-
PDF
- Split View
-
Views
-
Cite
Cite
Tomohiro Sugimoto, Ai Yorishima, Naoya Oka, Sho Masuda, Naoki Nakamoto, Genki Kidoguchi, Hirofumi Watanabe, Yusuke Yoshida, Sho Mokuda, Shintaro Hirata, Appearance of anti-MDA5 antibody-positive dermatomyositis after COVID-19 vaccination, Modern Rheumatology Case Reports, Volume 7, Issue 1, January 2023, Pages 108–112, https://doi.org/10.1093/mrcr/rxac064
Close - Share Icon Share
ABSTRACT
The direct causes of dermatomyositis, a common autoimmune disease, have not yet been accurately identified, but several studies have linked this condition to various patient-associated and environmental factors, such as viral infections and area of residence. In the present report, we describe our experience with a patient presenting with anti-melanoma differentiation–associated gene 5 (MDA5) antibody-positive dermatomyositis, which developed after vaccination against coronavirus disease 2019 (COVID-19). This patient was simultaneously diagnosed with anti-glutamic acid decarboxylase antibody-positive slowly progressive insulin-dependent diabetes (SPIDDM); her human leucocyte antigen test revealed that she expressed the DRB1*04:05 allele. This is important as this genotype is known to increase susceptibility to both anti-MDA5 antibody-positive dermatomyositis and type I diabetes. To the best of our knowledge, this is the first case of dermatomyositis complicated by SPIDDM identified after COVID-19 vaccination against COVID-19 and presenting with an underlying susceptible genotype. The patient’s genetic predisposition may also be important for the development of autoimmune disease after COVID-19 vaccination.
Introduction
Despite the short period since its first reported case in December 2019, there have been significant changes in the clinical environment surrounding coronavirus disease 2019 (COVID-19). These changes have been driven in large part by the production of several effective vaccines against this virus and a better understanding of its underlying biology. However, even though these vaccines have the potential to prevent the occurrence and aggravation of coronavirus infections, there have been several reports of relatively frequent post-vaccine side reactions [1]. In addition, there have been several other reports describing the exacerbation or induction of various autoimmune diseases in response to these vaccines [2–11]. However, there is little to no information available describing any risk factors associated with these outcomes.
Dermatomyositis is an autoimmune disease predominantly affecting the skin and muscles. Many myositis-specific autoantibodies have been identified, including anti-melanoma differentiation–associated gene 5 (MDA5) autoantibodies. Anti-MDA5 antibody-positive dermatomyositis is associated with rapidly progressive interstitial lung disease, which leads to a poor prognosis [12]. Environmental factors, including viral infections, have been suggested as the root cause of this disease, and its onset and severity have been linked to both the season and place of residence [13].
In addition, human leucocyte antigen (HLA) types, which are commonly associated with various diseases, including rheumatoid arthritis (RA), can also affect these outcomes. One recent study linked HLA-DRB1*04:05 to the development of RA, type I diabetes, and anti-MDA5 antibody-positive dermatomyositis, making it a linked genotype [14–16]. Here, we describe a case of anti-MDA5 antibody-positive dermatomyositis and slowly progressive insulin-dependent diabetes mellitus (SPIDDM) after COVID-19 vaccination. To the best of our knowledge, there have been no previous reports of SPIDDM after COVID-19 vaccination, making this the first reported case of dermatomyositis and SPIDDM after COVID-19 vaccination. This report evaluated both the physical and genetic presentations of this patient to better understand the risk factors associated with severe adverse reactions to COVID-19 prophylaxis.
