-
PDF
- Split View
-
Views
-
Cite
Cite
A Faure, F Lique, J Loreau, The effect of CO−H2O collisions in the rotational excitation of cometary CO, Monthly Notices of the Royal Astronomical Society, Volume 493, Issue 1, March 2020, Pages 776–782, https://doi.org/10.1093/mnras/staa242
Close - Share Icon Share
ABSTRACT
We present the first accurate rate coefficients for the rotational excitation of CO by H2O in the kinetic temperature range 5–100 K. The statistical adiabatic channel method (SACM) is combined with a high-level rigid-rotor CO−H2O intermolecular potential energy surface. Transitions among the first 11 rotational levels of CO and the first 8 rotational levels of both para-H2O and ortho-H2O are considered. Our rate coefficients are compared to previous data from the literature and they are also incorporated in a simple non-LTE model of cometary coma including collision-induced transitions, solar radiative pumping and radiative decay. We find that the uncertainties in the collision data have significant influence on the CO population distribution for H2O densities in the range 103–108 cm−3. We also show that the rotational distribution of H2O plays an important role in CO excitation (owing to correlated energy transfer in both CO and H2O), while the impact of the ortho-to-para ratio of H2O is found to be negligible.
1 INTRODUCTION
Rate coefficients (or cross sections) for molecular collisional excitation are needed to model both thermal balance and spectral line formation in astrophysical objects such as interstellar clouds, protoplanetary discs, and planetary/cometary atmospheres. In such environments, the density can be so low (n ≪ 1010 cm−3) that collisions indeed cannot maintain a local thermodynamical equilibrium (LTE) everywhere. Until recently, the accuracy of theoretical collision data was mainly inferred indirectly by comparison with related processes such as line broadening cross-sections (see e.g. Mengel, Flatin & De Lucia 2000; Wiesenfeld & Faure 2010). In the last decade, there has been huge progress in measuring state-to-state collision cross-sections at very low energy (Ecol ∼ 1 meV) (see Naulin & Bergeat 2018, for a recent review). Detailed comparisons between quantum calculations and crossed -beam measurements have thus allowed experimentalists to verify theoretical cross-sections in the ‘cold’ regime (equivalent temperature ∼10 K) where quantum effects such as resonances are predominant. Theory has passed all experimental tests so far, establishing the reliability of modern collisional data used in astrophysical models (Chefdeville et al. 2013, 2015; Bergeat et al. 2020; Gao et al. 2019). These theoretical values have been obtained for ‘simple’ collision systems such as rigid rotors excited by light atomic or diatomic perturbers, in particular He atoms and H2 molecules which are the most abundant collisional partners in the interstellar medium (ISM).
However, collision rate coefficients are also needed for systems involving heavy and even polyatomic perturbers, which challenge current computational capabilities. For instance, in the thermosphere of Titan, N2, and CH4 are the most abundant collisional perturbers (Rezac et al. 2013). In cometary comas, collisional excitation is generally dominated by H2O (and/or electrons) and, for comets at large heliocentric distances, by CO (Bockelée-Morvan et al. 2004). The main difficulty with such systems is the excessively large number of angular couplings between the target and the projectile which make full quantum calculations prohibitively expensive, both in terms of computer time and memory requirements. As a result, to the best of our knowledge, the only sets of rotationally resolved rate coefficients for the water perturber are those of Green (1993) for CO−H2O, those of Buffa et al. (2000) for H2O−H2O, and those of Dubernet & Quintas-Sánchez (2019) for HCN−H2O. The two former sets were estimated using crude treatments while the latter was obtained using only partially converged coupled-states (CS) calculations.
In this paper, we present the first quantum calculations for the rotational excitation of CO by H2O molecules. This is also the first study to consider the two nuclear-spin isomers of H2O, ortho-, and para-H2O (hereafter referred as o-H2O and p-H2O), as two distinct perturbers. The statistical adiabatic channel model (SACM) is used as a validated alternative to prohibitive close-coupling calculations (Loreau, Lique & Faure 2018a, 2018b) and it is combined here with the recent ab initio CO−H2O interaction potential computed by Kalugina et al. (2018). The CO−H2O collision system is of great importance in comets but also in protoplanetary and debris discs when the gas originates from ices in planetesimals (Matrà et al. 2015). In addition to providing collision data for the first 11 rotational levels of CO, we also present a non-LTE model for the CO excitation in comets, including collision-induced transitions due to p-H2O and o-H2O, radiative pumping of the v = 0 → 1 band due to solar photons and radiative decay due to spontaneous emission. A key objective is to assess the impact of accurate CO−H2O collisional data on the rotational distribution of cometary CO. The next section describes the CO−H2O scattering calculations. Section 3 compares the present results with previous collisional data for CO. In Section 4, we present simple non-equilibrium calculations for the excitation of CO in comets. Concluding remarks are made in Section 5.
