-19 vaccines have been rapidly delivered worldwide. In Japan, the mRNA vaccines “BNT162b2” (Pfizer Biotech) and “mRNA-1273” (Moderna) have been approved since 2021. These vaccines are effective in reducing the morbidity and severity of the disease. Although minor adverse drug reactions are common, serious adverse drug reactions, such as thrombosis with thrombocytopenia and myocarditis, have rarely been reported [1]. In addition, the development of autoimmune diseases, such as rheumatoid arthritis and lupus nephritis, has been reported following COVID-19 vaccination [2, 3]. Furthermore, several investigators reported vasculitis following the vaccination [4, 5].
Herein, we report a possible case of polyarteritis nodosa (PN) with epididymitis following COVID-19 mRNA vaccination.
Case presentation
A 61-year-old healthy man presented with fever and testicular pain. He had no past history of chronic disease or allergies, no new medications, and no infectious symptoms before vaccination. He and his family had no documented history of COVID-19.
In September 2021, he received the first dose of the Pfizer vaccine. Seventeen days following the first dose of Pfizer vaccination, fever and testicular pain developed, which did not resolve. He visited the clinic and received antibiotics; however, the treatment did not improve his condition. Therefore, he was referred to our hospital.
On admission, his body temperature was 38.5°C, pulse rate 105 beats per minute, blood pressure 127/91 mmHg, respiratory rate 18 breaths per minute, and oxygen saturation 98% (room air). His weight was 83.0 kg, which had reduced by 6 kg compared to 3 weeks prior. Physical examinations showed no conjunctival hyperaemia, palpable cervical lymph nodes, difference in breath sounds, obvious pulmonary or cardiac murmur, tenderness of the abdominal region, bilateral leg oedema, skin rash, or abnormal neurological findings. However, he had grasping pain in the lower leg and scrotum.
Blood tests showed white blood cells 12,890/µL, red blood cells 549 × 104/µL, haemoglobin 13.8 g/dL, haematocrit 41.2%, platelets 35.5 × 104/µL, total protein 7.3 g/dL, albumin 3.4 g/dL, total bilirubin 1.3 mg/dL, direct bilirubin 0.4 mg/dL, aspartate transaminase 14 U/L, alanine transaminase 13 U/L, alkaline phosphatase 58 U/L, lactate dehydrogenase 141 U/L, γ-glutamyltransferase 20 U/L, cholinesterase 252 U/L, creatine kinase 78 U/L, uric acid 3.3 mg/dL, blood urea nitrogen 17.0 mg/dL, creatinine 1.1 mg/dL, C-reactive protein 22.9 mg/dL, erythrocyte sedimentation rate 83 mm/h, total prostate-specific antigen 2.04 ng/mL, free prostate-specific antigen 0.35 ng/mL, antinuclear antibody 1:40 (homogeneous), myeloperoxidase-anti neutrophil cytoplasmic antibody (ANCA) <1.0 U/mL, and proteinase 3-ANCA <1.0 U/mL, and urinalysis showed no occult blood, 1–4 red blood cells/high power field, 1–4 white blood cells/high power field, and no proteins. The patient was negative for Hepatitis B antigen. The T-SPOT-TB test and urine mycobacterium culture were negative. The polymerase chain reaction (PCR) test for chlamydia and gonorrhoea as per the urine sample was negative. His PCR test for COVID-19 was also negative. His blood and urine cultures were negative. Whole-body computed tomography (CT) showed no specific lesions, and CT angiography showed no aneurysm. Testicular ultrasonography showed that the left spermatic cord was markedly more prominent than the right spermatic cord, with a perineal hyperechoic area but no increased blood flow (Figure 1). He was initially administered meropenem for the high fever, but his condition did not improve. Thus, further examinations were conducted. T2-weighted magnetic resonance imaging (MRI) for his scrotum showed enlargement of the left epididymis and dilation of the venous system (Figure 2(a)). Gadolinium-contrasted T1-weighted MRI findings for a sagittal section of the epididymis showed soft tissue thickening around the epididymis (Figure 2(b)). These MRI findings were consistent with epididymitis but did not show vasculitis change. The MRI for his lower extremities showed a diffuse high-intensity area in his adductor femoris, hamstrings, quadriceps, and triceps surae muscles (Figure 3). In addition, the MRI showed that perivascular high-signal area and vessel wall thickening were seen in the femoral artery and posterior tibial artery, which showed inflammation of the arteries. The patient refused a biopsy for his testicular vein, and a biopsy was performed for his lateral great muscle, which showed mild lymphocyte infiltration of the muscle fibres with degeneration and atrophic change, but the biopsy did not show necrotising vasculitis.
