-
PDF
- Split View
-
Views
-
Cite
Cite
Kenta Misaki, Naoto Tamura, Takanori Azuma, Koichiro Shinoda, Masao Tanaka, Hiroshi Fujiwara, Hideki Tsuboi, Tsuyoshi Kasama, Ryusuke Yoshimi, Tadamasa Hanyu, Yoshiaki Kusaka, Makoto Hirao, Makoto Onishi, Ayumi Uchino, Tomomasa Izumiyama, Kwang-Seok Yang, Noriyoshi Ogawa, Kiyoshi Matsui, Kazuhiro Kurasawa, Satoshi Kawaai, Hidekata Yasuoka, Noriaki Okumura, Yo Ueda, Eiichi Tanaka, Eisuke Inoue, Katsuki Tsuritani, Shigeru Matsumoto, Hisashi Yamanaka, Masayoshi Harigai, Associations of disease duration and anti-citrullinated peptide antibody status with the effectiveness of abatacept in biologic-naïve patients with rheumatoid arthritis: Post hoc analysis of a multicentre, real-world observational study in Japan (ORIGAMI), Modern Rheumatology, Volume 34, Issue 2, March 2024, Pages 297–306, https://doi.org/10.1093/mr/road045
Close - Share Icon Share
ABSTRACT
The aim of the article is to investigate the associations of disease duration and anti-cyclic citrullinated peptide antibody (ACPA) status with the effectiveness of abatacept in biologic-naïve patients with rheumatoid arthritis (RA).
We performed post hoc analyses of the Orencia® Registry in Geographically Assembled Multicenter Investigation (ORIGAMI) study of biologic-naïve RA patients aged ≥20 years with moderate disease activity who were prescribed abatacept. Changes in the Simplified Disease Activity Index (SDAI) and Japanese Health Assessment Questionnaire (J-HAQ) at 4, 24, and 52 weeks of treatment were analysed in patients divided according to ACPA serostatus (positive/negative), disease duration (<1/≥1 year), or both.
SDAI scores decreased from baseline in all groups. SDAI scores tended to decrease more in the ACPA-positive group and disease duration <1-year group than in the ACPA-negative group and disease duration ≥1-year group, respectively. In the disease duration <1-year group, SDAI tended to decrease more in the ACPA-positive group than in the ACPA-negative group. Disease duration was independently associated with the change in SDAI and SDAI remission at Week 52 in multivariable regression models.
These results suggest that starting abatacept within 1 year of diagnosis was associated with greater effectiveness of abatacept in biologic-naïve patients with RA and moderate disease activity.
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune disease that is characterised by joint inflammation and erosion of cartilage and bone due to immune dysfunction and generation of autoantibodies [1]. The introduction of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as methotrexate (MTX), biologic DMARDs (bDMARDs), and targeted synthetic DMARDs (tsDMARDs), over the last two decades has led to profound improvements in the prognosis of RA [1]. However, the efficacies of these therapies vary among individual patients, and patients with low efficacy require changes in their therapy, such as adding another drug or switching to a different regimen, especially in patients not at target despite maximally tolerated doses of MTX [2–5].
Some patients with RA develop biologic-refractory disease and require cycling of bDMARDs [6]. The identification of factors that may predict the response to bDMARDs may facilitate personalised medicine and help achieve the optimal therapy sooner to avoid excessive cycling of bDMARDs. It has been suggested that the retention rates differ across the first bDMARD [7] and that low disease activity and bDMARD persistence (both at 1 year) are significant predictors of long-term bDMARD continuation [8]. Therefore, being able to predict the effectiveness of bDMARDs is important in terms of guiding therapeutic decisions.
Abatacept is a recombinant soluble fusion protein that comprises the extracellular domain of human cytotoxic T-lymphocyte-associated protein 4 and the fragment crystallizable domain of human immunoglobulin G1 [9]. It binds to CD80 and CD86 expressed on the surface of antigen-presenting cells and modulates immunity by inhibiting the co-stimulatory signal through CD28 required for T-cell activation. In Japan, intravenous abatacept was approved for the treatment of RA in 2010 and for juvenile idiopathic arthritis in 2018, and a subcutaneous formulation was approved for the treatment of RA in 2013 [10, 11].
Several studies have sought to identify potential biomarkers, including patient characteristics, for predicting the response to abatacept in patients with RA. For example, several clinical studies, registries, and postmarketing surveillance have documented that the efficacy/effectiveness of abatacept is greater in patients with anti-citrullinated peptide antibody (ACPA), especially patients with high ACPA titres [12–15].
It is thought that the adaptive immune system may play a more pronounced role in the pathogenesis of RA in ACPA-positive patients than in ACPA-negative patients [16], which may help explain why abatacept was often more effective in ACPA-positive patients [12–15].
Several studies have also revealed that shorter disease duration is associated with greater improvements in clinical parameters (e.g. Clinical Disease Activity Index) in patients treated with bDMARDs, including abatacept [17–19].
Despite the knowledge accumulated to date, there remain several unanswered questions. In particular, few studies have examined biomarkers in RA patients with moderate disease activity. Furthermore, it is unknown whether multiple patient/disease factors act together in predicting the response to bDMARDs. The Orencia® Registry in Geographically Assembled Multicenter Investigation (ORIGAMI) study is an ongoing, multicentre, prospective, observational study evaluating the effectiveness and safety of subcutaneous abatacept in patients with biologic-naïve RA and moderate disease activity [Simplified Disease Activity Index (SDAI) >11 to ≤26].
Because the ORIGAMI study is being conducted in real-world settings, data obtained from this study can be used to assess the associations between patient background/clinical factors and the effectiveness of abatacept and thus contribute to personalised medicine. We performed post hoc analyses of the ORIGAMI study to investigate whether disease duration and ACPA serostatus are associated with the effectiveness of abatacept by using data collected from patients treated for up to 52 weeks.
Patients and methods
Study design and patients
The ORIGAMI study is a multicentre, prospective, observational study that enrolled 325 patients with RA who started treatment with subcutaneous abatacept at 64 centres throughout Japan. The design of the study is described in more detail in the previous report [20].
Briefly, patients with RA were eligible for the study if they were ≥20 years old at the time of providing consent, had moderate disease activity (SDAI > 11 to ≤26), had an inadequate response to ≥1 csDMARD, had not previously been treated with a bDMARD, met the blood test criteria (white blood cell count ≥4000/mm3, lymphocyte count ≥1000/mm3, and β-d-glucan negative), and provided written informed consent.
Effectiveness, safety, and patient-reported outcomes data were collected at visits scheduled at baseline, Week 4, Week 24, and Week 52. Further visits are planned every 6 months through to 5 years. Assessments included SDAI, Disease Activity Score-28 for RA with C-reactive protein (DAS28-CRP), and the Japanese Health Assessment Questionnaire (J-HAQ; the Japanese version of the Stanford Health Assessment Questionnaire [21]).
Abatacept was administered subcutaneously, once weekly (125 mg per dose). Treatment could be discontinued in case of consent withdrawal, significant protocol deviation, administration missed three consecutive times, adverse events requiring discontinuation, ineligibility for the study, or at the physician’s discretion.
Data analysis
For the purpose of this study, we analysed data for 270 patients with a confirmed baseline ACPA serostatus (positive/negative) and titre out of 325 patients enrolled in ORIGAMI. The patients were divided into groups according to their baseline ACPA serostatus (positive: ≥4.5 U/ml; negative: <4.5 U/ml as used in the prior report [20]) and disease duration (<1 year, ≥1 year). Patient and disease characteristics were compared between the two groups using the Wilcoxon test or the Mann–Whitney test as appropriate for continuous variables and χ2 tests for categorical variables.
Changes in SDAI over time and from baseline, proportions of patients with disease remission (SDAI score ≤3.3), and changes in J-HAQ were evaluated at each post-baseline visit through to Week 52. However, statistical comparisons between each group were not performed owing to the different patient backgrounds and the post hoc, exploratory nature of these analyses. Missing values were imputed using the last observation carried forward method.
Factors associated with the change in SDAI were analysed by multivariable regression analysis in all patients. The model included the following baseline variables: ACPA serostatus (positive vs negative), disease duration (<1 vs ≥1 year), age, sex, presence of comorbidities (yes vs no), J-HAQ score, rheumatoid factor (RF) status (positive vs negative), glucocorticoid (GC) use (yes vs no), MTX use (yes vs no), and use of a csDMARD other than MTX (yes vs no). These variables were defined prior to conducting the analysis. The presence of an interaction between ACPA serostatus and disease duration for the change in SDAI was also analysed by including an interaction term in multiple regression analysis. R (version 4.0) was used for data analyses.