2 QUANTUM STATISTICAL CALCULATIONS
The computation of inelastic cross-sections from first principles consists of two main steps. First, the electronic Schrödinger equation is solved using quantum chemistry methods. Within the Born–Oppenheimer approximation, a single intermolecular potential energy surface (PES) governing the motion of the nuclei is defined as function of nuclear coordinates. The nuclear motion is solved separately, in a second step, using quantum or (semi)classical scattering methods. We describe in this section our calculations for the CO−H2O system.
2.1 Potential energy surface
The CO−H2O scattering calculations presented below are based on the high-level ab initio intermolecular potential recently determined by Kalugina et al. (2018). The interacting molecules CO and H2O are considered as rigid-rotors, with geometries corresponding to their ground vibrational state. The PES was calculated at 93 000 relative orientations using the explicitly correlated single- and double-excitation coupled cluster theory with a non-iterative perturbative treatment of triple excitations [CCSD(T)-F12a] along with the augmented correlation-consistent aug-cc-pVTZ basis sets (AVTZ hereafter). The intermolecular energies were then fitted to a body-fixed expansion expressed as a sum of spherical harmonic products and a Monte Carlo estimator was employed to select the largest expansion terms. This CO−H2O PES has a global well depth De = 646.1 cm−1 for an intermolecular centre-of-mass separation R = 7.42 a0 and a planar configuration OC−H2O, with a weak hydrogen bond between the C atom of CO and one H atom of H2O. Bound-state rovibrational energy calculations using this PES have been found in very good agreement with experiments (Barclay et al. 2019), confirming the good accuracy of the potential. We note that a full-dimensional CO−H2O PES was recently computed at the same level (CCSD(T)-F12a/AVTZ) by Liu & Li (2019). These authors found a similar but deeper well depth (De = 685.5 cm−1), which is due to the basis set superposition error (BSSE) not corrected by Liu & Li (2019).
2.2 The statistical adiabatic channel model
Rigourous close-coupling quantum scattering calculations become impracticable when the number of coupled channels exceed typically 10 000. In such situation, statistical methods may provide a useful alternative. Statistical theories generally apply when a collisional system can access a deep well on the PES and the lifetime of the complex is sufficiently long. We have recently (Loreau et al. 2018a) developed a statistical approach inspired by the SACM of Quack & Troe (1975). In our implementation, we diagonalize the Hamiltonian excluding the kinetic term in the basis of rotational functions for the collision. In this case, the angular functions are products of Wigner rotation matrices for H2O and spherical harmonics for CO as well as for the relative orbital motion (Phillips, Maluendes & Green 1995). This expansion corresponds to the coupling of the angular momenta |${\boldsymbol{j}_1}$| of H2O, |${\boldsymbol{j}_2}$| of CO, and |${\boldsymbol{\ell }}$| for the relative motion to form the total angular momentum |$\boldsymbol{ J = j_1+j_2+\ell }$|. Upon diagonalization, for each value of J we obtain a set of adiabatic rotational curves as a function of the intermolecular distance R with an asymptotic value given by the sum of rotational energies of H2O and CO. These curves possess centrifugal barriers that depend on the orbital quantum number ℓ, and for a given initial state, the collision is assumed to take place if the collision energy is larger than the height of the barrier. The probability is then divided equally among the channels for which the energy is larger than their respective centrifugal barriers, allowing us to compute state-to-state cross-sections and rate coefficients.
This procedure was found to provide rate coefficients with an average accuracy better than 50 per cent for ion–molecule systems up to room temperature (Loreau et al. 2018a). In the case of CO−H2O, the SACM partial rate coefficients (computed for a total angular momentum J = 0) were shown to be accurate to within 40 per cent up to 100 K (Loreau et al. 2018b). Full comparisons between SACM and close-coupling calculations can be found in the two papers by Loreau and co-workers.
