-
PDF
- Split View
-
Views
-
Cite
Cite
Ken-ei Sada, Shinya Kaname, Tomoaki Higuchi, Shunsuke Furuta, Kenji Nagasaka, Toshihiro Nanki, Naotake Tsuboi, Koichi Amano, Hiroaki Dobashi, Keiju Hiromura, Masashi Bando, Takashi Wada, Yoshihiro Arimura, Hirofumi Makino, Masayoshi Harigai, for the Research Committee of Intractable Vasculitis Syndrome (JPVAS) and Research Committee of Intractable Renal Disease of the Ministry of Health, Labour, and Welfare of Japan, Validation of new ACR/EULAR 2022 classification criteria for anti-neutrophil cytoplasmic antibody–associated vasculitis, Modern Rheumatology, Volume 34, Issue 1, January 2024, Pages 144–150, https://doi.org/10.1093/mr/road017
Close - Share Icon Share
ABSTRACT
The objective of this study was to compare the American College of Rheumatology/European Alliance of Associations for Rheumatology 2022 criteria with the previous classification algorithm for anti-neutrophil cytoplasmic antibody–associated vasculitis.
We used data from two nationwide, prospective, inception cohort studies. The enrolled patients were classified as having eosinophilic granulomatosis with polyangiitis (EGPA), granulomatosis with polyangiitis (GPA), or microscopic polyangiitis (MPA) according to the new criteria; these criteria were compared with Watts’ algorithm.
Among 477 patients, 10.7%, 9.9%, and 75.6% were classified as having EGPA, GPA, and MPA, respectively; 6.1% were unclassifiable. Three patients met both the EGPA and MPA criteria, and eight patients met both the GPA and MPA criteria. Of 78 patients with GPA classified using Watts’ algorithm, 27 (34.6%) patients were reclassified as having MPA. Ear, nose, and throat involvement was significantly less frequent in patients reclassified as having MPA than in those reclassified as having GPA. Of 73 patients unclassifiable using Watts’ algorithm, 62 were reclassified as having MPA. All patients reclassified as having MPA were myeloperoxidase-anti-neutrophil cytoplasmic antibody positive, and 46 had interstitial lung disease.
Although the American College of Rheumatology/European Alliance of Associations for Rheumatology 2022 criteria cause overlapping multiple criteria fulfilments in some patients, those items contribute to classifying unclassifiable patients using Watts’ algorithm into MPA.
Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) is characterized by ANCA production and small and medium blood vessel inflammation, and eosinophilic granulomatosis with polyangiitis (EGPA), granulomatosis with polyangiitis (GPA), and microscopic polyangiitis (MPA) are the major categories of AAV [1].
The AAV classification criteria have been developed for clinical trials and epidemiological studies. In 1990, the classification criteria for GPA and EGPA were published by the American College of Rheumatology (ACR) [2]; however, these criteria did not include ANCA status and MPA. Therefore, a combination of the ACR 1990 criteria for EGPA and GPA and the Chapel Hill Consensus Conference (CHCC) definition (although this is not a classification criterion) has been recommended for inclusion in clinical trials for a long time [3]. For epidemiological studies, a four-step algorithm was developed in 2007 to classify patients in the Western population with AAV and polyarteritis nodosa into a single category [4]. In this algorithm (Watts’ algorithm, hereafter), EGPA was first classified using the ACR and Lanham criteria [5]. Subsequently, patients were classified as having GPA if they met the ACR criteria for GPA or the CHCC histological definition for GPA or showed histology compatible with the CHCC definition for MPA or ANCA positivity with at least one of the GPA surrogate markers for vasculitis defined by this algorithm. The remaining patients were classified as having MPA if they had clinical features and histology compatible with small-vessel vasculitis without GPA surrogate markers. In addition, ANCA-positive patients with surrogate markers for renal vasculitis defined by this algorithm were classified as having renal-limited vasculitis, a variant of MPA. Previously, we applied Watts’ algorithm to the Japanese AAV population and reported that half of the patients with GPA tested positive for myeloperoxidase (MPO)-ANCA, and ∼20% of patients were unclassifiable [6]. The clinical phenotypes of patients with AAV are different across countries or regions, and MPO-ANCA and MPA are more dominant than proteinase (PR) 3-ANCA and GPA, respectively, in Japan [7]. Because this algorithm includes ANCA positivity but not ANCA types and GPA is initially classified and followed by MPA, Watts’ algorithm might not be suitable for the classification of Japanese patients with AAV.
Recently, the ACR/European Alliance of Associations for Rheumatology (EULAR) proposed new classification criteria for EGPA, GPA, and MPA on the basis of the diagnostic and classification criteria for vasculitis (DCVAS) study [8–10]. These classification criteria were the first to adopt ANCA types as items. Because the DCVAS study also included a Japanese population, this classification criterion might be more suitable for application to Japanese patients with AAV than Watts’ algorithm.
In the present study, we applied the new ACR/EULAR criteria to patients with AAV in Japan who were enrolled in two previous prospective cohort studies and compared the results with those obtained using Watts’ algorithm.