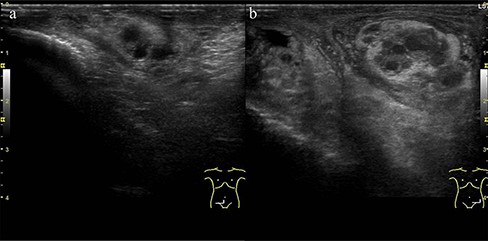
Ultrasound test for his scrotum: (a) right spermatic cord and (b) left spermatic cord. Left spermatic cord was markedly more prominent than the right spermatic cord, with a perineal hyperechoic area.
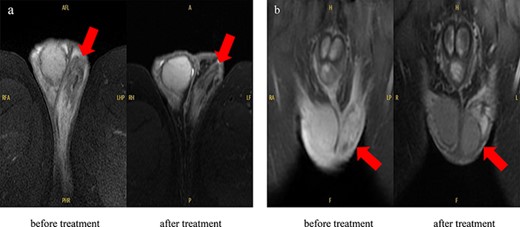
MRI findings in the scrotum: (a) T2-weighted MRI image, which showed enlargement of the left epididymis and dilation of the venous system but improved the size and vasodilation of the epididymis after treatment. (b) Gadolinium-contrasted T1-weighted MRI findings for a sagittal section of the epididymis, which showed soft tissue thickening around the epididymis but improved after treatment.
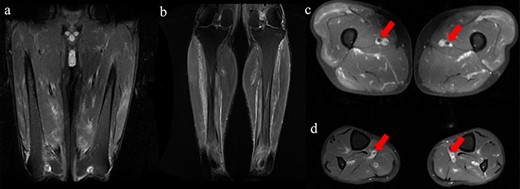
MRI findings for his lower extremities. All images were fat-suppressed T2-weighted MRI findings. (a and c) MRI of femur. (b and d) MRI of lower legs. (a and b) A high intensity area in the adductor femoris, hamstrings, quadriceps, and triceps surae muscles. (c and d) Perivascular high-signal with thickening of the vessel wall was seen in femoral and posterior tibial artery.
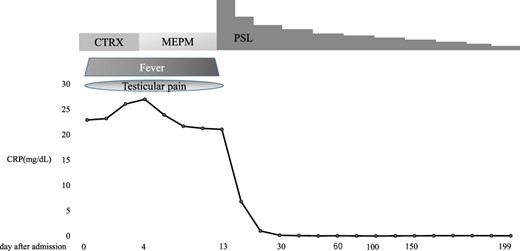
Although the pathological findings did not show vasculitis, the patient fulfilled the American College of Rheumatology 1990 criteria for the classification of PN [6]. In addition, he had myalgia with diffuse hyperintensity area of muscle and inflammatory change of the femoral and posterior tibial arteries on MRI. Furthermore, scrotum MRI showed epididymitis, and infections that cause epididymitis, such as gonorrhoea, chlamydia, and tuberculosis, were denied in blood or urine examinations. Considering these results, we diagnosed him PN with epididymitis and treated with prednisolone (PSL) 60 mg/day. The clinical course is shown in Figure 4. After the treatment, his testicular pain improved within a few days after PSL treatment, and his condition improved. He was discharged from the hospital 31 days after admission. Five months later, his condition was stable with the treatment of PSL 5 mg/day. His MRI for the scrotum at last observation improved compared to that of before treatment (Figure 2).
Discussion
We experienced a possible case of PN with epididymitis following COVID-19 vaccination. Through our experience of this case, we can make two clinically important observations.
First, our patient developed following COVID-19 vaccination. The COVID-19 vaccine is an mRNA vaccine, with a different mechanism from that of existing vaccines. Viral mRNA is administered into human cells in the epithelium encapsulated in lipid nanoparticles (LNPs), which undergo cellular translation to form the target spike protein and promote antibody formation [7]. Vaccines based on mRNA-containing LNPs are a promising new platform. However, injection of LNPs leads to rapid and robust inflammatory responses, characterised by neutrophil infiltration, activation of diverse inflammatory pathways, and production of various inflammatory cytokines and chemokines [8].