In this paper, the cross-sections were calculated for transitions among the first 11 energy levels of CO (up to j2 = 10) and the first 8 energy levels of both p-H2O and o-H2O (up to j1 = 3) and for collision energies between 0 and 500 cm−1 in order to derive rate coefficients up to T = 100 K. This requires to obtain all the adiabatic curves up to a total energy of 750 cm −1. The basis set of angular functions included levels up to j1 = 5 and j2 = 15, and tests were performed to ensure the convergence of the adiabatic curveswith respect to the size of the basis set. The rotational constant of CO was taken to be B0 = 1.92253 cm−1, while the energy of the rotational levels of H2O, |$E_{j_1k_ak_c}$|, was obtained using the effective Hamiltonian of Kyrö (1981). They are labelled with j1, ka, and kc, where ka and kc are pseudo-quantum numbers corresponding to the projection of the angular momentum j1 along the axis of least and greatest moment of inertia, respectively. Adiabatic curves were generated for values of the total angular momentum from J = 0 up to J = 100 for o-H2O and p-H2O separately. High total angular momentum values were found to be necessary as the cross-section converges very slowly with J due to the deep well in the PES as well as to the large reduced mass of H2O–CO.
Two sets of rate coefficients describing the excitation of CO by H2O were considered: the first consists of rate coefficients for which H2O is in the ground rotational state of its nuclear-spin isomer, i.e. |$k_{j_2\rightarrow j_2^\prime }(T)=k_{0_{00}, j_2 \rightarrow 0_{00}, j_2^\prime }(T)$| or |$k_{1_{01}, j_2 \rightarrow 1_{01}, j_2^\prime }(T)$| for p-H2O and o-H2O, respectively. This set of ‘ground-state’ rate coefficients neglects the simultaneous excitation of CO and H2O as well as the fact that excited rotational states of H2O can be significantly populated. Such an approximation is therefore expected to be accurate only at very low temperatures.
3 RESULTS
Rate coefficients for the rotational excitation of CO by H2O are presented below at three kinetic temperatures, 10, 50, and 100 K, for transitions out of the ground rotational state of CO (j2 = 0) as functions of the final j2 (|$j^\prime _2=1\!-\!10$|). In Fig. 1, four sets of data are presented, corresponding to the ‘ground-state’ and ‘thermalized’ SACM rate coefficients defined above with p-H2O and o-H2O as colliders. It is seen that all four sets have very similar rate coefficients at 10 K and that the transition j2 = 0 → 1 dominates. Small differences between thermalized and ground-state rate coefficients were expected at 10 K because (i) only the ground-state of p-H2O and o-H2O is significantly populated at thermal equilibrium and (ii) the excitation of H2O is inhibited because the rotational thresholds are larger than 10 K (23 and 54 K for the first excitation in o-H2O and p-H2O, respectively). In fact, however, substantial differences (more than a factor of 2) are found for transitions with |$j^\prime _2\gt 3$| whose rate coefficients are smaller than 10−11 cm3 s−1. For instance, the thermalized and ground-state rate coefficients for the excitation j2 = 0 → 4 (ΔE = 38.4 cm−1) by p-H2O are 1.26 × 10−12 and 3.16 × 10−13 cm3 s−1, respectively. The much larger thermalized value is due to the correlation between the CO excitation j2 = 0 → 4 (ΔE = 38.4 cm−1) and the simultaneous H2O de-excitation j1kakc = 111 → 000 (ΔE = 37.1 cm−1). This particular process provides a major contribution to the thermalized rate coefficient, despite the low population of the 111 level at 10 K (∼1 per cent). We also note that the near-resonant character of this correlated (de)excitation is not accounted for by SACM calculations and that close-coupling calculations would predict an even larger thermalized rate coefficient. At 50 and 100 K, the differences between the thermalized and the ground-state rate coefficients are clearly visible, reflecting both the larger population of excited H2O levels (thus pair correlations) and the opening of excitation channels in H2O. In particular, the transitions j2 = 0 → 2, 3 are preferred in the thermalized data at 100 K while j2 = 0 → 1 is favoured in the ground-state data. We also note that the difference between p-H2O and o-H2O is very small, both for thermalized and ground-state data, suggesting that the ortho-to-para ratio (OPR) of H2O should not play a major role in the excitation of CO (see Section 4 below).