Methods
Database
This study used data from two nationwide, prospective, inception cohort studies: the Remission Induction Therapy in Japanese Patients with AAV (RemIT-JAV) study and the Remission Induction Therapy in Japanese Patients with AAV and Rapidly Progressive Glomerulonephritis (RemIT-JAV-RPGN) study. Twenty-two university and referring hospitals and 53 institutions participated in the RemIT-JAV and RemIT-JAV-RPGN studies, respectively. Newly diagnosed 156 patients with AAV were prospectively enrolled from April 2009 to December 2010 in the RemIT-JAV study, and 321 patients were enrolled from April 2011 to March 2014 in the RemIT-JAV-RPGN study. The inclusion criteria for the RemIT-JAV and RemIT-JAV-RPGN studies were as follows: (1) clinical diagnosis of AAV by site investigators, (2) fulfilment of the algorithm criteria for primary systemic vasculitis [4], and (3) initiation of immunosuppressive treatment at the discretion of site investigators. The exclusion criteria for the RemIT-JAV and RemIT-JAV-RPGN studies were as follows: (1) age <20 years, (2) serological evidence of hepatitis B or C virus infection, and (3) history of a malignancy.
Clinical variables
In the present study, age, sex, medical history, ANCA phenotype (PR3-ANCA or MPO-ANCA), Birmingham Vasculitis Activity Score (BVAS) 2003 [11], serum C-reactive protein and creatinine levels, and presence of interstitial lung disease (ILD) were used as clinical variables. ANCA was evaluated using commercial assays according to the manufacturers’ instructions. During the study period, the fluorescent enzyme immunoassay, chemiluminescent enzyme immunoassay, and enzyme-linked immunosorbent assay had been available in clinical practice in Japan, respectively.
Disease classification
In the RemIT-JAV and RemIT-JAV-RPGN studies, patients were classified according to Watts’ algorithm [4]. In the present study, patients were reclassified according to the ACR/EULAR 2022 criteria for AAV [8–10]. As some items in the ACR/EULAR 2022 criteria could not be thoroughly evaluated, alternative items were used (Supplementary Table S1).
Statistical analysis
First, the enrolled patients were classified according to the ACR/EULAR 2022 criteria. Subsequently, patient characteristics were compared across the MPA, GPA, and EGPA groups, excluding those meeting more than one criterion. To detect possible problems with the ACR/EULAR 2022 criteria, the positivity of classification items was described for patients meeting two classification criteria or only one criterion. Finally, AAV types, classified using the ACR/EULAR 2022 criteria, were compared with those classified using Watts’ algorithm. Continuous variables were compared using the Mann–Whitney U test or Student’s t-test, depending on data distribution, and categorical variables were compared using the chi-square test or Fisher’s direct probability test, as appropriate. Statistical significance was set at P < .05. All statistical analyses were performed using Statistical Package of Stata (version 17.0; StataCorp, College Station, TX, USA).
Ethics approval and consent to participate
This study was approved by the ethics committee of Kochi Medical School (2022–2038) and was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Epidemiological Research in Japan. Written informed consent was obtained from each participant, and the study protocol was approved by the ethics committee of each participating hospital. The RemIT-JAV and RemIT-JAV-RPGN studies were registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000001648 and 000005136).
Results
Classification using the ACR/EULAR 2022 criteria
Using the ACR/EULAR 2022 criteria, we classified a total of 477 patients as having EGPA (51, 10.7%), GPA (47, 9.9%), or MPA (361, 75.6%) (Figure 1). Twenty-nine (6.1%) patients were unclassifiable. Table 1 shows the characteristics of patients meeting only one criterion. MPO- and PR3-ANCA positivity rates were 45.8% and 2.1% in EGPA, 12.8% and 82.1% in GPA, and 100% and 2.0% in MPA, respectively. Organ involvement evaluated using the BVAS was compared among patients with EGPA, GPA, and MPA (Figure 2), with significant differences in general (P = .048); cutaneous (P < .001); mucous membrane and eye (P < .001); ear, nose, and throat (ENT) (P < .001); renal (P < .001); and nervous system (P = .048) symptoms. The application of each criteria item for the patients meeting only one criterion is shown in Table 1. Although 22 of 48 (46%) of the patients classified as EGPA had nasal symptoms in GPA criteria, other items of GPA criteria could differentiate these patients from GPA. In the 350 patients classified as MPA, 7 patients were PR3-ANCA positive and 4 patients showed granulomatous inflammation in histology. All four patients classified as MPA with granulomatous inflammation were MPO-ANCA positive and exhibited renal symptom, while only one patient had ENT symptom.
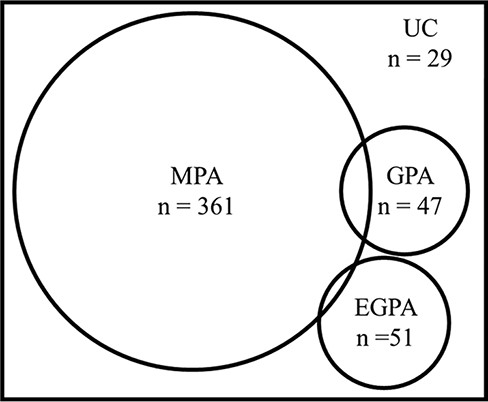
The classification of ANCA-associated vasculitis according to the ACR/EULAR 2022 criteria.