Results
Association between ACPA serostatus and the effectiveness of abatacept
Patient characteristics
Of 270 patients with a known ACPA serostatus, 226 were positive for ACPA (83.7%) and 44 were negative for ACPA (16.3%). The baseline characteristics of ACPA-positive and ACPA-negative patients are summarised in Table 1. The mean age of the ACPA-positive group was younger than that of the ACPA-negative group. Several baseline factors were significantly greater in the ACPA-positive group, including CRP, erythrocyte sedimentation rate (ESR), swollen joint count (SJC), DAS28-CRP, RF titre, and proportion of RF-positive patients (all P < .05). The mean ± standard deviation (SD) SDAI at baseline was also numerically greater in the ACPA-positive group (20.1 ± 5.6 vs 18.1 ± 5.7; P = .066). Although the mean number of csDMARDs used was significantly greater in the ACPA-positive group than in the ACPA-negative group (1.9 ± 1.0 vs 1.5 ± 0.8; P = .004), the percentages of patients using MTX or GCs, and their mean doses, tended to be similar in both groups. The treatment retention rate at Week 52 was 72.1% in the ACPA-positive group and 58.7% in the ACPA-negative group (Supplementary Figure S1(a)).
| . | ACPA-negative . | ACPA-positive . | . | ||
|---|---|---|---|---|---|
| Variable . | N . | Value . | N . | Value . | P-value . |
| Age, years | 44 | 69.6 ± 14.1 | 226 | 66.5 ± 12.1 | .028 |
| Sex, female | 44 | 39 (88.6) | 226 | 179 (79.2) | .214 |
| Body weight, kg | 44 | 52.2 ± 9.7 | 225 | 52.8 ± 10.6 | .701 |
| Disease duration, years | 44 | 226 | .475 | ||
| <1 | 11 (25.0) | 56 (24.8) | |||
| ≥1 to <2 | 6 (13.6) | 32 (14.2) | |||
| ≥2 to <3 | 4 (9.1) | 13 (5.8) | |||
| ≥3 to <5 | 7 (15.9) | 21 (9.3) | |||
| ≥5 to <10 | 8 (18.2) | 34 (15.0) | |||
| ≥10 | 8 (18.2) | 70 (31.0) | |||
| Comorbidities | 44 | 34 (77.3) | 226 | 184 (81.4) | .668 |
| Pulmonary disease | 44 | 8 (18.2) | 226 | 55 (24.3) | .491 |
| Interstitial pneumonitis | 44 | 2 (4.5) | 226 | 21 (9.3) | .461 |
| Other | 44 | 6 (13.6) | 226 | 40 (17.7) | .662 |
| Cardiovascular disease | 44 | 4 (9.1) | 226 | 15 (6.6) | .795 |
| Renal disease | 44 | 1 (2.3) | 226 | 17 (7.5) | .344 |
| Diabetes | 44 | 6 (13.6) | 226 | 25 (11.1) | .817 |
| Osteoporosis | 44 | 15 (34.1) | 226 | 55 (24.3) | .245 |
| Sjogren’s syndrome | 44 | 5 (11.4) | 226 | 11 (4.9) | .187 |
| Other | 44 | 7 (15.9) | 226 | 63 (27.9) | .142 |
| Past history | 44 | 14 (31.8) | 226 | 68 (30.1) | .961 |
| Infectious disease | 44 | 5 (11.4) | 226 | 18 (8.0) | .657 |
| Malignancy | 44 | 0 (0.0) | 226 | 5 (2.2) | .700 |
| Other | 44 | 9 (20.5) | 226 | 46 (20.4) | 1.000 |
| MTX use | 44 | 26 (59.1) | 226 | 116 (51.3) | .436 |
| Dose, mg/week | 26 | 9.0 ± 3.6 | 116 | 8.9 ± 3.1 | .855 |
| GC use | 44 | 25 (56.8) | 226 | 99 (43.8) | .156 |
| Dose, mg/day | 25 | 4.6 ± 3.1 | 99 | 4.9 ± 3.4 | .857 |
| Use of non-MTX csDMARD | 44 | 18 (40.9) | 226 | 126 (55.8) | .101 |
| Total number of csDMARDs used (including MTX) | 44 | 1.5 ± 0.8 | 226 | 1.9 ± 1.0 | .004 |
| CRP, mg/dl | 44 | 0.9 ± 1.1 | 225 | 1.8 ± 2.4 | <.001 |
| ESR, mm/h | 35 | 26.3 ± 20.8 | 177 | 47.2 ± 28.1 | <.001 |
| TJC | 44 | 4.7 ± 3.5 | 226 | 4.3 ± 3.1 | .718 |
| SJC | 44 | 4.1 ± 2.8 | 226 | 5.4 ± 3.1 | .018 |
| PGA | 44 | 38.6 ± 15.0 | 226 | 40.4 ± 14.7 | .243 |
| SDAI | 44 | 18.1 ± 5.7 | 223 | 20.1 ± 5.6 | .066 |
| DAS28-CRP | 44 | 3.8 ± 0.8 | 223 | 4.1 ± 0.7 | .036 |
| ACPA, U/ml | 44 | 0.9 ± 0.7 | 226 | 280.3 ± 376.8 | <.001 |
| RF, U/ml | 44 | 20.9 ± 61.7 | 226 | 174.8 ± 302.6 | <.001 |
| RF-positive (>15 U/ml) | 44 | 6 (13.6) | 226 | 191 (84.5) | <.001 |
| J-HAQ (0–3) | 44 | 1.2 ± 0.8 | 226 | 1.2 ± 0.7 | .563 |
| EQ-5D | 44 | 0.7 ± 0.1 | 221 | 0.7 ± 0.2 | .357 |
| Pain VAS (0–100) | 44 | 44.8 ± 26.9 | 224 | 47.8 ± 23.6 | .520 |
| Global VAS (0–100) | 44 | 45.2 ± 26.0 | 224 | 45.3 ± 23.0 | .895 |
| . | ACPA-negative . | ACPA-positive . | . | ||
|---|---|---|---|---|---|
| Variable . | N . | Value . | N . | Value . | P-value . |
| Age, years | 44 | 69.6 ± 14.1 | 226 | 66.5 ± 12.1 | .028 |
| Sex, female | 44 | 39 (88.6) | 226 | 179 (79.2) | .214 |
| Body weight, kg | 44 | 52.2 ± 9.7 | 225 | 52.8 ± 10.6 | .701 |
| Disease duration, years | 44 | 226 | .475 | ||
| <1 | 11 (25.0) | 56 (24.8) | |||
| ≥1 to <2 | 6 (13.6) | 32 (14.2) | |||
| ≥2 to <3 | 4 (9.1) | 13 (5.8) | |||
| ≥3 to <5 | 7 (15.9) | 21 (9.3) | |||
| ≥5 to <10 | 8 (18.2) | 34 (15.0) | |||
| ≥10 | 8 (18.2) | 70 (31.0) | |||
| Comorbidities | 44 | 34 (77.3) | 226 | 184 (81.4) | .668 |
| Pulmonary disease | 44 | 8 (18.2) | 226 | 55 (24.3) | .491 |
| Interstitial pneumonitis | 44 | 2 (4.5) | 226 | 21 (9.3) | .461 |
| Other | 44 | 6 (13.6) | 226 | 40 (17.7) | .662 |
| Cardiovascular disease | 44 | 4 (9.1) | 226 | 15 (6.6) | .795 |
| Renal disease | 44 | 1 (2.3) | 226 | 17 (7.5) | .344 |
| Diabetes | 44 | 6 (13.6) | 226 | 25 (11.1) | .817 |
| Osteoporosis | 44 | 15 (34.1) | 226 | 55 (24.3) | .245 |
| Sjogren’s syndrome | 44 | 5 (11.4) | 226 | 11 (4.9) | .187 |
| Other | 44 | 7 (15.9) | 226 | 63 (27.9) | .142 |
| Past history | 44 | 14 (31.8) | 226 | 68 (30.1) | .961 |
| Infectious disease | 44 | 5 (11.4) | 226 | 18 (8.0) | .657 |
| Malignancy | 44 | 0 (0.0) | 226 | 5 (2.2) | .700 |
| Other | 44 | 9 (20.5) | 226 | 46 (20.4) | 1.000 |
| MTX use | 44 | 26 (59.1) | 226 | 116 (51.3) | .436 |
| Dose, mg/week | 26 | 9.0 ± 3.6 | 116 | 8.9 ± 3.1 | .855 |
| GC use | 44 | 25 (56.8) | 226 | 99 (43.8) | .156 |
| Dose, mg/day | 25 | 4.6 ± 3.1 | 99 | 4.9 ± 3.4 | .857 |
| Use of non-MTX csDMARD | 44 | 18 (40.9) | 226 | 126 (55.8) | .101 |
| Total number of csDMARDs used (including MTX) | 44 | 1.5 ± 0.8 | 226 | 1.9 ± 1.0 | .004 |
| CRP, mg/dl | 44 | 0.9 ± 1.1 | 225 | 1.8 ± 2.4 | <.001 |
| ESR, mm/h | 35 | 26.3 ± 20.8 | 177 | 47.2 ± 28.1 | <.001 |
| TJC | 44 | 4.7 ± 3.5 | 226 | 4.3 ± 3.1 | .718 |
| SJC | 44 | 4.1 ± 2.8 | 226 | 5.4 ± 3.1 | .018 |
| PGA | 44 | 38.6 ± 15.0 | 226 | 40.4 ± 14.7 | .243 |
| SDAI | 44 | 18.1 ± 5.7 | 223 | 20.1 ± 5.6 | .066 |
| DAS28-CRP | 44 | 3.8 ± 0.8 | 223 | 4.1 ± 0.7 | .036 |
| ACPA, U/ml | 44 | 0.9 ± 0.7 | 226 | 280.3 ± 376.8 | <.001 |
| RF, U/ml | 44 | 20.9 ± 61.7 | 226 | 174.8 ± 302.6 | <.001 |
| RF-positive (>15 U/ml) | 44 | 6 (13.6) | 226 | 191 (84.5) | <.001 |
| J-HAQ (0–3) | 44 | 1.2 ± 0.8 | 226 | 1.2 ± 0.7 | .563 |
| EQ-5D | 44 | 0.7 ± 0.1 | 221 | 0.7 ± 0.2 | .357 |
| Pain VAS (0–100) | 44 | 44.8 ± 26.9 | 224 | 47.8 ± 23.6 | .520 |
| Global VAS (0–100) | 44 | 45.2 ± 26.0 | 224 | 45.3 ± 23.0 | .895 |
Data are expressed as the mean ± SD or n (%). Variance tests were used for continuous variables, and χ2 tests for categorical variables.