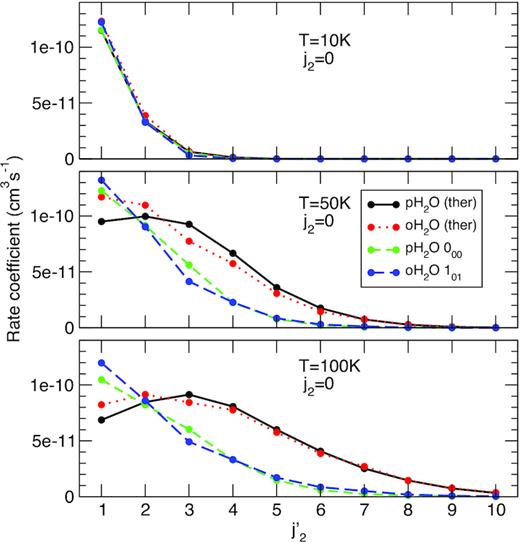
SACM rate coefficients for the rotational excitation of CO by H2O from the ground state (j2 = 0) to the lowest 10 excited states. The projectiles p-H2O and o-H2O are assumed to be either thermalized at the kinetic temperature (10, 50, or 100 K) or in their ground rotational states 000 and 101, respectively. See text for details.
In Fig. 2, the above set of thermalized rate coefficients is compared to previous data from the literature: those of Biver et al. (1999) (denoted as B99) and those of Green (1993) (denoted as G93). Biver et al. (1999) assumed a total cross-section of σc = 2 × 10−14 cm−2, with no energy dependence. This value was derived from room temperature pressure broadening measurements on H2O–H2O (Biver 1997), with an estimated uncertainty of a factor of ∼2. The total collisional rate coefficient was computed as kc(T) = σc〈v(T)〉, where T is the kinetic temperature and 〈v(T)〉 is the thermal mean velocity of the CO molecule relative to the cometary gas. State-to-state rate coefficients from an initial CO level j2 to a final level |$j^\prime _2$| were then obtained as |$k_{j_2\rightarrow j^\prime _2}(T) = k_cP_{j^\prime _2}(T)$|, where |$P_{j^\prime _2}(T)$| is the Boltzmann population of the final level |$j^\prime _2$| at temperature T. This recipe corresponds to assuming that each collision redistributes the molecule according to the Boltzmann distribution, which has no physical basis, except that it satisfies the detailed balance principle (by construction). Green (1993), on his side, combined line shape measurements on N2–H2O with a rate-law derived from the infinite-order-sudden (IOS) approximation. The total excitation rates were expected to be rather accurate (about 10 per cent) at room temperature, where the rate-law parameters were fitted. It should be noted that in contrast to our SACM calculations, the B99 and G93 collision data sets do not distinguish between p-H2O and o-H2O. It is found in Fig. 2 that the magnitude and the |$\Delta j_2 = j^\prime _2-j_2$| dependence of the rate coefficients are reasonably described by the B99 and G93 data sets: the largest rate coefficients are ∼10−10 cm3 s−1 and they (globally) slowly decrease with increasing Δj2. The overall agreement is somewhat surprising, in particular for the B99 data set given the simplicity of the prescription. We note, however, that differences larger than a factor of 2 are observed for transitions with Δj2 > 2 and, more generally, that the B99 set overestimates while the G93 set tends to underestimate the SACM rate coefficients.