Patient characteristics classified according to the ACR/EULAR 2022 criteria and application of criteria items.
| . | EGPA (n = 48) . | GPA (n = 39) . | MPA (n = 350) . | Unclassifiable (n = 29) . |
|---|---|---|---|---|
| Sex, female subjects/male subjects, n | 31/17 | 20/19 | 202/148 | 15/14 |
| Age, median (IQR), years | 61.5 (47–71) | 63 (60–73) | 73 (65–78) | 71 (60–76) |
| BVAS, median (IQR) | 14 (12–19.5) | 16 (12–19) | 15 (12–21) | 20 (13–25) |
| CRP, median (IQR), mg/dl | 3.05 (0.93–5.62) | 7.20 (2.86–12.80) | 7.10 (1.89–12.05) | 5.25 (2.20–11.28) |
| Serum creatinine, median (IQR), mg/dl | 0.62 (0.48–0.80) | 0.84 (0.60–1.30) | 1.50 (0.82–3.51) | 0.83 (0.60–2.00) |
| Clinical criteria | ||||
| Obstructive airway disease, n (%) | 39 (81.3) | 2 (5.1) | 16 (4.6) | 2 (6.9) |
| Nasal polyps, n (%) | 5 (10.4) | 1 (2.6) | 7 (2.0) | 0 |
| Mononeuritis multiplex, n (%) | 45 (93.8) | 6 (15.4) | 83 (23.7) | 8 (27.6) |
| Nasal bloody discharge, ulcers, crusting, congestion or blockage, or nasal septal defect/perforation, n (%) | 22 (45.8) | 34 (87.2) | 10 (2.9) | 17 (58.6) |
| Cartilaginous involvement, n (%) | 0 | 3 (7.7) | 1 (0.3) | 0 |
| Conductive or sensorineural hearing loss, n (%) | 3 (6.3) | 11 (28.2) | 22 (6.3) | 6 (20.7) |
| Laboratory and biopsy criteria | ||||
| MPO-ANCA, n (%) | 22 (45.8) | 4 (12.8) | 350 (100) | 19 (65.5) |
| PR3-ANCA, n (%) | 1 (2.1) | 32 (82.1) | 7 (2.0) | 2 (6.9) |
| Eosinophil count ≥1 × 109/l, n (%) | 46 (95.8) | 1 (2.6) | 9 (2.6) | 7 (24.1) |
| Haematuria, n (%) | 9 (18.8) | 19 (48.7) | 270 (77.1) | 15 (51.7) |
| Pulmonary nodules, mass, or cavitation, n (%) | 5 (10.4) | 18 (46.2) | 12 (3.4) | 4 (13.8) |
| Fibrosis or interstitial lung disease, n (%) | 11 (22.9) | 3 (7.7) | 176 (50.3) | 2 (6.9) |
| Inflammation, consolidation, or effusion of the nasal/paranasal sinuses or mastoiditis | N/A | N/A | N/A | N/A |
| Extravascular eosinophilic-predominant inflammation, n (%) | 28 (58.3) | 0 | 1 (0.3) | 0 |
| Granuloma, extravascular granulomatous inflammation, or giant cells, n (%) | 0 | 14 (35.9) | 4 (1.1) | 6 (20.7) |
| Pauci-immune glomerulonephritis, n (%) | 1 (2.1) | 5 (12.8) | 155 (44.3) | 3 (10.3) |
| . | EGPA (n = 48) . | GPA (n = 39) . | MPA (n = 350) . | Unclassifiable (n = 29) . |
|---|---|---|---|---|
| Sex, female subjects/male subjects, n | 31/17 | 20/19 | 202/148 | 15/14 |
| Age, median (IQR), years | 61.5 (47–71) | 63 (60–73) | 73 (65–78) | 71 (60–76) |
| BVAS, median (IQR) | 14 (12–19.5) | 16 (12–19) | 15 (12–21) | 20 (13–25) |
| CRP, median (IQR), mg/dl | 3.05 (0.93–5.62) | 7.20 (2.86–12.80) | 7.10 (1.89–12.05) | 5.25 (2.20–11.28) |
| Serum creatinine, median (IQR), mg/dl | 0.62 (0.48–0.80) | 0.84 (0.60–1.30) | 1.50 (0.82–3.51) | 0.83 (0.60–2.00) |
| Clinical criteria | ||||
| Obstructive airway disease, n (%) | 39 (81.3) | 2 (5.1) | 16 (4.6) | 2 (6.9) |
| Nasal polyps, n (%) | 5 (10.4) | 1 (2.6) | 7 (2.0) | 0 |
| Mononeuritis multiplex, n (%) | 45 (93.8) | 6 (15.4) | 83 (23.7) | 8 (27.6) |
| Nasal bloody discharge, ulcers, crusting, congestion or blockage, or nasal septal defect/perforation, n (%) | 22 (45.8) | 34 (87.2) | 10 (2.9) | 17 (58.6) |
| Cartilaginous involvement, n (%) | 0 | 3 (7.7) | 1 (0.3) | 0 |
| Conductive or sensorineural hearing loss, n (%) | 3 (6.3) | 11 (28.2) | 22 (6.3) | 6 (20.7) |
| Laboratory and biopsy criteria | ||||
| MPO-ANCA, n (%) | 22 (45.8) | 4 (12.8) | 350 (100) | 19 (65.5) |
| PR3-ANCA, n (%) | 1 (2.1) | 32 (82.1) | 7 (2.0) | 2 (6.9) |
| Eosinophil count ≥1 × 109/l, n (%) | 46 (95.8) | 1 (2.6) | 9 (2.6) | 7 (24.1) |
| Haematuria, n (%) | 9 (18.8) | 19 (48.7) | 270 (77.1) | 15 (51.7) |
| Pulmonary nodules, mass, or cavitation, n (%) | 5 (10.4) | 18 (46.2) | 12 (3.4) | 4 (13.8) |
| Fibrosis or interstitial lung disease, n (%) | 11 (22.9) | 3 (7.7) | 176 (50.3) | 2 (6.9) |
| Inflammation, consolidation, or effusion of the nasal/paranasal sinuses or mastoiditis | N/A | N/A | N/A | N/A |
| Extravascular eosinophilic-predominant inflammation, n (%) | 28 (58.3) | 0 | 1 (0.3) | 0 |
| Granuloma, extravascular granulomatous inflammation, or giant cells, n (%) | 0 | 14 (35.9) | 4 (1.1) | 6 (20.7) |
| Pauci-immune glomerulonephritis, n (%) | 1 (2.1) | 5 (12.8) | 155 (44.3) | 3 (10.3) |
Patients who overlapped the two criteria were excluded.