On the other hand, in patients with COVID-19, the cooperative action of NF-kB and STAT3 activates the amplification circuit of interleukin 6, resulting in an abnormal increase in the production of inflammatory cytokines [9]. In addition, patients with multiorgan failure had coagulation abnormalities, and these patients were associated with poor prognosis because they had microthrombi of the lungs, lower limbs, hands, brain, heart, liver, and kidneys, while vaccine-induced inflammation is not associated with microthrombi [8, 9].
Although the mechanism of the development of vasculitis following vaccination is unknown, a number of cases of vasculitis have been reported following influenza vaccination [10]. One report showed that 64.6% of systemic vasculitis following influenza vaccination was small vessel vasculitis [11]. Small vessel vasculitis following COVID-19 vaccination has also been reported, mainly ANCA-associated vasculitis (AAV) [4]. In addition, one report showed that AAV patients with the second vaccination were higher than those of the first dose, and it is possible that booster vaccination might have induced the development of AAV [12]. However, medium vessel vasculitis is a rare complication. We found four cases of medium-sized vasculitis following COVID-19 vaccination, including our case, and summarised them in Table 1 [13–15]. Three patients were males, and two patients received mRNA vaccination. Three patients developed vasculitis after the first dose of vaccination. All the patients experienced adverse effects within 30 days of vaccination. Two male patients experienced orchitis. The visceral involvement was renal, mesenteric artery, skin, muscle, and epididymis. Biopsy was performed in three cases, two of which showed necrotising vasculitis. ANCA was negative in all cases. All patients were treated with systemic therapy, including glucocorticoids and immunosuppressive drugs, and all recovered.
| . | Case 1 . | Case 2 . | Case 3 . | Case 4 . |
|---|---|---|---|---|
| Age (years) | 73 | 46 | 52 | 63 |
| Sex | Male | Male | Female | Male |
| Vaccination type | N/A | Pfizer | Astra Zeneca | Pfizer |
| Past history | HBV | HT | None | None |
| Vaccine dose | First | Second | First | First |
| Days after vaccination | 21 | 7 | 28 | 17 |
| CRP (mg/dL) | N/A | 20.3 | Normal | 22.9 |
| ESR (mm/h) | N/A | 11 | Normal | 83 |
| Symptom | Fever, arthralgia, purpura, and orchitis | Abdominal pain | Erythematous subcutaneous nodule | Fever and orchitis |
| Visceral involvement | Kidney | Celiac artery | Cutaneous | Muscle epididymis |
| Treatment | HD, GC, and CY | IVMP, GC, and AZP | GC and MTX | GC |
| Outcome | Improved | Improved | Improved | Improved |
| Reference | [13] | [14] | [15] | This case |
| . | Case 1 . | Case 2 . | Case 3 . | Case 4 . |
|---|---|---|---|---|
| Age (years) | 73 | 46 | 52 | 63 |
| Sex | Male | Male | Female | Male |
| Vaccination type | N/A | Pfizer | Astra Zeneca | Pfizer |
| Past history | HBV | HT | None | None |
| Vaccine dose | First | Second | First | First |
| Days after vaccination | 21 | 7 | 28 | 17 |
| CRP (mg/dL) | N/A | 20.3 | Normal | 22.9 |
| ESR (mm/h) | N/A | 11 | Normal | 83 |
| Symptom | Fever, arthralgia, purpura, and orchitis | Abdominal pain | Erythematous subcutaneous nodule | Fever and orchitis |
| Visceral involvement | Kidney | Celiac artery | Cutaneous | Muscle epididymis |
| Treatment | HD, GC, and CY | IVMP, GC, and AZP | GC and MTX | GC |
| Outcome | Improved | Improved | Improved | Improved |
| Reference | [13] | [14] | [15] | This case |
Abbreviations: AZP, azathioprine; CRP, C-reactive protein; CY, cyclophosphamide; ESR, erythrocyte sedimentation rate; GC, glucocorticoid; HBV, hepatitis B virus; HD, haemodialysisemodialysis; HT, hypertension; IVMP, intravenous methylprednisolone; MTX, methotrexate; N/A, not available.