Case presentation
A healthy 62-year-old woman received the Pfizer-BioNTech COVID-19 vaccine in July and August 2021 with no major adverse reactions. She undergoes a medical examination every year and has not been pointed out to have any abnormalities. Most recently, she was examined in November 2021, and her blood tests showed a haemoglobin A1c (HbA1c) level of 5.8% and a blood glucose level of 95 mg/dl. She received her third dose of this vaccine in February 2022, after which she developed a low-grade fever. Six days later, she developed erythema on the back of her hands and elbows and began to experience swelling in her fingers. She also started to have difficulty standing up from her chair, and at 13 days post-inoculation, her finger swelling worsened, and her ring dug into her hand, forcing her to go to the emergency department at her nearest hospital. She was then diagnosed with interstitial pneumonia using chest computed tomography (CT) and treated with symptomatic support [Figure 1(a)]. On Day 19 post-vaccination, she was diagnosed with dermatomyositis based on the development of clear Gottron’s signs on both her hands and elbows, inverse Gottron’s signs on her palms, periungual erythema, muscle pain, muscle weakness, and anti-MDA5 antibody positivity (Figure 2). She underwent a skin biopsy from the site of erythema on her left elbow; the skin pathology revealed pathological findings suggestive of dermatomyositis. No muscle biopsy was performed. She also presented with hypoxaemia and received a 1000 mg infusion of methylprednisolone for 3 days before initiating oral tacrolimus (TAC) treatment. Intravenous cyclophosphamide (IVCY) therapy was also initiated; the prednisolone (PSL) dose was adjusted to 1.0 mg/kg, and the TAC dose was adjusted to a trough value of 10–12 ng/ml after the initial steroid pulse. These treatment methods were based on a report from Tsujii et al. describing similar cases [17]. Plasmapheresis was initiated 24 days after vaccination, and the nailfold capillaries for each finger were evaluated by nailfold video-capillaroscopy (NVC). These evaluations revealed limited vasodilation and only mild bleeding (Figure 3). Simultaneous blood tests revealed that the antibody titre for MDA-5 was 2190, the ferritin level was 1772 ng/ml, Krebs von den Lungen(KL)-6 was 544 U/ml, creatine kinase level was 269 U/l, and C-reactive protein level was 2.63 mg/dl. The rheumatoid factor, anti-cyclic citrullinated peptide (CCP) antibody, anti-Sjögren's-syndrome-related antigen A (anti-SS-A) antibody, anti-DNA antibody, and anti-neutrophil cytoplasmic antibody (ANCA) tests yielded negative results. In addition, despite undergoing regular medical examinations and never being diagnosed with diabetes, this patient was now found to be presenting with an abnormal HbA1c level (6.9%). She repeatedly reported blood glucose levels of >200 mg/dl for up to 2 h after eating, and subsequent fasting blood tests revealed a fasting blood glucose level of 113 mg/dl and an insulin and C-peptide concentration of 18.5 µIU/ml and 4.4 ng/ml, respectively. Evaluations revealed that insulin secretion was maintained, but there was an abnormal increase in anti-glutamic acid decarboxylase (GAD) antibodies (225.8 U/ml). Given these results, we determined that this patient was experiencing SPIDDM. By the 33rd day post-vaccination, five plasma exchange therapies had been administered, and plasmapheresis was terminated because the antibody titre of MDA5 improved to 85. This change coincided with improved myalgia, but the interstitial opacities on the CT and oxygenation on the patient’s saturation monitor gradually worsened [Figure 1(b)]. We were then forced to revive the methylprednisolone 500 mg intravenous therapy at Day 40 post-vaccination, but by Day 45 post-vaccination, the MDA-5 antibody titre had increased to 865. After 46 days of vaccination, the patient presented with even more pronounced hypoxaemia, and CT showed subcutaneous emphysema and mediastinal emphysema [Figure 1(c)]. These observations coincided with the development of a consciousness disorder, forcing us to complete a magnetic resonance imaging examination of her head. These evaluations revealed a high-intensity area in the diffusion-weighted imaging, facilitating a diagnosis of cerebral infarction. This made it difficult to continue TAC, IVCY, and additional plasmapheresis, but with time the consciousness disorder improved but the shadows on the lung field persisted, accompanied by reduced oxygenation. Unfortunately, despite the concerted effort of the team, this patient died 57 days post-vaccination after continuous unsustainable oxygenation.
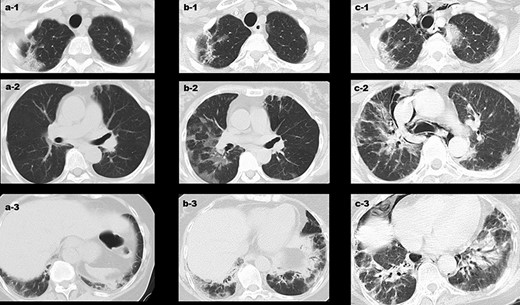
Changes in the CT findings around the patient’s interstitial lung disease at various time points. (a) 13 days post-vaccination, interstitial opacities were observed at the apex and bottom of the lung; (b) 34 days post-vaccination, emergence of a new ground glass shadow and the shadow on the bottom of the lung were exacerbated; and (c) 46 days post-vaccination, the interstitial pneumonia shadow across the entire lung field was exacerbated, and there were clear signs of mediastinal emphysema.