CRP, C-reactive protein; IQR, interquartile range; N/A, not applicable.
Patient characteristics classified according to the ACR/EULAR 2022 criteria and application of criteria items.
| . | EGPA (n = 48) . | GPA (n = 39) . | MPA (n = 350) . | Unclassifiable (n = 29) . |
|---|---|---|---|---|
| Sex, female subjects/male subjects, n | 31/17 | 20/19 | 202/148 | 15/14 |
| Age, median (IQR), years | 61.5 (47–71) | 63 (60–73) | 73 (65–78) | 71 (60–76) |
| BVAS, median (IQR) | 14 (12–19.5) | 16 (12–19) | 15 (12–21) | 20 (13–25) |
| CRP, median (IQR), mg/dl | 3.05 (0.93–5.62) | 7.20 (2.86–12.80) | 7.10 (1.89–12.05) | 5.25 (2.20–11.28) |
| Serum creatinine, median (IQR), mg/dl | 0.62 (0.48–0.80) | 0.84 (0.60–1.30) | 1.50 (0.82–3.51) | 0.83 (0.60–2.00) |
| Clinical criteria | ||||
| Obstructive airway disease, n (%) | 39 (81.3) | 2 (5.1) | 16 (4.6) | 2 (6.9) |
| Nasal polyps, n (%) | 5 (10.4) | 1 (2.6) | 7 (2.0) | 0 |
| Mononeuritis multiplex, n (%) | 45 (93.8) | 6 (15.4) | 83 (23.7) | 8 (27.6) |
| Nasal bloody discharge, ulcers, crusting, congestion or blockage, or nasal septal defect/perforation, n (%) | 22 (45.8) | 34 (87.2) | 10 (2.9) | 17 (58.6) |
| Cartilaginous involvement, n (%) | 0 | 3 (7.7) | 1 (0.3) | 0 |
| Conductive or sensorineural hearing loss, n (%) | 3 (6.3) | 11 (28.2) | 22 (6.3) | 6 (20.7) |
| Laboratory and biopsy criteria | ||||
| MPO-ANCA, n (%) | 22 (45.8) | 4 (12.8) | 350 (100) | 19 (65.5) |
| PR3-ANCA, n (%) | 1 (2.1) | 32 (82.1) | 7 (2.0) | 2 (6.9) |
| Eosinophil count ≥1 × 109/l, n (%) | 46 (95.8) | 1 (2.6) | 9 (2.6) | 7 (24.1) |
| Haematuria, n (%) | 9 (18.8) | 19 (48.7) | 270 (77.1) | 15 (51.7) |
| Pulmonary nodules, mass, or cavitation, n (%) | 5 (10.4) | 18 (46.2) | 12 (3.4) | 4 (13.8) |
| Fibrosis or interstitial lung disease, n (%) | 11 (22.9) | 3 (7.7) | 176 (50.3) | 2 (6.9) |
| Inflammation, consolidation, or effusion of the nasal/paranasal sinuses or mastoiditis | N/A | N/A | N/A | N/A |
| Extravascular eosinophilic-predominant inflammation, n (%) | 28 (58.3) | 0 | 1 (0.3) | 0 |
| Granuloma, extravascular granulomatous inflammation, or giant cells, n (%) | 0 | 14 (35.9) | 4 (1.1) | 6 (20.7) |
| Pauci-immune glomerulonephritis, n (%) | 1 (2.1) | 5 (12.8) | 155 (44.3) | 3 (10.3) |
| . | EGPA (n = 48) . | GPA (n = 39) . | MPA (n = 350) . | Unclassifiable (n = 29) . |
|---|---|---|---|---|
| Sex, female subjects/male subjects, n | 31/17 | 20/19 | 202/148 | 15/14 |
| Age, median (IQR), years | 61.5 (47–71) | 63 (60–73) | 73 (65–78) | 71 (60–76) |
| BVAS, median (IQR) | 14 (12–19.5) | 16 (12–19) | 15 (12–21) | 20 (13–25) |
| CRP, median (IQR), mg/dl | 3.05 (0.93–5.62) | 7.20 (2.86–12.80) | 7.10 (1.89–12.05) | 5.25 (2.20–11.28) |
| Serum creatinine, median (IQR), mg/dl | 0.62 (0.48–0.80) | 0.84 (0.60–1.30) | 1.50 (0.82–3.51) | 0.83 (0.60–2.00) |
| Clinical criteria | ||||
| Obstructive airway disease, n (%) | 39 (81.3) | 2 (5.1) | 16 (4.6) | 2 (6.9) |
| Nasal polyps, n (%) | 5 (10.4) | 1 (2.6) | 7 (2.0) | 0 |
| Mononeuritis multiplex, n (%) | 45 (93.8) | 6 (15.4) | 83 (23.7) | 8 (27.6) |
| Nasal bloody discharge, ulcers, crusting, congestion or blockage, or nasal septal defect/perforation, n (%) | 22 (45.8) | 34 (87.2) | 10 (2.9) | 17 (58.6) |
| Cartilaginous involvement, n (%) | 0 | 3 (7.7) | 1 (0.3) | 0 |
| Conductive or sensorineural hearing loss, n (%) | 3 (6.3) | 11 (28.2) | 22 (6.3) | 6 (20.7) |
| Laboratory and biopsy criteria | ||||
| MPO-ANCA, n (%) | 22 (45.8) | 4 (12.8) | 350 (100) | 19 (65.5) |
| PR3-ANCA, n (%) | 1 (2.1) | 32 (82.1) | 7 (2.0) | 2 (6.9) |
| Eosinophil count ≥1 × 109/l, n (%) | 46 (95.8) | 1 (2.6) | 9 (2.6) | 7 (24.1) |
| Haematuria, n (%) | 9 (18.8) | 19 (48.7) | 270 (77.1) | 15 (51.7) |
| Pulmonary nodules, mass, or cavitation, n (%) | 5 (10.4) | 18 (46.2) | 12 (3.4) | 4 (13.8) |
| Fibrosis or interstitial lung disease, n (%) | 11 (22.9) | 3 (7.7) | 176 (50.3) | 2 (6.9) |
| Inflammation, consolidation, or effusion of the nasal/paranasal sinuses or mastoiditis | N/A | N/A | N/A | N/A |
| Extravascular eosinophilic-predominant inflammation, n (%) | 28 (58.3) | 0 | 1 (0.3) | 0 |
| Granuloma, extravascular granulomatous inflammation, or giant cells, n (%) | 0 | 14 (35.9) | 4 (1.1) | 6 (20.7) |
| Pauci-immune glomerulonephritis, n (%) | 1 (2.1) | 5 (12.8) | 155 (44.3) | 3 (10.3) |
Patients who overlapped the two criteria were excluded.