It is now interesting to compare the efficiency of H2O relative to other colliders for exciting CO. In Fig. 3, the thermalized SACM rate coefficients for CO−H2O are compared to the corresponding data for CO−H (Yang et al. 2013), CO−He (Cecchi-Pestellini et al. 2002), CO−p-H2, and CO−o-H2 (Yang et al. 2010). These latter four sets of data were obtained from quantum close-coupling calculations combined with modern PES. Their accuracy at the state-to-state level is expected to be very good (about 20 per cent), as verified experimentally for CO−He and CO−H2 (Carty et al. 2004; Chefdeville et al. 2015). We can first observe that, at 10 K, p-H2O and o-H2O are the most effective colliders with a rate coefficient for the transition j2 = 0 → 1 that is a factor of ∼2 larger than those for the other colliders. Atomic hydrogen is the less effective, with rate coefficients lower than 10−11 cm3 s−1. At larger temperatures, the Δj2 dependence of the rate coefficients is found to be very different for H2O than for H and H2: while the water rate coefficients slowly decrease with increasing Δj2, a strong propensity rule with Δj2 = 2 is observed for atomic and molecular hydrogen. As a result, the largest rate coefficients at 100 K are those of H and H2 for the transition j2 = 0 → 2. For transitions with Δj2 > 2, however, H2O is seen to be more than twice as effective as the other colliders. The magnitude of the rate coefficients for the excitation of CO by H2O is thus rather similar to that of other neutral colliders. The main difference is the lack of strong propensity rules and the relatively slow decrease of the rate coefficients with increasing Δj2. This reflects mainly the larger potential well depth but also the fact that SACM calculations are not able to catch resonance and interference effects (Loreau et al. 2018a, 2018b). In particular, as discussed above, SACM calculations are expected to underestimate near-resonant rotational energy transfers.
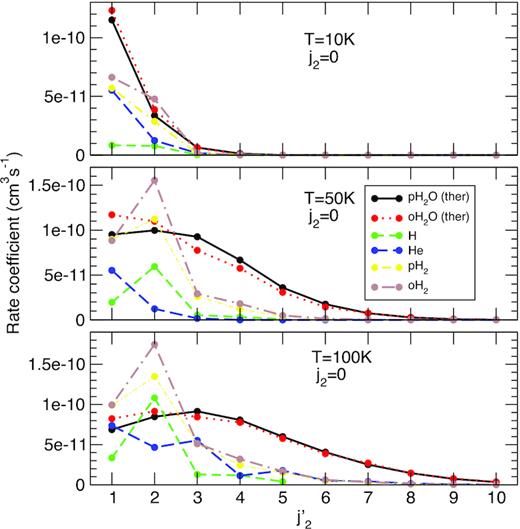
Same as Fig. 1. The thermalized SACM data for p-H2O and o-H2O are compared to the data for other projectiles: H, He, p-H2, and o-H2.
We note, finally, that free electrons provide another source of CO rotational excitation in astronomical environments. Owing to the small dipole of CO (0.11 D), however, rate coefficients for electron-impact rotational excitation do no exceed ∼2 × 10−8 cm3 s−1. This means that electrons compete with neutrals in exciting CO only when the electron-to-neutral number density ratio is larger than ∼10−2.
4 NON-LTE MODEL OF CO IN COMETS
The input parameters to RADEX are the kinetic temperature, Tk, the column density of CO, N(CO), the line width, Δv, and the density of the collider, here |$n_{\rm H_2O}$|. Three kinetic temperatures were selected: 10, 50, and 100 K. The column density was fixed at N(CO) = 1014 cm−2, which is the typical magnitude at r ∼ 1000 km for a CO/H2O abundance ratio of ∼10 per cent (e.g. Lupu et al. 2007). We have checked, however, that the relative populations are not sensitive to the column density provided that N(CO) < 1016 cm−2 (i.e. the lines are optically thin). The water density is varied in the large range 10−3–1013 cm−3 corresponding to nucleocentric distances between 1 and 106 km in the Haser model, as plotted in Fig. 4. The line width is fixed at 1.6 km s−1, i.e. Δv = 2 × vexp. Finally, the background radiation field includes both the 2.73 K cosmic microwave background (CMB) and the Sun radiation. This latter contribution is taken as a diluted blackbody of 5770 K with the dilution factor W = ΩS/|$4\pi$|, where ΩS ∼ 6.79 × 10−5 sr is the Sun solid angle seen at Rh = 1 au.