ACPA: anti-cyclic citrullinated peptide antibody; csDMARD: conventional synthetic disease-modifying antirheumatic drug; CRP: C-reactive protein; DAS28-CRP: Disease Activity Score-28 with CRP; EQ-5D: EuroQOL 5 dimension questionnaire; ESR: erythrocyte sedimentation rate; J-HAQ: Japanese Health Assessment Questionnaire; MTX: methotrexate; PGA: physician’s global assessment of disease activity; RF: rheumatoid factor; SDAI: Simplified Disease Activity Index; SJC: swollen joint count; TJC: tender joint count; VAS: visual analogue scale.
| . | ACPA-negative . | ACPA-positive . | . | ||
|---|---|---|---|---|---|
| Variable . | N . | Value . | N . | Value . | P-value . |
| Age, years | 44 | 69.6 ± 14.1 | 226 | 66.5 ± 12.1 | .028 |
| Sex, female | 44 | 39 (88.6) | 226 | 179 (79.2) | .214 |
| Body weight, kg | 44 | 52.2 ± 9.7 | 225 | 52.8 ± 10.6 | .701 |
| Disease duration, years | 44 | 226 | .475 | ||
| <1 | 11 (25.0) | 56 (24.8) | |||
| ≥1 to <2 | 6 (13.6) | 32 (14.2) | |||
| ≥2 to <3 | 4 (9.1) | 13 (5.8) | |||
| ≥3 to <5 | 7 (15.9) | 21 (9.3) | |||
| ≥5 to <10 | 8 (18.2) | 34 (15.0) | |||
| ≥10 | 8 (18.2) | 70 (31.0) | |||
| Comorbidities | 44 | 34 (77.3) | 226 | 184 (81.4) | .668 |
| Pulmonary disease | 44 | 8 (18.2) | 226 | 55 (24.3) | .491 |
| Interstitial pneumonitis | 44 | 2 (4.5) | 226 | 21 (9.3) | .461 |
| Other | 44 | 6 (13.6) | 226 | 40 (17.7) | .662 |
| Cardiovascular disease | 44 | 4 (9.1) | 226 | 15 (6.6) | .795 |
| Renal disease | 44 | 1 (2.3) | 226 | 17 (7.5) | .344 |
| Diabetes | 44 | 6 (13.6) | 226 | 25 (11.1) | .817 |
| Osteoporosis | 44 | 15 (34.1) | 226 | 55 (24.3) | .245 |
| Sjogren’s syndrome | 44 | 5 (11.4) | 226 | 11 (4.9) | .187 |
| Other | 44 | 7 (15.9) | 226 | 63 (27.9) | .142 |
| Past history | 44 | 14 (31.8) | 226 | 68 (30.1) | .961 |
| Infectious disease | 44 | 5 (11.4) | 226 | 18 (8.0) | .657 |
| Malignancy | 44 | 0 (0.0) | 226 | 5 (2.2) | .700 |
| Other | 44 | 9 (20.5) | 226 | 46 (20.4) | 1.000 |
| MTX use | 44 | 26 (59.1) | 226 | 116 (51.3) | .436 |
| Dose, mg/week | 26 | 9.0 ± 3.6 | 116 | 8.9 ± 3.1 | .855 |
| GC use | 44 | 25 (56.8) | 226 | 99 (43.8) | .156 |
| Dose, mg/day | 25 | 4.6 ± 3.1 | 99 | 4.9 ± 3.4 | .857 |
| Use of non-MTX csDMARD | 44 | 18 (40.9) | 226 | 126 (55.8) | .101 |
| Total number of csDMARDs used (including MTX) | 44 | 1.5 ± 0.8 | 226 | 1.9 ± 1.0 | .004 |
| CRP, mg/dl | 44 | 0.9 ± 1.1 | 225 | 1.8 ± 2.4 | <.001 |
| ESR, mm/h | 35 | 26.3 ± 20.8 | 177 | 47.2 ± 28.1 | <.001 |
| TJC | 44 | 4.7 ± 3.5 | 226 | 4.3 ± 3.1 | .718 |
| SJC | 44 | 4.1 ± 2.8 | 226 | 5.4 ± 3.1 | .018 |
| PGA | 44 | 38.6 ± 15.0 | 226 | 40.4 ± 14.7 | .243 |
| SDAI | 44 | 18.1 ± 5.7 | 223 | 20.1 ± 5.6 | .066 |
| DAS28-CRP | 44 | 3.8 ± 0.8 | 223 | 4.1 ± 0.7 | .036 |
| ACPA, U/ml | 44 | 0.9 ± 0.7 | 226 | 280.3 ± 376.8 | <.001 |
| RF, U/ml | 44 | 20.9 ± 61.7 | 226 | 174.8 ± 302.6 | <.001 |
| RF-positive (>15 U/ml) | 44 | 6 (13.6) | 226 | 191 (84.5) | <.001 |
| J-HAQ (0–3) | 44 | 1.2 ± 0.8 | 226 | 1.2 ± 0.7 | .563 |
| EQ-5D | 44 | 0.7 ± 0.1 | 221 | 0.7 ± 0.2 | .357 |
| Pain VAS (0–100) | 44 | 44.8 ± 26.9 | 224 | 47.8 ± 23.6 | .520 |
| Global VAS (0–100) | 44 | 45.2 ± 26.0 | 224 | 45.3 ± 23.0 | .895 |
| . | ACPA-negative . | ACPA-positive . | . | ||
|---|---|---|---|---|---|
| Variable . | N . | Value . | N . | Value . | P-value . |
| Age, years | 44 | 69.6 ± 14.1 | 226 | 66.5 ± 12.1 | .028 |
| Sex, female | 44 | 39 (88.6) | 226 | 179 (79.2) | .214 |
| Body weight, kg | 44 | 52.2 ± 9.7 | 225 | 52.8 ± 10.6 | .701 |
| Disease duration, years | 44 | 226 | .475 | ||
| <1 | 11 (25.0) | 56 (24.8) | |||
| ≥1 to <2 | 6 (13.6) | 32 (14.2) | |||
| ≥2 to <3 | 4 (9.1) | 13 (5.8) | |||
| ≥3 to <5 | 7 (15.9) | 21 (9.3) | |||
| ≥5 to <10 | 8 (18.2) | 34 (15.0) | |||
| ≥10 | 8 (18.2) | 70 (31.0) | |||
| Comorbidities | 44 | 34 (77.3) | 226 | 184 (81.4) | .668 |
| Pulmonary disease | 44 | 8 (18.2) | 226 | 55 (24.3) | .491 |
| Interstitial pneumonitis | 44 | 2 (4.5) | 226 | 21 (9.3) | .461 |
| Other | 44 | 6 (13.6) | 226 | 40 (17.7) | .662 |
| Cardiovascular disease | 44 | 4 (9.1) | 226 | 15 (6.6) | .795 |