CRP, C-reactive protein; IQR, interquartile range; N/A, not applicable.
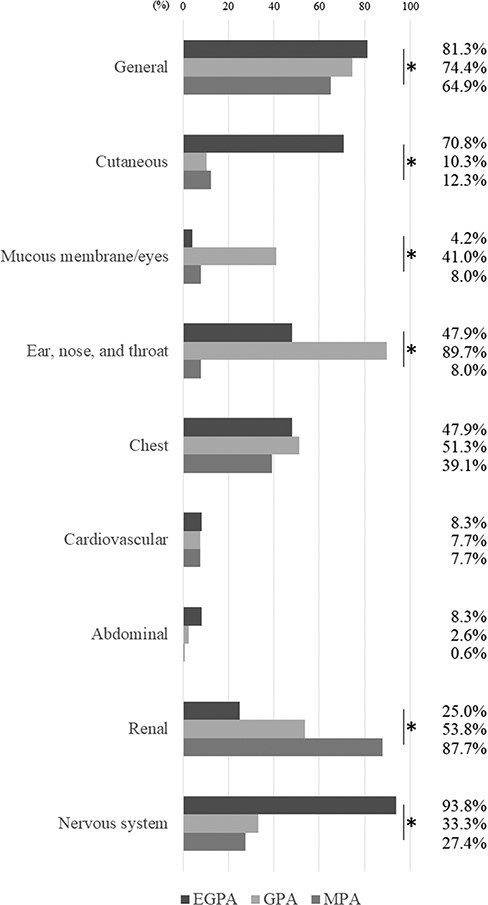
The organ involvement among AAV diseases classified using the ACR/EULAR 2022 criteria. *P < .05, chi-square test.
Eight patients met both the GPA and MPA criteria, whereas three patients met both the MPA and EGPA criteria. None met both the EGPA and GPA criteria. All three patients who met the criteria for EGPA and MPA were MPO-ANCA positive and PR3-ANCA negative. One of the three patients showed eosinophilic inflammation, while the other showed pauci-immune glomerulonephritis on biopsy. Two of the three patients had ILD (Supplementary Table S2). Two of the eight patients who met the criteria for GPA and MPA were MPO-ANCA positive, while six were both MPO-ANCA and PR3-ANCA positive. None of the patients showed granulomatous inflammation on biopsy. Five patients had nasal symptoms and ILD, while four had ear symptoms (Supplementary Table S3).
Comparison between the ACR/EULAR 2022 criteria and Watts’ algorithm
Using Watts’ algorithm, we identified 42 patients with EGPA, 86 patients with GPA, 276 patients with MPA, and 73 patients who were unclassifiable (Figure 3). A significant difference was observed in the proportion of patients with each disease classified using the ACR/EULAR 2022 criteria and Watts’ algorithm (chi-square test, P < .0001). By Watts’ algorithm, MPO-ANCA was detectable in 45.2% of patients with EGPA, in 59.3% of those with GPA, and in 98.2% of those with MPA. In contrast, PR3-ANCA was detectable in 2.4% of the patients with EGPA, in 39.5% of those with GPA, and in 3.3% of those with MPA.

The transition of classification of ANCA-associated vasculitis by Watts’ algorithm to those according to the ACR/EULAR 2022 criteria. Bold circle, the number of patients whose classification did not change; solid circle, the number of patients whose classification was changed (≥10 patients); and dotted square, the number of patients whose classification was changed (<10 patients).
Among the 42 patients classified as having EGPA and 276 as having MPA using Watts’ algorithm, 41 (97.6%) and 261 (95.2%) were reclassified as having EGPA and MPA using the ACR/EULAR 2022 criteria, respectively (Figure 3). On the other hand, 42 (48.8%) of the 86 patients classified as having GPA using Watts’ algorithm were reclassified as having GPA, and 35 (40.7%) were reclassified as having MPA using the ACR/EULAR 2022 criteria. After excluding seven patients who met both the GPA and MPA criteria and one patient who met both the EGPA and MPA criteria, ENT involvement was less frequent (100% vs. 56%, P < .001), while lung and renal involvement was more frequent (lung 81% vs. 57%, P = .042; renal 89% vs. 51%, P < .002) among 27 patients reclassified as having MPA than among those 35 with GPA according to the ACR/EULAR 2022 criteria. In the ENT involvement items, nasal symptoms were less frequent in patients reclassified as having MPA than in those with GPA (26% in MPA vs. 97% in GPA, P < .001), while ear symptoms were not different (37% in MPA vs. 31% in GPA, P = .64). MPO-ANCA positivity was 14% in patients reclassified as having GPA and 100% in those reclassified as having MPA (P < .001), whereas PR3-ANCA positivity was 80% and 0% in the former and latter groups, respectively (P < .001).