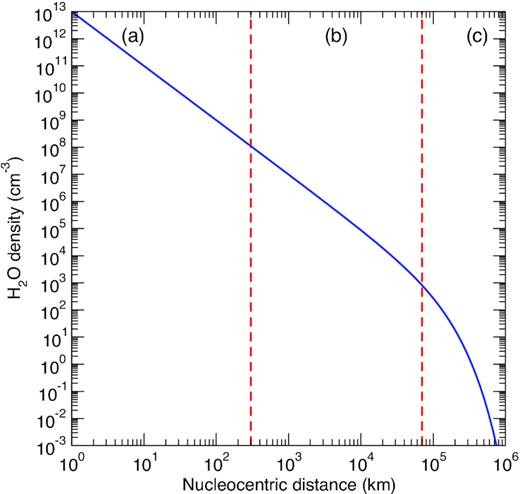
Coma density profile of H2O in the Haser’s model. The photodissociation rate was taken as |$\beta _{\rm H_2O} = 1.042\times 10^{-5}$| s−1 from Zakharov et al. (2007). The dashed lines delineate, for a production rate |$Q_{\rm H_2O} = 1\times 10^{29}$| s−1, the three approximate density regimes: (a) LTE equilibrium, (b) non-LTE regime, and (c) fluorescence equilibrium. See text for details.
Input data include the CO energy levels (v2, j2) (v2 is the CO vibrational quantum number), the spontaneous emission Einstein coefficients and the collisional rate coefficients. Level energies and Einstein coefficients were taken from the HITRAN data base1 (Gordon et al. 2017). Only the first excited vibrational level v2 = 1 was taken into account because the solar excitation rate of v2 = 1 is at least two orders of magnitude greater than those of v2 > 1 (Crovisier & Le Bourlot 1983). Our model thus include the lowest 80 ro-vibrational levels of CO, i.e. up to level (v2, j2) = (1, 32) which lies 4148 cm−1 above the ground-state (0, 0). Since our SACM rate coefficients are available for the lowest 11 rotational levels only, we had to extrapolate the collisional data above j2 = 10. It should be noted, however, that the corresponding levels are negligibly populated (less than 1 per cent) at the investigated temperatures and the extrapolated collisional data play only a minor role in the SE equations. In practice, the total de-excitation rotational rate coefficients were simply fixed at 10−10 cm3 s−1 with an equal distribution among final levels and no temperature dependence. Similarly the total de-excitation vibrational rate coefficients were fixed at 10−13 cm3 s−1 (with again an equal distribution among final levels), as suggested by the CO(v2 = 1) − H2O quenching measurements of Wang, Gu & Kong (1999) performed at 300 K. Finally, for pure rotational transitions involving rotational levels j2 ≤ 10 within v2 = 1, we adopted the corresponding rate coefficients within v2 = 0. Rotational rate coefficients indeed depend only weakly on the vibrational state (Carty et al. 2004). This extrapolation procedure was used for the SACM, the B99 and the G93 collision data sets.
The population distribution of the CO rotational levels j2 in v2 = 0 is thus governed by the balance between the collision-induced transitions, the vibrational excitation (v2 = 0 → 1) by the solar infrared radiation field, the rotational excitation by the CMB radiation field and the radiative decay due to spontaneous emission. The results of our non-LTE model are presented in Fig. 5 where the population of levels j2 is plotted as function of the H2O density for the selected kinetic temperatures 10, 50, and 100 K. The set of thermalized SACM rate coefficient is employed here with an OPR for H2O equal to 3. We can clearly observe the presence of three different regimes: the fluorescence equilibrium at low densities (|$n_{\rm H_2O}\lesssim 10^3$| cm−3), the non-LTE regime in the range |$10^3\lesssim n_{\rm H_2O}\lesssim 10^8$| cm−3, and the LTE-regime at larger densities. Thus, for a production rate |$Q_{\rm H_2O} = 1\times 10^{29}$| s−1, LTE applies at r ≲ 300 km, i.e. in the inner coma, while the fluorescence equilibrium distribution is completely established at r ≳ 7 × 104 km (see Fig. 4). The non-LTE regime extends typically from 300 to 70 000 km, representing about 10 per cent of all CO molecules in the coma. We can notice in Fig. 5 that the non-LTE regime of densities does not significantly vary with temperature. In contrast, the LTE population distribution is very sensitive to the temperature: at 10 K significant populations (>1 per cent) are found only for j2 = 0–3 while at 100 K all levels j2 = 0–10 are occupied above 2 per cent.