| Renal disease | 44 | 1 (2.3) | 226 | 17 (7.5) | .344 |
| Diabetes | 44 | 6 (13.6) | 226 | 25 (11.1) | .817 |
| Osteoporosis | 44 | 15 (34.1) | 226 | 55 (24.3) | .245 |
| Sjogren’s syndrome | 44 | 5 (11.4) | 226 | 11 (4.9) | .187 |
| Other | 44 | 7 (15.9) | 226 | 63 (27.9) | .142 |
| Past history | 44 | 14 (31.8) | 226 | 68 (30.1) | .961 |
| Infectious disease | 44 | 5 (11.4) | 226 | 18 (8.0) | .657 |
| Malignancy | 44 | 0 (0.0) | 226 | 5 (2.2) | .700 |
| Other | 44 | 9 (20.5) | 226 | 46 (20.4) | 1.000 |
| MTX use | 44 | 26 (59.1) | 226 | 116 (51.3) | .436 |
| Dose, mg/week | 26 | 9.0 ± 3.6 | 116 | 8.9 ± 3.1 | .855 |
| GC use | 44 | 25 (56.8) | 226 | 99 (43.8) | .156 |
| Dose, mg/day | 25 | 4.6 ± 3.1 | 99 | 4.9 ± 3.4 | .857 |
| Use of non-MTX csDMARD | 44 | 18 (40.9) | 226 | 126 (55.8) | .101 |
| Total number of csDMARDs used (including MTX) | 44 | 1.5 ± 0.8 | 226 | 1.9 ± 1.0 | .004 |
| CRP, mg/dl | 44 | 0.9 ± 1.1 | 225 | 1.8 ± 2.4 | <.001 |
| ESR, mm/h | 35 | 26.3 ± 20.8 | 177 | 47.2 ± 28.1 | <.001 |
| TJC | 44 | 4.7 ± 3.5 | 226 | 4.3 ± 3.1 | .718 |
| SJC | 44 | 4.1 ± 2.8 | 226 | 5.4 ± 3.1 | .018 |
| PGA | 44 | 38.6 ± 15.0 | 226 | 40.4 ± 14.7 | .243 |
| SDAI | 44 | 18.1 ± 5.7 | 223 | 20.1 ± 5.6 | .066 |
| DAS28-CRP | 44 | 3.8 ± 0.8 | 223 | 4.1 ± 0.7 | .036 |
| ACPA, U/ml | 44 | 0.9 ± 0.7 | 226 | 280.3 ± 376.8 | <.001 |
| RF, U/ml | 44 | 20.9 ± 61.7 | 226 | 174.8 ± 302.6 | <.001 |
| RF-positive (>15 U/ml) | 44 | 6 (13.6) | 226 | 191 (84.5) | <.001 |
| J-HAQ (0–3) | 44 | 1.2 ± 0.8 | 226 | 1.2 ± 0.7 | .563 |
| EQ-5D | 44 | 0.7 ± 0.1 | 221 | 0.7 ± 0.2 | .357 |
| Pain VAS (0–100) | 44 | 44.8 ± 26.9 | 224 | 47.8 ± 23.6 | .520 |
| Global VAS (0–100) | 44 | 45.2 ± 26.0 | 224 | 45.3 ± 23.0 | .895 |
Data are expressed as the mean ± SD or n (%). Variance tests were used for continuous variables, and χ2 tests for categorical variables.
ACPA: anti-cyclic citrullinated peptide antibody; csDMARD: conventional synthetic disease-modifying antirheumatic drug; CRP: C-reactive protein; DAS28-CRP: Disease Activity Score-28 with CRP; EQ-5D: EuroQOL 5 dimension questionnaire; ESR: erythrocyte sedimentation rate; J-HAQ: Japanese Health Assessment Questionnaire; MTX: methotrexate; PGA: physician’s global assessment of disease activity; RF: rheumatoid factor; SDAI: Simplified Disease Activity Index; SJC: swollen joint count; TJC: tender joint count; VAS: visual analogue scale.
Effectiveness of abatacept according to ACPA serostatus
The effectiveness of abatacept was evaluated in terms of the change in SDAI from baseline, the proportions of patients with SDAI remission, and the change in J-HAQ from baseline in the ACPA-positive and ACPA-negative groups. SDAI decreased over time in both groups (Figure 1(a,b)). Proportions of patients with SDAI remission in the ACPA-positive and ACPA-negative groups, respectively, increased from 5.8% and 2.3% at Week 4 to 21.2% and 15.9% at Week 52 (Figure 1(c)). In terms of physical function, J-HAQ also decreased over time in both groups (Figure 1(d)). There was no effect of ACPA titres on the change in SDAI at Week 52.
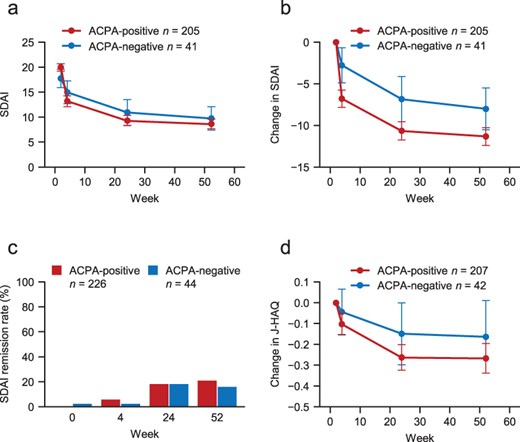
Changes in SDAI and J-HAQ in ACPA-positive and ACPA-negative patients.
Association between disease duration and the effectiveness of abatacept
Patient characteristics
Of the 270 analysed patients, 67 (24.8%) had a disease duration of <1 year and 203 (75.2%) had a disease duration of ≥1 year. The baseline characteristics of these two groups are presented in Table 2. The proportion of males and body weight were significantly greater, and the physician’s global assessment (PGA) score and the number of csDMARDs were significantly lower, in patients with a disease duration of <1 year than in patients with a disease duration of ≥1 year (all P < .05). The percentages of patients using MTX or GCs and their mean doses were similar in both groups. The treatment retention rate at Week 52 was 73.1% in patients with a disease duration of <1 year and 68.8% in patients with a disease duration of ≥1 year (Supplementary Figure S1(b)).