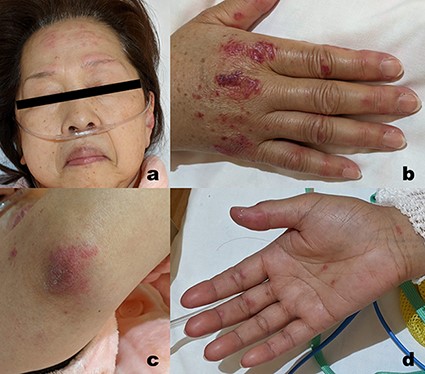
Catalogue of the skin lesions identified 19 days post-vaccination. (a) Erythema on both the forehead and pinna; (b) Gottron’s signs on the back of the hand and periungual erythema; (c) Gottron’s signs on the elbow; and (d) inverse Gottron’s signs on the palms.
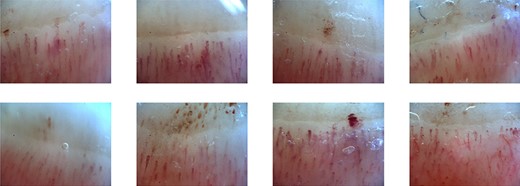
Nailfold capillary abnormalities as evaluated by NVC (OptiPix Capillaroscopy; Optilia Instruments AB, Sollentuna, Sweden). The NVC instrument facilitates a 200× magnification of the area allowing us to evaluate the nailfold capillaries for eight fingers, except thumbs, across both hands. These images revealed some vasodilation and only mild bleeding.
Discussion
This report was created to discuss the case of a 62-year-old patient presenting with sudden onset MDA5 antibody-positive dermatomyositis after their third exposure to the Pfizer-BioNTech COVID-19 vaccine. To the best of our knowledge, our case is the first case report of the occurrence of anti-MDA5 antibody-positive dermatomyositis after COVID-19 vaccination. Currently, there are only 15 reports discussing patients diagnosed with polymyositis or dermatomyositis after COVID-19 vaccination; this number includes the present case and excludes the more frequent cases of local myositis [3–11]. Table 1 summarises the descriptions for these 15 patients and facilitates our evaluation of the overarching themes in these cases. Eight patients (53.3%) presented with autoantibodies, while 10 of the 15 patients were female. In addition, most previous reports describe the initial onset after the first or second vaccination, while in our case, the onset occurred after the third ‘booster’ dose of the vaccine. In our case, the symptoms appeared 6 days after the third COVID-19 vaccination. Furthermore, among the 15 aforementioned cases, dermatomyositis/polymyositis occurred within 1 week in 13 of these cases (86.7%) and within 2 weeks in the remaining 2 cases. The occurrence of these conditions at short intervals from the vaccination suggests a causal relationship. In addition, the patient described in the present report underwent a medical examination every year. More recently, she had a medical examination 3 months before the onset; this examination showed that she neither had diabetes nor interstitial lung disease.
Summary of our study cohort including various cases of dermatomyositis/polymyositis after COVID-19 vaccinations.
| No. . | Age . | Sex . | Antibody . | Type of vaccine/manufacturer . | Number of doses . | Time to onset after vaccination . | Gottron’s sign . | Skin lesions . |
|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Female | PM-Scl | Vector/AstraZeneca | 1 | 4 days | − | + |
| 2 | 74 | Male | None | Vector/AstraZeneca | 1 | 2 days | − | − |
| 3 | 75 | Female | None | Vector/AstraZeneca | 1 | 2 days | − | − |
| 4 | 80 | Female | None | Vector/AstraZeneca | 2 | 2 days | − | − |
| 5 | 46 | Female | Jo-1, Ro-52 | Vector/AstraZeneca | 2 | 1 week | − | + |
| 6 | 47 | Female | None | mRNA/Moderna | 2 | 3 days | − | + |
| 7 | 51 | Male | None | mRNA/Moderna | 2 | 6 days | + | + |
| 8 | 26 | Male | None | mRNA/Moderna | 2 | 7 days | + | + |
| 9 | 55 | Female | Mi-2, Ro-52 | mRNA/Pfizer | 1 | 2 days | − | + |
| 10 | 72 | Female | Fibrillarin | mRNA/Pfizer | 2 | 1 day | − | − |
| 11 | 43 | Female | RNP | mRNA/Pfizer | 2 | 10 days | − | + |
| 12 | 53 | Male | NXP-2 | mRNA/Pfizer | 2 | 2 weeks | − | + |
| 13 | 30 | Male | None | mRNA/Pfizer | 2 | 6 days | + | + |
| 14 | 77 | Female | TIF-1γ | mRNA/Pfizer | 1 | 5 days | − | + |
| 15a | 62 | Female | MDA-5 | mRNA/Pfizer | 3 | 6 days | + | + |
| No. . | Age . | Sex . | Antibody . | Type of vaccine/manufacturer . | Number of doses . | Time to onset after vaccination . | Gottron’s sign . | Skin lesions . |