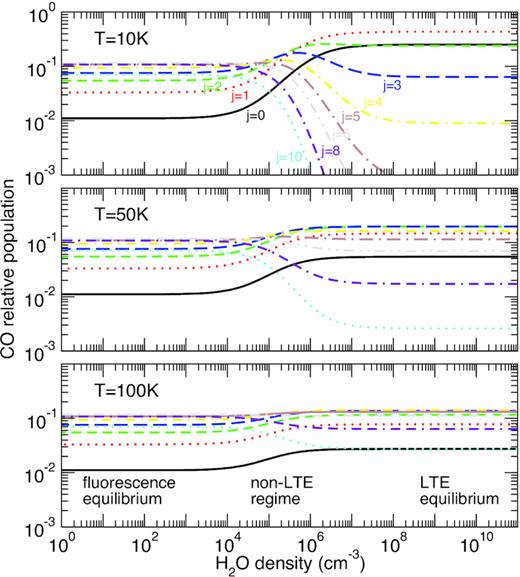
Level populations of CO (j = 0 − 6, 8, 10) as functions of H2O density for kinetic temperatures of 10, 50, and 100 K. The thermalized SACM rate coefficients are employed with an OPR of H2O fixed at 3.
In the following, the H2O density is fixed at |$n_{\rm H_2O} = 1.9\times 10^5$| cm−3, which is representative of the non-LTE regime. The corresponding nucleocentric distance is r ∼ 7000 km. In Fig. 6, the CO relative population is plotted as function of j2 for the two sets of SACM rate coefficients (thermalized and ground-state) and for an OPR of H2O equal to 1 and 3. The OPR of H2O in comets is debated but values are generally comprised between 1 and 3, with a median equal to 2.9 (see Faure et al. 2019, and references therein). We first observe that the impact of the OPR of water is negligible, as expected from the small differences seen in Fig. 1. It can be also noticed that the thermalized SACM rate coefficients predict a more peaked distribution than the ground-state rate coefficients, with a greater population for low j2 levels. The relative difference is found to reach ∼60 per cent at 10 K for j2 = 2. This effect is due to pair correlations between CO and H2O transitions, as discussed in Section 3. Perhaps surprisingly, the influence of the H2O rotational distribution is found to be less important at higher temperature. In fact, this mainly reflects the LTE distributions which are closer to the fluorescence equilibrium distribution at 50 K and mostly 100 K than at 10 K, attenuating the impact of collisional data.
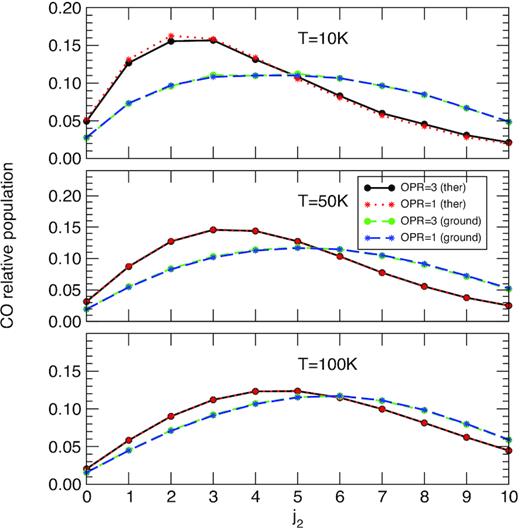
CO population distribution at a fixed H2O density of 1.9 × 105 cm−3 for the three kinetic temperatures 10, 50, and 100 K. The SACM thermalized and ground-state rate coefficients are employed with an OPR of H2O equal to 1 and 3. See text for details.
Finally, in Fig. 7 we compare the CO rotational populations as predicted by our thermalized SACM rate coefficients (with an OPR of H2O fixed at 3) and by the B99 and G93 rate coefficients. As in Fig. 6, the influence of collisional data is found to decrease with temperature, which again reflects the relative deviation between the LTE and the fluorescence equilibrium distribution. At 10 K, the uncertainties in collisional data have the largest impact with relative differences up to a factor of ∼3 for j2 = 1. The B99 set overestimates while the G93 set underestimates the populations of levels j2 ≲ 4, which is consistent with the trend in the rate coefficients shown in Fig. 2. For levels with j2 > 4, however, the reverse is observed, emphasizing the role of collisional transitions out of j2 > 0.