| . | Disease duration <1 year . | Disease duration ≥1 year . | . | ||
|---|---|---|---|---|---|
| Variable . | N . | Value . | N . | Value . | P-value . |
| Age, years | 67 | 67.2 ± 12.5 | 203 | 66.9 ± 12.5 | .782 |
| Sex, female | 67 | 48 (71.6) | 203 | 170 (83.7) | .046 |
| Body weight, kg | 67 | 55.1 ± 10.3 | 202 | 51.9 ± 10.4 | .025 |
| Disease duration, years | 67 | - | 203 | - | <.001 |
| <1 | 67 (100) | 0 (0) | |||
| ≥1 to <2 | 0 (0) | 38 (18.7) | |||
| ≥2 to <3 | 0 (0) | 17 (8.4) | |||
| ≥3 to <5 | 0 (0) | 28 (13.8) | |||
| ≥5 to <10 | 0 (0) | 42 (20.7) | |||
| ≥10 | 0 (0) | 78 (38.4) | |||
| Comorbidities | 67 | 55 (82.1) | 203 | 163 (80.3) | .885 |
| Pulmonary disease | 67 | 14 (20.9) | 203 | 49 (24.1) | .706 |
| Interstitial pneumonitis | 67 | 7 (10.4) | 203 | 16 (7.9) | .689 |
| Other | 67 | 11 (16.4) | 203 | 35 (17.2) | 1.000 |
| Cardiovascular disease | 67 | 5 (7.5) | 203 | 14 (6.9) | 1.000 |
| Renal disease | 67 | 2 (3.0) | 203 | 16 (7.9) | .267 |
| Diabetes | 67 | 5 (7.5) | 203 | 26 (12.8) | .333 |
| Osteoporosis | 67 | 14 (20.9) | 203 | 56 (27.6) | .356 |
| Sjogren’s syndrome | 67 | 2 (3.0) | 203 | 14 (6.9) | .380 |
| Other | 67 | 22 (32.8) | 203 | 48 (23.6) | .184 |
| Past history | 67 | 17 (25.4) | 203 | 65 (32.0) | .383 |
| Infectious disease | 67 | 5 (7.5) | 203 | 18 (8.9) | .917 |
| Malignancy | 67 | 1 (1.5) | 203 | 4 (2.0) | 1.000 |
| Other | 67 | 12 (17.9) | 203 | 43 (21.2) | .688 |
| MTX use | 67 | 38 (56.7) | 203 | 104 (51.2) | .523 |
| Dose, mg/week | 38 | 9.7 ± 2.5 | 104 | 8.7 ± 3.4 | .090 |
| GC use | 67 | 27 (40.3) | 203 | 97 (47.8) | .355 |
| Dose, mg/day | 27 | 5.4 ± 3.3 | 97 | 4.6 ± 3.3 | .136 |
| Use of non-MTX csDMARD | 67 | 30 (44.8) | 203 | 114 (56.2) | .139 |
| Total number of csDMARDs used (including MTX) | 67 | 1.4 ± 0.7 | 203 | 2.0 ± 1.0 | <.001 |
| CRP, mg/dl | 67 | 1.4 ± 1.9 | 202 | 1.8 ± 2.4 | .105 |
| ESR, mm/h | 53 | 43.3 ± 27.5 | 159 | 43.8 ± 28.4 | .987 |
| TJC | 67 | 4.6 ± 3.0 | 203 | 4.3 ± 3.2 | .325 |
| SJC | 67 | 4.7 ± 2.6 | 203 | 5.3 ± 3.2 | .258 |
| PGA | 67 | 36.1 ± 13.6 | 203 | 41.4 ± 14.9 | .005 |
| SDAI | 67 | 18.7 ± 5.6 | 200 | 20.1 ± 5.7 | .117 |
| DAS28-CRP | 67 | 4.0 ± 0.8 | 200 | 4.1 ± 0.7 | .418 |
| ACPA, U/ml | 67 | 209.6 ± 331.2 | 203 | 243.1 ± 369.2 | .468 |
| ACPA-positive (≥4.5 U/ml) | 67 | 56 (83.6) | 203 | 170 (83.7) | 1.000 |
| RF, U/ml | 67 | 127.0 ± 255.3 | 203 | 157.2 ± 292.6 | .840 |
| RF-positive (>15 U/ml) | 67 | 49 (73.1) | 203 | 148 (72.9) | 1.000 |
| J-HAQ | 67 | 1.1 ± 0.7 | 203 | 1.2 ± 0.8 | .292 |
| EQ-5D | 67 | 0.7 ± 0.1 | 198 | 0.6 ± 0.1 | .107 |
| Pain VAS | 67 | 44.5 ± 24.7 | 201 | 48.2 ± 24 | .285 |
| Global VAS | 67 | 43.3 ± 25.3 | 201 | 45.9 ± 22.8 | .522 |
| . | Disease duration <1 year . | Disease duration ≥1 year . | . | ||
|---|---|---|---|---|---|
| Variable . | N . | Value . | N . | Value . | P-value . |
| Age, years | 67 | 67.2 ± 12.5 | 203 | 66.9 ± 12.5 | .782 |
| Sex, female | 67 | 48 (71.6) | 203 | 170 (83.7) | .046 |
| Body weight, kg | 67 | 55.1 ± 10.3 | 202 | 51.9 ± 10.4 | .025 |
| Disease duration, years | 67 | - | 203 | - | <.001 |
| <1 | 67 (100) | 0 (0) | |||
| ≥1 to <2 | 0 (0) | 38 (18.7) | |||
| ≥2 to <3 | 0 (0) | 17 (8.4) | |||
| ≥3 to <5 | 0 (0) | 28 (13.8) | |||
| ≥5 to <10 | 0 (0) | 42 (20.7) | |||
| ≥10 | 0 (0) | 78 (38.4) | |||
| Comorbidities | 67 | 55 (82.1) | 203 | 163 (80.3) | .885 |
| Pulmonary disease | 67 | 14 (20.9) | 203 | 49 (24.1) | .706 |
| Interstitial pneumonitis | 67 | 7 (10.4) | 203 | 16 (7.9) | .689 |
| Other | 67 | 11 (16.4) | 203 | 35 (17.2) | 1.000 |
| Cardiovascular disease | 67 | 5 (7.5) | 203 | 14 (6.9) | 1.000 |
| Renal disease | 67 | 2 (3.0) | 203 | 16 (7.9) | .267 |
| Diabetes | 67 | 5 (7.5) | 203 | 26 (12.8) | .333 |
| Osteoporosis | 67 | 14 (20.9) | 203 | 56 (27.6) | .356 |
| Sjogren’s syndrome | 67 | 2 (3.0) | 203 | 14 (6.9) | .380 |
| Other | 67 | 22 (32.8) | 203 | 48 (23.6) | .184 |
| Past history | 67 | 17 (25.4) | 203 | 65 (32.0) | .383 |
| Infectious disease | 67 | 5 (7.5) | 203 | 18 (8.9) | .917 |
| Malignancy | 67 | 1 (1.5) | 203 | 4 (2.0) | 1.000 |
| Other | 67 | 12 (17.9) | 203 | 43 (21.2) | .688 |
| MTX use | 67 | 38 (56.7) | 203 | 104 (51.2) | .523 |
| Dose, mg/week | 38 | 9.7 ± 2.5 | 104 | 8.7 ± 3.4 | .090 |
| GC use | 67 | 27 (40.3) | 203 | 97 (47.8) | .355 |
| Dose, mg/day | 27 | 5.4 ± 3.3 | 97 | 4.6 ± 3.3 | .136 |
| Use of non-MTX csDMARD | 67 | 30 (44.8) | 203 | 114 (56.2) | .139 |
| Total number of csDMARDs used (including MTX) | 67 | 1.4 ± 0.7 | 203 | 2.0 ± 1.0 | <.001 |
| CRP, mg/dl | 67 | 1.4 ± 1.9 | 202 | 1.8 ± 2.4 | .105 |
| ESR, mm/h | 53 | 43.3 ± 27.5 | 159 | 43.8 ± 28.4 | .987 |
| TJC | 67 | 4.6 ± 3.0 | 203 | 4.3 ± 3.2 | .325 |
| SJC | 67 | 4.7 ± 2.6 | 203 | 5.3 ± 3.2 | .258 |
| PGA | 67 | 36.1 ± 13.6 | 203 | 41.4 ± 14.9 | .005 |
| SDAI | 67 | 18.7 ± 5.6 | 200 | 20.1 ± 5.7 | .117 |
| DAS28-CRP | 67 | 4.0 ± 0.8 | 200 | 4.1 ± 0.7 | .418 |
| ACPA, U/ml | 67 | 209.6 ± 331.2 | 203 | 243.1 ± 369.2 | .468 |
| ACPA-positive (≥4.5 U/ml) | 67 | 56 (83.6) | 203 | 170 (83.7) | 1.000 |
| RF, U/ml | 67 | 127.0 ± 255.3 | 203 | 157.2 ± 292.6 | .840 |
| RF-positive (>15 U/ml) | 67 | 49 (73.1) | 203 | 148 (72.9) | 1.000 |
| J-HAQ | 67 | 1.1 ± 0.7 | 203 | 1.2 ± 0.8 | .292 |
| EQ-5D | 67 | 0.7 ± 0.1 | 198 | 0.6 ± 0.1 | .107 |
| Pain VAS | 67 | 44.5 ± 24.7 | 201 | 48.2 ± 24 | .285 |
| Global VAS | 67 | 43.3 ± 25.3 | 201 | 45.9 ± 22.8 | .522 |
Data are expressed as mean ± SD or n (%). Variance tests were used for continuous variables, and χ2 tests for categorical variables.