|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Female | PM-Scl | Vector/AstraZeneca | 1 | 4 days | − | + |
| 2 | 74 | Male | None | Vector/AstraZeneca | 1 | 2 days | − | − |
| 3 | 75 | Female | None | Vector/AstraZeneca | 1 | 2 days | − | − |
| 4 | 80 | Female | None | Vector/AstraZeneca | 2 | 2 days | − | − |
| 5 | 46 | Female | Jo-1, Ro-52 | Vector/AstraZeneca | 2 | 1 week | − | + |
| 6 | 47 | Female | None | mRNA/Moderna | 2 | 3 days | − | + |
| 7 | 51 | Male | None | mRNA/Moderna | 2 | 6 days | + | + |
| 8 | 26 | Male | None | mRNA/Moderna | 2 | 7 days | + | + |
| 9 | 55 | Female | Mi-2, Ro-52 | mRNA/Pfizer | 1 | 2 days | − | + |
| 10 | 72 | Female | Fibrillarin | mRNA/Pfizer | 2 | 1 day | − | − |
| 11 | 43 | Female | RNP | mRNA/Pfizer | 2 | 10 days | − | + |
| 12 | 53 | Male | NXP-2 | mRNA/Pfizer | 2 | 2 weeks | − | + |
| 13 | 30 | Male | None | mRNA/Pfizer | 2 | 6 days | + | + |
| 14 | 77 | Female | TIF-1γ | mRNA/Pfizer | 1 | 5 days | − | + |
| 15a | 62 | Female | MDA-5 | mRNA/Pfizer | 3 | 6 days | + | + |
RNP, ribonucleoprotein; NXP-2, nuclear matrix protein 2; TIF-1γ, transcription intermediary factor 1γ; +, existence; −, none.
This case is in bold the one we experienced.
Summary of our study cohort including various cases of dermatomyositis/polymyositis after COVID-19 vaccinations.
| No. . | Age . | Sex . | Antibody . | Type of vaccine/manufacturer . | Number of doses . | Time to onset after vaccination . | Gottron’s sign . | Skin lesions . |
|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Female | PM-Scl | Vector/AstraZeneca | 1 | 4 days | − | + |
| 2 | 74 | Male | None | Vector/AstraZeneca | 1 | 2 days | − | − |
| 3 | 75 | Female | None | Vector/AstraZeneca | 1 | 2 days | − | − |
| 4 | 80 | Female | None | Vector/AstraZeneca | 2 | 2 days | − | − |
| 5 | 46 | Female | Jo-1, Ro-52 | Vector/AstraZeneca | 2 | 1 week | − | + |
| 6 | 47 | Female | None | mRNA/Moderna | 2 | 3 days | − | + |
| 7 | 51 | Male | None | mRNA/Moderna | 2 | 6 days | + | + |
| 8 | 26 | Male | None | mRNA/Moderna | 2 | 7 days | + | + |
| 9 | 55 | Female | Mi-2, Ro-52 | mRNA/Pfizer | 1 | 2 days | − | + |
| 10 | 72 | Female | Fibrillarin | mRNA/Pfizer | 2 | 1 day | − | − |
| 11 | 43 | Female | RNP | mRNA/Pfizer | 2 | 10 days | − | + |
| 12 | 53 | Male | NXP-2 | mRNA/Pfizer | 2 | 2 weeks | − | + |
| 13 | 30 | Male | None | mRNA/Pfizer | 2 | 6 days | + | + |
| 14 | 77 | Female | TIF-1γ | mRNA/Pfizer | 1 | 5 days | − | + |
| 15a | 62 | Female | MDA-5 | mRNA/Pfizer | 3 | 6 days | + | + |
| No. . | Age . | Sex . | Antibody . | Type of vaccine/manufacturer . | Number of doses . | Time to onset after vaccination . | Gottron’s sign . | Skin lesions . |
|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Female | PM-Scl | Vector/AstraZeneca | 1 | 4 days | − | + |
| 2 | 74 | Male | None | Vector/AstraZeneca | 1 | 2 days | − | − |
| 3 | 75 | Female | None | Vector/AstraZeneca | 1 | 2 days | − | − |
| 4 | 80 | Female | None | Vector/AstraZeneca | 2 | 2 days | − | − |
| 5 | 46 | Female | Jo-1, Ro-52 | Vector/AstraZeneca | 2 | 1 week | − | + |
| 6 | 47 | Female | None | mRNA/Moderna | 2 | 3 days | − | + |
| 7 | 51 | Male | None | mRNA/Moderna | 2 | 6 days | + | + |
| 8 | 26 | Male | None | mRNA/Moderna | 2 | 7 days | + | + |
| 9 | 55 | Female | Mi-2, Ro-52 | mRNA/Pfizer | 1 | 2 days | − | + |
| 10 | 72 | Female | Fibrillarin | mRNA/Pfizer | 2 | 1 day | − | − |
| 11 | 43 | Female | RNP | mRNA/Pfizer | 2 | 10 days | − | + |
| 12 | 53 | Male | NXP-2 | mRNA/Pfizer | 2 | 2 weeks | − | + |
| 13 | 30 | Male | None | mRNA/Pfizer | 2 | 6 days | + | + |
| 14 | 77 | Female | TIF-1γ | mRNA/Pfizer | 1 | 5 days | − | + |
| 15a | 62 | Female | MDA-5 | mRNA/Pfizer | 3 | 6 days | + | + |
RNP, ribonucleoprotein; NXP-2, nuclear matrix protein 2; TIF-1γ, transcription intermediary factor 1γ; +, existence; −, none.