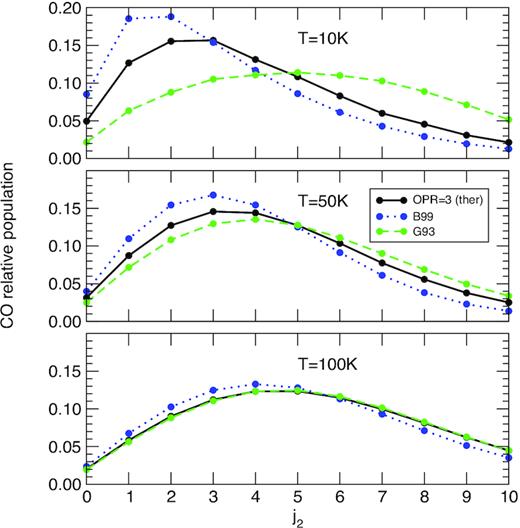
Same as Fig. 6. Three sets of collisional rate coefficients are employed: the thermalized SACM set with the OPR of H2O fixed at 3, the B99 set, and the G93 set. See text for details.
In summary, using a simple non-LTE model of CO in standard cometary conditions, we have found that uncertainties in the collisional rate coefficients have a significant impact on the CO population distribution in the non-LTE regime (|$n_{\rm H_2O}\sim 10^5$| cm−3). In particular, the CO rotational populations predicted by three different collisional data sets were found to differ by up to a factor of 3 at 10 K. Thanks to our SACM rate coefficients, we were also able to demonstrate that the H2O rotational distribution plays an important role, even at low temperature (due to correlated rotational energy transfers) while the OPR of H2O has an almost negligible influence.
5 CONCLUDING REMARKS
We have provided the first quantum rate coefficients for the rotational excitation of CO by p-H2O and o-H2O. These rate coefficients were obtained by combining SACM calculations with a recent ab initio CO−H2O PES computed at the CCSD(T)-F12a/AVTZ level. Rotational transitions among the first 11 energy levels of CO and the first 8 energy levels of both p-H2O and o-H2O were considered. Two different sets of rate coefficients were obtained: one corresponding to p-H2O and o-H2O in their ground rotational state and one corresponding to thermal distributions of each nuclear-spin isomer. Correlated energy transfer in both CO and H2O was shown to be important while the differences between p-H2O and o-H2O were found to be small. Our set of ‘thermalized’ rate coefficients was compared to previous data from the literature and significant differences were observed. We also found that H2O is more effective than other colliders (H, He, and H2) for exciting CO transitions with Δj2 > 2. Finally, we have assessed the impact of the collision data on the CO population distribution in a cometary coma using a simple non-LTE model including collision-induced transitions, radiative pumping and radiative decay. We have found that the uncertainties in the collision data can affect the rotational populations by large factors at low temperature and at H2O densities ∼103–108 cm−3. In addition, the rotational distribution of H2O was found to play a significant role so that a consistent non-LTE model of CO excitation should include a realistic (i.e. non-LTE) population distribution for H2O. This in turn would require the knowledge of accurate H2O-H2O rotational rate coefficients, which are lacking in the literature. On the other hand, the OPR of H2O was shown to have a negligible impact on the CO excitation, which removes one uncertainty from the interpretation of CO spectra.
Future studies will need to consider other linear targets such as CN, CS, HCO+ as well as non-linear species such as NH3, H2CO, etc. colliding with both H2O and CO. The present SACM approach should be therefore extended to treat two non-linear polyatomic collision partners. We note that many data are available for the electron-impact rotational excitation of cometary molecules (e.g. Harrison, Faure & Tennyson 2013). Comparing the efficiency of electrons relative to neutrals (H2O and CO) will be very useful to distinguish between the different collisional non-LTE regimes in a comet (H2O-, e-, or CO-dominated). Finally, we strongly encourage modellers to use the present CO−H2O data in any model of CO excitation in comets and, more generally, to use the ever-increasing amount of theoretical collision data available for astrophysical applications.
ACKNOWLEDGEMENTS
This research was supported by the Programme National ‘Physique et Chimie du Milieu Interstellaire’ (PCMI) of INSU, CNRS with INC/INP co-funded by CEA and CNES. Nicolas Biver, Jérémie Boissier and Lucas Paganini are acknowledged for very useful discussions.