ACPA: anti-cyclic citrullinated peptide antibody; csDMARD: conventional synthetic disease-modifying antirheumatic drug; CRP: C-reactive protein; DAS28-CRP: Disease Activity Score-28 with CRP; EQ-5D: EuroQol 5 Dimension questionnaire; ESR: erythrocyte sedimentation rate; J-HAQ: Japanese Health Assessment Questionnaire; MTX: methotrexate; PGA: physician’s global assessment of disease activity; RF: rheumatoid factor; SDAI: Simplified Disease Activity Index; SJC: swollen joint count; TJC: tender joint count; VAS: visual analogue scale.
| . | Disease duration <1 year . | Disease duration ≥1 year . | . | ||
|---|---|---|---|---|---|
| Variable . | N . | Value . | N . | Value . | P-value . |
| Age, years | 67 | 67.2 ± 12.5 | 203 | 66.9 ± 12.5 | .782 |
| Sex, female | 67 | 48 (71.6) | 203 | 170 (83.7) | .046 |
| Body weight, kg | 67 | 55.1 ± 10.3 | 202 | 51.9 ± 10.4 | .025 |
| Disease duration, years | 67 | - | 203 | - | <.001 |
| <1 | 67 (100) | 0 (0) | |||
| ≥1 to <2 | 0 (0) | 38 (18.7) | |||
| ≥2 to <3 | 0 (0) | 17 (8.4) | |||
| ≥3 to <5 | 0 (0) | 28 (13.8) | |||
| ≥5 to <10 | 0 (0) | 42 (20.7) | |||
| ≥10 | 0 (0) | 78 (38.4) | |||
| Comorbidities | 67 | 55 (82.1) | 203 | 163 (80.3) | .885 |
| Pulmonary disease | 67 | 14 (20.9) | 203 | 49 (24.1) | .706 |
| Interstitial pneumonitis | 67 | 7 (10.4) | 203 | 16 (7.9) | .689 |
| Other | 67 | 11 (16.4) | 203 | 35 (17.2) | 1.000 |
| Cardiovascular disease | 67 | 5 (7.5) | 203 | 14 (6.9) | 1.000 |
| Renal disease | 67 | 2 (3.0) | 203 | 16 (7.9) | .267 |
| Diabetes | 67 | 5 (7.5) | 203 | 26 (12.8) | .333 |
| Osteoporosis | 67 | 14 (20.9) | 203 | 56 (27.6) | .356 |
| Sjogren’s syndrome | 67 | 2 (3.0) | 203 | 14 (6.9) | .380 |
| Other | 67 | 22 (32.8) | 203 | 48 (23.6) | .184 |
| Past history | 67 | 17 (25.4) | 203 | 65 (32.0) | .383 |
| Infectious disease | 67 | 5 (7.5) | 203 | 18 (8.9) | .917 |
| Malignancy | 67 | 1 (1.5) | 203 | 4 (2.0) | 1.000 |
| Other | 67 | 12 (17.9) | 203 | 43 (21.2) | .688 |
| MTX use | 67 | 38 (56.7) | 203 | 104 (51.2) | .523 |
| Dose, mg/week | 38 | 9.7 ± 2.5 | 104 | 8.7 ± 3.4 | .090 |
| GC use | 67 | 27 (40.3) | 203 | 97 (47.8) | .355 |
| Dose, mg/day | 27 | 5.4 ± 3.3 | 97 | 4.6 ± 3.3 | .136 |
| Use of non-MTX csDMARD | 67 | 30 (44.8) | 203 | 114 (56.2) | .139 |
| Total number of csDMARDs used (including MTX) | 67 | 1.4 ± 0.7 | 203 | 2.0 ± 1.0 | <.001 |
| CRP, mg/dl | 67 | 1.4 ± 1.9 | 202 | 1.8 ± 2.4 | .105 |
| ESR, mm/h | 53 | 43.3 ± 27.5 | 159 | 43.8 ± 28.4 | .987 |
| TJC | 67 | 4.6 ± 3.0 | 203 | 4.3 ± 3.2 | .325 |
| SJC | 67 | 4.7 ± 2.6 | 203 | 5.3 ± 3.2 | .258 |
| PGA | 67 | 36.1 ± 13.6 | 203 | 41.4 ± 14.9 | .005 |
| SDAI | 67 | 18.7 ± 5.6 | 200 | 20.1 ± 5.7 | .117 |
| DAS28-CRP | 67 | 4.0 ± 0.8 | 200 | 4.1 ± 0.7 | .418 |
| ACPA, U/ml | 67 | 209.6 ± 331.2 | 203 | 243.1 ± 369.2 | .468 |
| ACPA-positive (≥4.5 U/ml) | 67 | 56 (83.6) | 203 | 170 (83.7) | 1.000 |
| RF, U/ml | 67 | 127.0 ± 255.3 | 203 | 157.2 ± 292.6 | .840 |
| RF-positive (>15 U/ml) | 67 | 49 (73.1) | 203 | 148 (72.9) | 1.000 |
| J-HAQ | 67 | 1.1 ± 0.7 | 203 | 1.2 ± 0.8 | .292 |
| EQ-5D | 67 | 0.7 ± 0.1 | 198 | 0.6 ± 0.1 | .107 |
| Pain VAS | 67 | 44.5 ± 24.7 | 201 | 48.2 ± 24 | .285 |
| Global VAS | 67 | 43.3 ± 25.3 | 201 | 45.9 ± 22.8 | .522 |
| . | Disease duration <1 year . | Disease duration ≥1 year . | . | ||
|---|---|---|---|---|---|
| Variable . | N . | Value . | N . | Value . | P-value . |
| Age, years | 67 | 67.2 ± 12.5 | 203 | 66.9 ± 12.5 | .782 |
| Sex, female | 67 | 48 (71.6) | 203 | 170 (83.7) | .046 |
| Body weight, kg | 67 | 55.1 ± 10.3 | 202 | 51.9 ± 10.4 | .025 |
| Disease duration, years | 67 | - | 203 | - | <.001 |
| <1 | 67 (100) | 0 (0) | |||
| ≥1 to <2 | 0 (0) | 38 (18.7) | |||
| ≥2 to <3 | 0 (0) | 17 (8.4) | |||
| ≥3 to <5 | 0 (0) | 28 (13.8) | |||
| ≥5 to <10 | 0 (0) | 42 (20.7) | |||
| ≥10 | 0 (0) | 78 (38.4) | |||
| Comorbidities | 67 | 55 (82.1) | 203 | 163 (80.3) | .885 |
| Pulmonary disease | 67 | 14 (20.9) | 203 | 49 (24.1) | .706 |
| Interstitial pneumonitis | 67 | 7 (10.4) | 203 | 16 (7.9) | .689 |
| Other | 67 | 11 (16.4) | 203 | 35 (17.2) | 1.000 |
| Cardiovascular disease | 67 | 5 (7.5) | 203 | 14 (6.9) | 1.000 |
| Renal disease | 67 | 2 (3.0) | 203 | 16 (7.9) | .267 |
| Diabetes | 67 | 5 (7.5) | 203 | 26 (12.8) | .333 |
| Osteoporosis | 67 | 14 (20.9) | 203 | 56 (27.6) | .356 |
| Sjogren’s syndrome | 67 | 2 (3.0) | 203 | 14 (6.9) | .380 |
| Other | 67 | 22 (32.8) | 203 | 48 (23.6) | .184 |
| Past history | 67 | 17 (25.4) | 203 | 65 (32.0) | .383 |
| Infectious disease | 67 | 5 (7.5) | 203 | 18 (8.9) | .917 |
| Malignancy | 67 | 1 (1.5) | 203 | 4 (2.0) | 1.000 |
| Other | 67 | 12 (17.9) | 203 | 43 (21.2) | .688 |
| MTX use | 67 | 38 (56.7) | 203 | 104 (51.2) | .523 |
| Dose, mg/week | 38 | 9.7 ± 2.5 | 104 | 8.7 ± 3.4 | .090 |
| GC use | 67 | 27 (40.3) | 203 | 97 (47.8) | .355 |
| Dose, mg/day | 27 | 5.4 ± 3.3 | 97 | 4.6 ± 3.3 | .136 |
| Use of non-MTX csDMARD | 67 | 30 (44.8) | 203 | 114 (56.2) | .139 |
| Total number of csDMARDs used (including MTX) | 67 | 1.4 ± 0.7 | 203 | 2.0 ± 1.0 | <.001 |
| CRP, mg/dl | 67 | 1.4 ± 1.9 | 202 | 1.8 ± 2.4 | .105 |
| ESR, mm/h | 53 | 43.3 ± 27.5 | 159 | 43.8 ± 28.4 | .987 |
| TJC | 67 | 4.6 ± 3.0 | 203 | 4.3 ± 3.2 | .325 |
| SJC | 67 | 4.7 ± 2.6 | 203 | 5.3 ± 3.2 | .258 |
| PGA | 67 | 36.1 ± 13.6 | 203 | 41.4 ± 14.9 | .005 |
| SDAI | 67 | 18.7 ± 5.6 | 200 | 20.1 ± 5.7 | .117 |
| DAS28-CRP | 67 | 4.0 ± 0.8 | 200 | 4.1 ± 0.7 | .418 |
| ACPA, U/ml | 67 | 209.6 ± 331.2 | 203 | 243.1 ± 369.2 | .468 |
| ACPA-positive (≥4.5 U/ml) | 67 | 56 (83.6) | 203 | 170 (83.7) | 1.000 |
| RF, U/ml | 67 | 127.0 ± 255.3 | 203 | 157.2 ± 292.6 | .840 |
| RF-positive (>15 U/ml) | 67 | 49 (73.1) | 203 | 148 (72.9) | 1.000 |
| J-HAQ | 67 | 1.1 ± 0.7 | 203 | 1.2 ± 0.8 | .292 |
| EQ-5D | 67 | 0.7 ± 0.1 | 198 | 0.6 ± 0.1 | .107 |
| Pain VAS | 67 | 44.5 ± 24.7 | 201 | 48.2 ± 24 | .285 |
| Global VAS | 67 | 43.3 ± 25.3 | 201 | 45.9 ± 22.8 | .522 |
Data are expressed as mean ± SD or n (%). Variance tests were used for continuous variables, and χ2 tests for categorical variables.