Of the 73 unclassifiable patients using Watts’ algorithm, 6 were reclassified as having EGPA (one patient met both the EGPA and MPA criteria), 2 as having GPA, and 63 as having MPA (one patent overlapped as EGPA). The ACR/EULAR 2022 criteria were applied to patients who were reclassified as having one of the three AAV diseases, and the positivity of each item is shown in Supplementary Table S4. Most patients reclassified as having EGPA had mononeuritis multiplex, eosinophilia, and eosinophilic inflammation on biopsy. Two patients reclassified as having GPA had PR3-ANCA positivity and lung involvement. All patients reclassified as having MPA were MPO-ANCA positive, and 46 of 62 (74.2%) patients had ILD.
Discussion
We applied the new ACR/EULAR 2022 criteria to Japanese patients with AAV enrolled in two previous prospective cohort studies, and the results were compared with those made using Watts’ algorithm. The enrolled patients were classified as having EGPA (10.7%), GPA (9.9%), or MPA (75.6%), while 6.1% were unclassifiable. Eight patients met both the GPA and MPA criteria, whereas three met both the MPA and EGPA criteria. After excluding eight patients meeting two criteria, 27 (34.6%) of the 78 patients classified as having GPA using Watts’ algorithm were reclassified as having MPA. Of the 73 patients unclassifiable using Watts’ algorithm, 62 were reclassified as having MPA.
In the present study, MPO-ANCA–positive AAV cases with ILD experienced some difficulty with disease classification. Of the three patients who met both the MPA and EGPA criteria, all were MPO-ANCA positive, two had eosinophilia, two had ILD, and two had biopsy-proven renal vasculitis. According to the ACR/EULAR 2022 criteria for MPA, the presence of eosinophilia is an important factor in differentiating MPA from EGPA. MPO-ANCA–positive AAV patients with eosinophilia were less likely to be classified as having MPA using the four main points of eosinophilia in the ACR/EULAR 2022 criteria for MPA. However, MPO-ANCA–positive AAV patients with ILD and eosinophilia were more likely to be classified as having MPA. Similarly, eight patients who met both MPA and GPA criteria were MPO-ANCA positive, and five had ILD. Nasal involvement and PR3-ANCA positivity are highly important for differentiating GPA from MPA in the ACR/EULAR 2022 criteria. However, AAV patients classified as having GPA according to the ACR/EULAR 2022 criteria can also be classified as having MPA if they have MPO-ANCA and ILD. MPO-ANCA positivity and ILD are relatively common characteristics of the Japanese AAV population [6]; 47.4%, 9.0%, and 14.3% of patients with MPA, GPA, and EGPA had ILD in a Japanese cohort study, respectively. Differentiating GPA or EGPA patients with ILD from MPA patients may be challenging. Patients with EGPA have been reported as having eosinophilic and vasculitic injuries [12]. Given that eosinophilia was occasionally found in the patients who met both MPA and EGPA criteria or unclassifiable patients, the selection of treatment should take into account eosinophilic and vasculitic disorders in these individuals.
Patients with MPO-ANCA and otitis media could be classified as having MPA using the ACR/EULAR 2022 criteria but as having GPA using Watts’ algorithm. In the present study, ∼35% of the patients with GPA classified using Watts’ algorithm were reclassified as having MPA using the ACR/EULAR 2022 criteria. All patients reclassified as having MPA were MPO-ANCA positive. In Watts’ algorithm, GPA was initially classified, followed by MPA; the ANCA type was not specified, and nasal and ear symptoms were grouped as surrogate markers for GPA. We previously reported that half of the patients with GPA diagnosed using Watts’ algorithm were MPO-ANCA positive [6]. Recently, otitis media with AAV (OMAAV) has been recognized as a clinical phenotype of AAV irrespective of the ANCA type, and approximately half of the patients with OMAAV were MPO-ANCA positive [13]. In the ACR/EULAR 2022 criteria, nasal involvement and ANCA type are essential for distinguishing GPA from MPA. MPO-ANCA–positive patients with OMAAV can be classified as having MPA according to the ACR/EULAR 2022 criteria if they have no items with negative scores.
The number of unclassifiable patients apparently decreased according to the ACR/EULAR 2022 criteria. Of the 73 unclassifiable patients using Watts’ algorithm, 84.9% were reclassified as having MPA. All patients reclassified as having MPA were MPO-ANCA positive, and 74.2% of the patients had ILD. ILD is less common in AAV populations in Western countries than in Japan [14] and is not included in the BVAS item [11] or Watts’ algorithm. Enrolment of the Asian population in the DCVAS study may result in a decreased number of unclassifiable patients in the present study.