| . | Case 1 . | Case 2 . | Case 3 . | Case 4 . |
|---|---|---|---|---|
| Age (years) | 73 | 46 | 52 | 63 |
| Sex | Male | Male | Female | Male |
| Vaccination type | N/A | Pfizer | Astra Zeneca | Pfizer |
| Past history | HBV | HT | None | None |
| Vaccine dose | First | Second | First | First |
| Days after vaccination | 21 | 7 | 28 | 17 |
| CRP (mg/dL) | N/A | 20.3 | Normal | 22.9 |
| ESR (mm/h) | N/A | 11 | Normal | 83 |
| Symptom | Fever, arthralgia, purpura, and orchitis | Abdominal pain | Erythematous subcutaneous nodule | Fever and orchitis |
| Visceral involvement | Kidney | Celiac artery | Cutaneous | Muscle epididymis |
| Treatment | HD, GC, and CY | IVMP, GC, and AZP | GC and MTX | GC |
| Outcome | Improved | Improved | Improved | Improved |
| Reference | [13] | [14] | [15] | This case |
| . | Case 1 . | Case 2 . | Case 3 . | Case 4 . |
|---|---|---|---|---|
| Age (years) | 73 | 46 | 52 | 63 |
| Sex | Male | Male | Female | Male |
| Vaccination type | N/A | Pfizer | Astra Zeneca | Pfizer |
| Past history | HBV | HT | None | None |
| Vaccine dose | First | Second | First | First |
| Days after vaccination | 21 | 7 | 28 | 17 |
| CRP (mg/dL) | N/A | 20.3 | Normal | 22.9 |
| ESR (mm/h) | N/A | 11 | Normal | 83 |
| Symptom | Fever, arthralgia, purpura, and orchitis | Abdominal pain | Erythematous subcutaneous nodule | Fever and orchitis |
| Visceral involvement | Kidney | Celiac artery | Cutaneous | Muscle epididymis |
| Treatment | HD, GC, and CY | IVMP, GC, and AZP | GC and MTX | GC |
| Outcome | Improved | Improved | Improved | Improved |
| Reference | [13] | [14] | [15] | This case |
Abbreviations: AZP, azathioprine; CRP, C-reactive protein; CY, cyclophosphamide; ESR, erythrocyte sedimentation rate; GC, glucocorticoid; HBV, hepatitis B virus; HD, haemodialysisemodialysis; HT, hypertension; IVMP, intravenous methylprednisolone; MTX, methotrexate; N/A, not available.
Second, our patient had PN with epididymitis. A previous report showed that approximately 20% of patients with PN had testicular pain, which was associated with epididymitis [16]. In addition, autopsy cases of patients with PN showed that 60–86% had intra-scrotal lesions, which were then identified as common lesions in these patients [17]. On the other hand, several investigators reported epididymitis following vaccination, in particular, measles–mumps–rubella vaccination [18, 19]. The epididymitis following mumps vaccination is considered to be an immune-mediated adverse reaction in which specific lymphocytes for the vaccine antigen or wild-type mumps virus are pre-existing in the testis and react immediately when the vaccine antigen reaches the testis [18]. Testicular pain and epididymitis were also observed in patients with COVID-19, and one report showed that patients with severe COVID-19 developed epididymitis more frequently [20]. In addition, patients with mild to moderate COVID-19 without testicular symptoms also had ultrasonographic findings indicative of epididymitis [21]. The novel COVID-19 has an affinity for angiotensin-converting enzyme 2 (ACE-2) receptors, and angiotensin-converting enzyme 2 (ACE-2), which is frequently found in the COVID-19 pathology of pneumonia, was also found in testicular tissue [22].
On the other hand, a retrospective study showed that COVID-19 vaccination decreased the risk of epididymitis [23]. In addition, the Society for Male Reproduction and Urology and the Society for the Study of Male Reproduction have reported that the COVID-19 vaccination should not be avoided for men desiring fertility [24, 25]. However, there is no solid data indicating whether COVID-19 vaccination can help decrease the risk of epididymitis, and it is possible that the vaccination may induce vasculitis, as seen in our case [26]. Further study is warranted to investigate the association between COVID-19 vaccination and vasculitis.
In conclusion, COVID-19 vaccination may induce vasculitis, and clinicians should be aware of the disease following the vaccination.
Acknowledgements
The authors would like to thank the patient for participating in this report.
Conflict of interest
None declared.
Funding
No specific funding was received from any bodies in the public, commercial, or not-for-profit sectors to carry out the work described in this article.
Patient consent
A written informed consent for this case report has been obtained from the patient.
Ethical approval
Not applicable.
Author contributions
All authors approved the final version of this article. Y.O. had full access to all the data. S.-i.O. was responsible for the organisation and coordination of the case.