ACPA: anti-cyclic citrullinated peptide antibody; csDMARD: conventional synthetic disease-modifying antirheumatic drug; CRP: C-reactive protein; DAS28-CRP: Disease Activity Score-28 with CRP; EQ-5D: EuroQol 5 Dimension questionnaire; ESR: erythrocyte sedimentation rate; J-HAQ: Japanese Health Assessment Questionnaire; MTX: methotrexate; PGA: physician’s global assessment of disease activity; RF: rheumatoid factor; SDAI: Simplified Disease Activity Index; SJC: swollen joint count; TJC: tender joint count; VAS: visual analogue scale.
Effectiveness of abatacept according to disease duration
SDAI and J-HAQ were also evaluated in patients with a disease duration of <1 year or ≥1 year. As shown in Figure 2(a,b), SDAI decreased over time in both groups. The proportions of patients with SDAI remission in the <1- and ≥1-year groups, respectively, increased from 9.0% and 3.9% at Week 4 to 35.8% and 15.3% at Week 52 (Figure 2(c)). J-HAQ also decreased over time in both groups (Figure 2(d)).
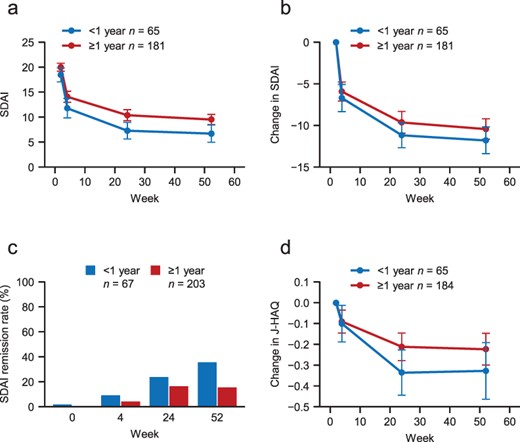
Changes in SDAI and J-HAQ in patients with a disease duration of <1 year or ≥1 year.
Independent associations of disease duration with the effectiveness of abatacept at Week 52
Multiple regression analysis was performed to assess whether ACPA serostatus and disease duration were independently associated with the change in SDAI at Week 52 (linear regression) and with SDAI remission (logistic regression) as the outcome variables. In the multiple linear regression model, disease duration was independently associated with the change in SDAI at Week 52, with an estimate of −2.84 [95% confidence interval (CI): −4.90 to −0.78; P = .007] for disease duration of <1 year (vs ≥1 year) (Figure 3(a)). We next performed sensitivity analyses by changing the cut-off values for disease duration. Using cut-off values of 2 and 5 years, the estimates decreased to −1.42 (P = .130) and −0.09 (P = .923), respectively (Supplementary Figure S2). Therefore, the cut-off value of 1 year had the greatest impact on the effectiveness of abatacept. Similarly, the multiple logistic regression model revealed that disease duration using the cut-off value of 1 year was significantly and independently associated with SDAI remission at Week 52 (Figure 3(b)). On the other hand, ACPA serostatus was not significantly associated with the change in either SDAI or SDAI remission at Week 52 in multivariable analyses (Figure 3(a,b)). Similar results were obtained when we used ACPA cut-off values of 13.5 and 100 U/ml (data not shown).
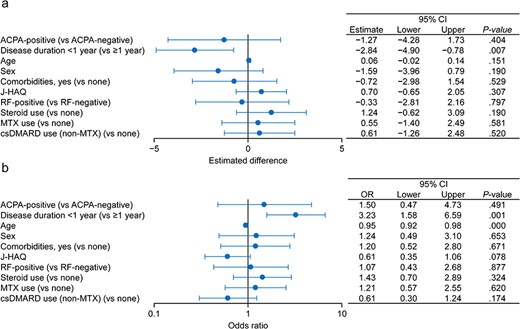
Factors associated with the change in SDAI and SDAI remission at Week 52.
Association between ACPA combined with disease duration and the effectiveness of abatacept
We next investigated whether ACPA could be combined with disease duration as a potential biomarker for the response to abatacept. Among patients with a disease duration of <1 year, SDAI steadily decreased over time in ACPA-positive and ACPA-negative patients (Figure 4(a)), with a tendency for greater changes in the ACPA-positive group than in the ACPA-negative group throughout the 52-week study period (Figure 4(b)). However, the multivariable regression analysis did not reveal a significant interaction between these variables (estimate 0.93; 95% CI −2.58 to 8.44; data not shown). SDAI also decreased among patients with a disease duration of ≥1 year (Supplementary Figure S3), with a trend towards greater numerical changes in ACPA-positive patients.
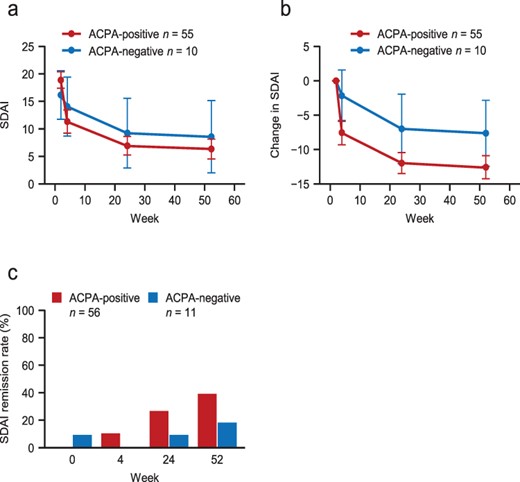
Changes in SDAI over time in ACPA-positive and ACPA-negative patients with a disease duration of <1 year.
Changes in ACPA titres according to disease duration
Finally, we examined the changes in ACPA titres among ACPA-positive patients according to the disease duration. As shown in Figure 5, the ACPA titre decreased among patients with a disease duration of <1 year (∼30% decrease), whereas no clear change was observed in patients with a disease duration of ≥1 year.
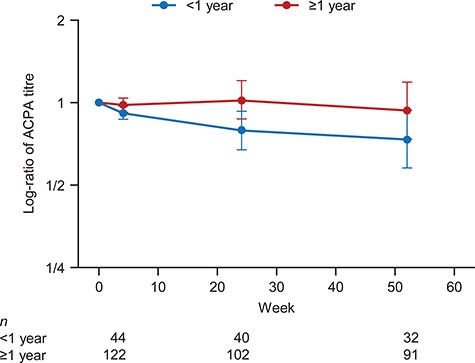
Changes in ACPA titres (log-ratio) from baseline according to disease duration in ACPA-positive patients.
Discussion
In this post hoc analysis, we examined the effects of disease duration and ACPA, which have already been reported to be associated with efficacy, on SDAI and HAQ in patients treated with abatacept. In addition, we investigated whether patient and clinical characteristics at baseline, including disease duration and ACPA status, were associated with the effectiveness of abatacept in terms of the improvement in SDAI.
The present analyses revealed that a disease duration of <1 year showed the strongest association with the change in SDAI from baseline and the proportion of patients with SDAI remission at Week 52. Furthermore, patients with a disease duration of <1 year tended to show greater decreases in J-HAQ scores, suggestive of improved physical function. Nevertheless, improvements in SDAI and J-HAQ were also observed in patients with a disease duration of ≥1 year, underscoring the effectiveness of abatacept in patients with longer disease duration. These findings are consistent with those of earlier studies in which shorter disease duration was associated with better outcomes in patients treated with abatacept [18, 22] or other bDMARDs, supporting the early introduction of these drugs [17, 19].