This study has some limitations. First, we could not precisely evaluate some items of the ACR/EULAR 2022 criteria because of a lack of information; this could have increased the number of unclassifiable patients. Nevertheless, only ∼6% of patients were unclassifiable. This lack of information could cause a little misclassification. Actually, 4 of 350 patients classified as MPA showed granulomatous inflammation histologically. All of those patients were MPO-ANCA positive, and most had no ENT symptoms. As such, MPO-ANCA–positive GPA patients without ENT involvement may be misclassified as GPA according to the ACR/EULAR 2022 criteria. Second, vasculitis was not confirmed in any of the patients. In the present study, AAV was clinically diagnosed by site investigators using Watts’ criteria [4]. The ACR/EULAR 2022 criteria should reportedly be applied when a diagnosis of small- or medium-vessel vasculitis has been made. MPA can be classified only with MPO-ANCA positivity using the ACR/EULAR 2022 criteria. Therefore, MPA classification might be overestimated using the new criteria, especially for patients classified as unclassifiable using Watts’ algorithm. Third, ILD was diagnosed by site investigators but not by pulmonologists or radiologists. The difference between eosinophilic pneumonia and ILD cannot be easily determined using imaging. Therefore, ILD and eosinophilic pneumonia in EGPA patients may not be able to be distinguished by non-specialists.
In conclusion, the ACR/EULAR 2022 criteria showed a better ability to classify Japanese patients with AAV in terms of decreasing unclassifiable patients; however, it may be difficult to differentiate EGPA or GPA patients with ILD from MPA patients.
Acknowledgements
The authors thank Tomomi Maruyama, Haruki Watanabe, Yoshia Miyawaki, Keiji Ohashi, Michiko Morishita, Eri Katsuyama, Takayuki Katsuyama, Mariko Narazaki, and Yoshinori Matsumoto for their assistance in data management as well as patients and physicians who attended RemIT-JAV and RemIT-JAV-RPGN (see the Appendix).
Supplementary data
Supplementary data are available at Modern Rheumatology online.
Conflict of interest
Ken-ei Sada received a speaker’s fee from Astra Zeneca K. K. and research grants from Pfizer Inc. Division of Multidisciplinary Management of Rheumatic Diseases is an endowment department, supported with an unrestricted grant from Ayumi Pharmaceutical Corp., Asahi Kasei Corp., Taisho Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., and AbbVie Japan GK. Toshihiro Nanki received grant/research support from Chugai Pharmaceutical Co., Eisai Co., Ltd., Teijin Pharma Ltd., Bristol-Myers K.K., Asahikasei Pharma Corp., Mitsubishi-Tanabe Pharma Co., Ayumi Pharmaceutical Corporation, AbbVie GK, Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharmaceutical Co., Ltd. and GlaxoSmithKline plc., consultant fees from Eisai Co., Ltd. and Chugai Pharmaceutical Co., and speakers fees from Eisai Co., Ltd., Astellas Pharma Inc., Janssen Pharmaceutical K.K., Ayumi Pharmaceutical Corporation, Pfizer Japan Inc., Asahikasei Pharma Corp., Eli Lilly Japan K.K., Takeda Pharmaceutical Co., Nippon Boehringer Ingelheim Co., Ltd., Chugai Pharmaceutical Co., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Taisho Pharmaceutical Co., Mochida Pharmaceutical Co., Ltd. and AbbVie GK. Naotake Tsuboi received consigned research fund from Zenyaku Kogyo Co., Ltd., research support from Kissei Pharmaceutical Co., Ltd., and consulting/lecture fees from Kissei Pharmaceutical Co., Ltd. Keiju Hiromura received research grants from Bayer, Chugai, GSK, and Kyowa Kirin; speaker’s fees from Astellas, AstraZeneca, Chugai, GSK, and Sanofi; a consultancy fee from GSK. Takashi Wada has received honoraria for lectures from Mitsubishi Tanabe Pharma Corporation and Kyowa Kirin Co. Ltd.; research funding from Shiseido Company, Ltd. and Kyowa Kirin Co. Ltd.; and research grant from Chugai Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd., MSD K.K., GlaxoSmithKline plc., and Bayer Yakuhin Ltd. Hirofumi Makino is a consultant for AbbVie, Boehringer Ingelheim, and Bristol Myers Squibb Co. Kissei Pharmaceutical Co. Ltd. and Teijin Pharma. Masayoshi Harigai served on the steering committee for AbbVie and Teijin and the advisory boards for Boehringer Ingelheim and Travere Therapeutics. Masayoshi Harigai received research grants from AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc. and Ayumi Pharmaceutical Co. Bayer Yakuhin Co., Ltd., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., and Chugai Pharmaceutical Co. Daiichi-Sankyo, Inc., Eisai Co. Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co. Ltd., Kissei Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., Nippon Shinyaku Co. Ltd., Taisho Pharmaceutical Co. Ltd., Teijin Pharma Ltd., UCB Japan Co. Ltd., and Viatris Japan. Additionally, Masayoshi Harigai received speaker fees from AbbVie Japan GK, Asahi Kasei Corp., Astra Zeneca K.K., and Ayumi Pharmaceutical Co. Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd., Eli Lilly Japan K.K., GlaxoSmithKline K.K., Gilead Sciences Inc., Janssen Pharmaceutical K.K., Kissei Pharmaceutical Co. Ltd., and Mitsubishi Tanabe Pharma Co. Mochida Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., Nippon Shinyaku Co. Ltd., Novartis Japan, Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co. Ltd., Teijin Pharma Ltd., and UCB Japan.
Funding
This work was supported by grants for Research on Rare and Intractable Diseases, the Ministry of Health, Labour and Welfare, Japan (grant numbers H29-nannti-ippann-018 and 20FC1044), and the study group for strategic exploration of drug seeds for ANCA-associated vasculitis and construction of clinical evidence from the Japan Agency for Medical Research and Development, AMED (grant number 17ek0109104h0003).