This case is in bold the one we experienced.
This patient was diagnosed with SPIDDM and while her insulin secretory capacity was maintained, her anti-GAD antibody was activated. Because this patient developed both SPIDDM and dermatomyositis, we hypothesised that there may be some underlying genetic risk factor in this case increasing their susceptibility to autoimmune disorders. This was shown to be the case when our evaluations revealed an HLA type of HLA-DRB1*04:05; this was further supported by the fact that this haplotype is strongly associated with type I diabetes and RA [14, 15]. This genotype is also known to act as a sensitivity marker for anti-MDA5 antibody-positive dermatomyositis [16], confirming that our patient had a genetic predisposition to developing dermatomyositis. Other reports also suggest that subacute thyroiditis may be induced after COVID-19 vaccination in patients with HLA-B*35:03 and HLA-C*04:01 genotypes [18]. In addition, two case reports suggest that type I diabetes may be induced after COVID-19 vaccination, one of which had HLA-DRB1*04:05 [19, 20].
There are also reports that there are many adverse reactions in people with specific HLA genotypes after COVID-19 vaccination and that immunogenicity differs depending on these haplotypes [21, 22]. These reports also suggest that the COVID-19 vaccine response may interact with patient-specific factors to produce a unique spectrum of adverse events, as we see here. In this case, the combination of a high-risk HLA genotype and COVID-19 vaccination-induced dermatomyositis resulted in the development of anti-MDA5 antibody-positive dermatomyositis.
Unfortunately, this was a particularly severe case of anti-MDA5 antibody-positive dermatomyositis, and the patient failed to recover despite numerous applications of plasma exchange therapy and early triple therapy intervention. We previously reported that cases with fewer nailfold capillary abnormalities may have a higher severity of lung lesions [23], which was confirmed in this patient, where she presented with mild nailfold abnormalities and rapidly progressing interstitial lung disease.
Previous reports have also linked type I interferon (IFN) to the pathophysiology of anti-MDA5 antibody-positive dermatomyositis [24], while other studies suggest that messenger RNA (mRNA)-mediated COVID-19 vaccines may induce strong type I IFN reactions, which when combined with other factors may create a perfect storm for certain adverse events [25]. These findings were further supported by an earlier case report in which we described a worsening in type I IFN responses in a patient with systemic lupus erythematosus (SLE), after vaccination with a COVID-19 vaccine [2]. Given this, we suggest that autoimmune diseases associated with abnormalities in type I IFN may be adversely affected after COVID-19 vaccination. Type I IFN is known to be involved in both SLE and anti-MDA5 antibody-positive dermatomyositis, with both being positively affected after the addition of Janus kinase (JAK) inhibitors [26, 27].
Thus, our data suggest that while a third COVID-19 vaccination provides increased immunity in many groups, it may also increase the potential for the development of adverse side effects. In addition, recent data show a reduced effect when evaluating a fourth dose of vaccine in low-risk groups, suggesting that additional inoculations and vaccine boosters should be evaluated in more detail to ensure proper efficacy [28]. Thus, we suggest that additional immunisations beyond the standard 2 shot inoculation and a single booster should be evaluated on a case-by-case basis.
Acknowledgements
We would like to thank Editage (www.editage.com) for their assistance with English language editing.
Conflict of interest
None declared.
Funding
This work has not received any funding.
Patient consent
We acquired written informed consent for the publication of this report from the patient’s husband.
Ethics statement
The Ethics Committee at Hiroshima University Hospital does not require ethical approval for the production of case reports. However, we were still obligated to obtain informed consent from the specific participants. This study was then carried out in accordance with the guidelines published by the Ministry of Health, Labour, and Welfare for Japan.