Abatacept suppresses T-cell activation by inhibiting antigen presentation from antigen-presenting cells [9]. In ACPA-positive RA, the presentation of citrullinated peptides from dendritic cells is thought to affect the development and progression of RA [16]. The pronounced activation of T cells in early RA may allow abatacept to exert good clinical benefits at this time. Interestingly, we found that the ACPA titre decreased over time in patients with a disease duration of <1 year, but not in patients with a longer disease duration. This finding is consistent with a previous study in which the decrease in ACPA over time was greater in abatacept responders than in non-responders [23]. These findings provide further evidence that abatacept is more effective when it is used in the early stages of RA, such as within 1 year of diagnosis [18].
Several prior studies have demonstrated that ACPA (or anti-CCP antibody) seropositivity and titre are associated with the effectiveness of abatacept in various patient populations [12–15]. Therefore, we hypothesised this association could be found in data from the ORIGAMI study. Although the changes in SDAI and J-HAQ over time tended to be greater in ACPA-positive patients than in ACPA-negative patients (Figure 1), the baseline ACPA serostatus was not an independent factor in the multivariable logistic regression analysis (Figure 3). This inconsistency between the current analysis and previous studies could be due to differences in the adjustment for confounding factors in the multivariable analyses that included radiographic erosion, tobacco use [12], measures of serostatus (ACPA and/or RF) [13], a comorbidity index, and the number of prior bDMARDs [14]. By comparison, we used different confounding factors (as described in the Patients and methods), all of which were defined prior to performing these post hoc analyses. In addition, the characteristics of the patients and the small number of ACPA-negative patients may contribute to the discordance between the current study and previous reports.
It has also been suggested that combinations of two or more factors, including ACPA serostatus and disease duration, may predict the effectiveness of abatacept [24, 25]. We also investigated an interaction between ACPA serostatus and disease duration in the multiple regression models. However, the interaction term was not significant. This lack of an interaction might be related to the imbalance in the number of patients in each group. In particular, the subgroup of ACPA-negative patients with a disease duration of <1 year comprised only 10 patients.
This study has several strengths and limitations. Strengths of the study include the involvement of a large number of institutions across Japan, which should reduce any facility bias and increase generalisability of the results. Furthermore, all of the patients were biologic-naïve and the use of concomitant csDMARDs and GCs was generally comparable among the study subgroups by ACPA status and disease duration, limiting potential bias due to the use of other drugs. However, some limitations of this study include its observational design and it may not be possible to account for other background factors not recorded in the study. The sample size was also relatively small with an imbalance in the numbers of patients between the subgroups.
In conclusion, the results of these analyses of the ORIGAMI study revealed that starting abatacept within 1 year of diagnosis was associated with greater effectiveness of abatacept in terms of the change in SDAI from baseline or SDAI remission in biologic-naïve patients with RA and moderate disease activity. Analyses of the ORIGAMI study at later follow-up dates may reveal further insight into the factors associated with the long-term effectiveness of abatacept for treating RA.
Acknowledgements
The authors thank the patients and their families for participating in this study. The authors thank Nicholas D. Smith (EMC K.K., Japan) for medical writing support and Mebix (Tokyo, Japan) for their role as the contract research organisation, which were funded by Bristol-Myers Squibb K.K. and Ono Pharmaceutical Co., Ltd.
Supplementary data
Supplementary data are available at Modern Rheumatology online.
Conflict of interest
K.M. reported grants from Ono Pharmaceutical, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astellas Pharma, Bayer, Boehringer Ingelheim, Eli Lilly Japan, Chugai Pharmaceutical, Sanofi, Kyowa Kirin, AbbVie, Pfizer Japan, Ono Pharmaceutical, UCB Japan, Mitsubishi Tanabe Pharma, Gilead Sciences, Nippon Shinyaku, Novartis Pharma, Eisai, Janssen Pharmaceutical, Teijin Pharma, and Asahi Kasei.
N.T. reported grants or contracts from AbbVie, Astellas Pharma, Asahi Kasei Pharma, Ayumi Pharma, Chugai Pharmaceutical. Eisai, and Mitsubishi Tanabe Pharma; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie, Astellas Pharma, Asahi Kasei Pharma, Chugai Pharmaceutical, Eisai, and Mitsubishi Tanabe Pharma.
M.T. reported support for this study from Bristol-Myers Squibb and Ono Pharmaceutical; grants or contracts from Nagahama City, Mitsubishi Tanabe Pharma, Ayumi Pharmaceutical, UCB Japan, AbbVie, Taisho Pharmaceutical, Toyooka City, Chugai Pharmaceutical, Asahi Kasei Pharma, Daiichi-Sankyo, Kyowa Kirin, and Teijin Pharma; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie, Astellas Pharma, Daiichi-Sankyo, Eli Lilly Japan, Pfizer Japan, UCB Japan, Asahi Kasei Pharma, Chugai Pharmaceutical, Eisai, Janssen Pharmaceutical, and Mitsubishi Tanabe Pharma.
R.Y. reported grants from Eli Lilly Japan, Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, and Bristol-Myers Squibb; and payment or honoraria for lectures from Sanofi, Eli Lilly Japan, Asahi Kasei Pharma, AbbVie, Janssen Pharmaceutical, Eisai, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Astellas Pharma, Chugai Pharmaceutical, Towa Pharmaceutical, UCB Japan, Pfizer Japan, and Ayumi Pharmaceutical.
K.-S.Y. reported presentation fees from Eisai and AbbVie.
K.M. reported grants or contracts from Chugai Pharmaceutical and Asahi Kasei Pharma.
K.K. reported payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Asahi Kasei Pharma, Pfizer Japan, GlaxoSmithKline, Ono Pharmaceutical, Eisai, and Eli Lilly Japan.
H.Y. reported payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Asahi Kasei Pharma, Mitsubishi Tanabe Pharma, Chugai Pharmaceutical, Astellas Pharma, Eisai, Bayer Yakuhin, and Ono Pharmaceutical.
E.T. reported lecture fees or consulting fees from AbbVie Japan, Asahi Kasei Pharma, Astellas Pharma, Ayumi Pharmaceutical, Nippon Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi-Sankyo, Eisai, Eli Lilly Japan, GlaxoSmithKline, Kyowa Pharma Chemical, Janssen Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Pfizer Japan, Takeda Pharmaceutical, Teijin Pharma, and Viatris.
E.I. reported honoraria for lectures from Bristol-Myers Squibb and Pfizer Japan; consulting fees from Nippontect Systems.
K.T. is an employee of Bristol-Myers Squibb.
S.M. is an employee of Ono Pharmaceutical.
H.Y. reported consulting fees or honoraria and payment towards review activities, such as data monitoring boards or statistical analysis, from Bristol-Myers Squibb.
M.H. reported research grants from Bristol-Myers Squibb and Ono Pharmaceutical; as well as grants from AbbVie, Asahi Kasei Pharma, Astellas Pharma, Ayumi Pharmaceutical, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Kaken Pharmaceutical, Mitsubishi Tanabe Pharma, Nippon Kayaku, Sekisui Medical, and Taisho Pharmaceutical; consulting fees from AbbVie and Bristol-Myers Squibb; and honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Ayumi Pharmaceutical, Bristol-Myers Squibb, Chugai Pharmaceutical, Eisai, Eli Lilly Japan, Gilead Sciences, Janssen Pharmaceutical, Nippon Kayaku, Nippon Shinyaku, Pfizer Japan, Mitsubishi Tanabe Pharma, and UCB Japan.
H.F. reported lecture fees from AbbVie Japan, Eisai, Astellas Pharma, UCB Japan, Bristol-Myers Squibb, Chugai Pharmaceutical, Mitsubishi Tanabe Pharma, Pfizer Japan, Takeda Pharmaceutical, and Viatris.
T.A., K.S., H.T., T.K., T.H., Y.K., M.H., M.O., A.U., T.I., N.O., S.K., N.O., and Y.U. declared no conflicts of interest.
Funding
This study was funded by Bristol-Myers Squibb K.K. and Ono Pharmaceutical Co., Ltd.
Ethical Approval
The study was registered on the University Hospital Medical Information Network Clinical Trials Registry (UMIN000021263) and was approved by an ethics committee or other relevant body at each participating site. The study was performed in accordance with relevant local/international guidelines for medical research and the Declaration of Helsinki.
References
Author notes
Selected data from this article were presented as an abstract and poster (POS0603) at the EULAR European Congress of Rheumatology 2021, June 2–5 (virtual).