References
Appendix
Japan Research Committee of the Ministry of Health, Labour, and Welfare for Intractable Vasculitis (JPVAS) and Research Committee of Intractable Renal Disease of the Ministry of Health, Labour, and Welfare of Japan: In addition to the authors, the following investigators and institutions participated in this study: Department of Internal Medicine and Rheumatology, Juntendo University School of Medicine (Yoshinari Takasaki); Department of Hemovascular Medicine and Artificial Organs, Faculty of Medicine, University of Miyazaki (Shouichi Fujimoto); Department of Nephrology, Faculty of Medicine, University of Tsukuba (Joichi Usui and Kunihiro Yamagata); Department of Rheumatology, Endocrinology and Nephrology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University (Tatsuya Atsumi); Department of Medicine and Rheumatology, Tokyo Metropolitan Geriatric Hospital (Takahiko Sugihara); Department of Nephrology, Internal Medicine, Nagoya University Graduate School of Medicine (Seiichi Matsuo); Department of Human Resource Development of Dialysis Therapy for Kidney Disease, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences (Hitoshi Sugiyama); Faculty of Health Sciences, Hokkaido University (Akihiro Ishizu); Department of the Control for Rheumatic Diseases, Graduate School of Medicine, Kyoto University (Takao Fujii); Department of Pathology, Keio University School of Medicine (Yasunori Okada); Department of Respiratory Medicine, Toho University Omori Medical Center (Sakae Homma); Department of Clinical Pathology and Immunology, Kobe University Graduate School of Medicine (Shunichi Kumagai); Department of Nephrology and Dialysis, Kitano Hospital, Tazuke Kofukai Medical Research Institute (Eri Muso); Department of Rheumatology, Shimane University Faculty of Medicine (Yohko Murakawa); Division of Rheumatology, Department of Medical Oncology and Immunology, Nagoya City University Graduate School of Medical Science (Shogo Banno); Department of Hematology, Clinical Immunology and Infectious Diseases, Ehime University Graduate School of Medicine (Hitoshi Hasegawa); Division of Nephrology, Department of Internal Medicine, Jichi Medical University (Wako Yumura); Department of Cardiovascular Medicine, Kyoto Prefectural University School of Medicine (Hiroaki Matsubara); Division of Nephrology, Tokyo Medical University Hachioji Medical Center (Masaharu Yoshida); Department of Dermatology, Kitasato University School of Medicine (Kensei Katsuoka); Division of Immunology and Rheumatology, Department of Internal Medicine 3, Hamamatsu University School of Medicine (Noriyoshi Ogawa); Department of Hematology, Oncology, Nephrology, and Rheumatology, Akita University Graduate School of Medicine (Atsushi Komatsuda); Department of Rheumatology, Niigata Rheumatic Center (Satoshi Ito); Department of Immunology and Rheumatology, Division of Advanced Preventive Medical Sciences, Nagasaki University Graduate School of Biomedical Sciences (Atsushi Kawakami); Department of Nephrology, Iwate Prefectural Central Hospital (Izaya Nakaya); Division of Nephrology and Rheumatology, Department of Internal Medicine, Fukuoka University School of Medicine (Takao Saito); Shimane University, Faculty of Medicine, Division of Nephrology (Takafumi Ito); Department of Hemodialysis and Apheresis, Yokohama City University Medical Center (Nobuhito Hirawa); Center for Rheumatology, Okayama Saiseikai General Hospital (Masahiro Yamamura); Department of Medical Technology, School of Health Sciences, Faculty of Medicine, Niigata University (Masaaki Nakano); Department of Medicine, Kidney Center, Tokyo Women’s Medical University (Kosaku Nitta); Division of Nephrology and Hypertension, Kashiwa Hospital, Jikei University (Makoto Ogura); Department of Respiratory Medicine, Allergy and Clinical Immunology, Nagoya City University Graduate School of Medical Sciences (Taio Naniwa); Division of Rheumatology and Allergology, Department of Internal Medicine, St. Marianna University School of Medicine (Shoichi Ozaki); Department of Nephrology and Endocrinology, Graduate School of Medicine, The University of Tokyo (Junichi Hirahashi); Division of Kidney and Hypertension, Department of Internal Medicine, Jikei University School of Medicine (Tatsuo Hosoya); Division of Nephrology, Department of Internal Medicine, Juntendo University Faculty of Medicine (Satoshi Horikoshi); Institute of Rheumatology, Tokyo Women’s Medical University (Yasushi Kawaguchi); Division of Clinical Immunology, Graduate School of Comprehensive Human Sciences, University of Tsukuba (Taichi Hayashi); Department of Nephrology, Hypertension, Diabetology, Endocrinology and Metabolic, Fukushima Medical University (Tsuyoshi Watanabe); Department of Nephrology, Japanese Red Cross Nagoya Daini Hospital (Daijo Inaguma); Department of Integrated Therapy for Chronic Kidney Disease, Kyushu University (Kazuhiko Tsuruya); Niigata Prefectural Shibata Hospital (Noriyuki Homma); Division of Rheumatology, Department of Internal Medicine, Keio University School of Medicine (Tsutomu Takeuchi); Division of Cardiology, Nephrology, Pulmonology and Neurology, Department of Internal Medicine, Asahikawa Medical University (Naoki Nakagawa); Kurobe City Hospital (Shinichi Takeda); National Fukuoka Higashi Medical Center (Ritsuko Katafuchi); Division of Nephrology, Department of Medicine, Faculty of Medical Sciences, University of Fukui (Masayuki Iwano); and Tokyo Medical University Ibaraki Medical Center (Masaki Kobayashi).



