-
PDF
- Split View
-
Views
-
Cite
Cite
Sara M Wilson, Andrei S B Lojek, Grettel J Zamora-Berridi, John A Hodgson, Not Your Average Mediastinal Mass: A Case of a Large Mediastinal Teratoma in a Patient With a History of Polio Disease, Military Medicine, Volume 188, Issue 7-8, July/August 2023, Pages e2693–e2696, https://doi.org/10.1093/milmed/usab343
Close - Share Icon Share
ABSTRACT
Mediastinal masses can be challenging to the surgical team and anesthetic considerations vary according to the location, pathology, surgical approach, and patient comorbidities. We report the case of a 21 cm symptomatic intrathoracic teratoma in a postpartum patient with a history of poliomyelitis. Significant challenges were presented for anesthetic induction, potential extracorporeal membrane oxygenation, and the use of neuraxial pain techniques and neuromuscular blockade. This case report demonstrates techniques to safely manage a patient with a large symptomatic mediastinal mass and potential neuromuscular disease.
INTRODUCTION
Mediastinal masses can be challenging to the anesthesiologist and considerations vary according to the location, pathology, surgical approach, and comorbidities. Management must be individualized. We report a case of a mediastinal teratoma in a patient with a history of poliomyelitis.
CASE REPORT
A 41-year-old woman (height 160 cm, 5ʹ3ʹ; weight 47.7 kg) presented for bilateral thoracotomy for intrathoracic mass excision. She had a history of childhood poliomyelitis and was otherwise healthy. Her childhood symptoms included total body weakness with lower extremity involvement greater than upper extremity. Her lower extremity weakness had not fully resolved, and she presented with bilateral gastrocnemius weakness and atrophy. Surgery was four months postpartum, although the mass was discovered when she presented with fatigue and shortness of breath at a 20 week pregnancy visit. A chest radiograph revealed a large chest mass. A follow-up computed tomography (CT) scan (Fig. 1) revealed a well-circumscribed 21.4 cm × 12.8 cm heterogeneous mass. CT-guided biopsy revealed benign teratoma. She had a positron emission tomography scan that did not demonstrate any malignant features. She had pulmonary function tests that were remarkable for a forced vital capacity (FVC) 35% of predicted, forced expiratory volume over 1 second (FEV1) 43% of predicted, forced expiratory volume over 1 second/forced vital capacity ratio123% of predicted, total lung capacity 48% of predicted volume, functional residual capacity 52% of predicted, residual volume 77% of predicted, vital capacity 35% of predicted, and expiratory reserve volume 16% of predicted.
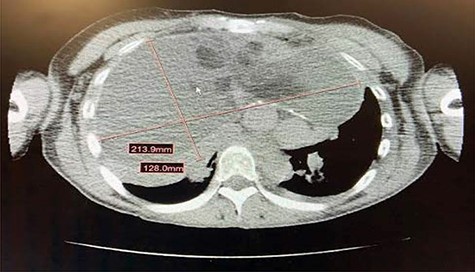
Large missed attenuation anterior mediastinal mass measuring approximately 21.4 cm x 12.8 cm. The mass had a large fluid component with loculations and scattered areas of fat. There were also scattered wall calcifications of the mass. There was leftward shift of the heart secondary to mass effect and the tumor was noted to be in close proximity to arch vessels as well as the SVC.
On the day of surgery, pre-operative vital signs were normal. Physical exam revealed decreased lung sounds bilaterally without wheezing, rales, or rhonchi. She did not require supplemental oxygen. She had minimal dyspnea in the seated position, but did experience dyspnea while supine. Exam revealed decreased bilateral gastrocnemius strength of 4/5, as well as bilateral gastrocnemius atrophy. She did not have a pre-operative echocardiogram. Her pre-operative labs were unremarkable.
In the operating room, electrocardiogram, pulse oximeter, and noninvasive blood pressure monitors were applied. She was given midazolam 2 mg intravenously for pre-medication. An awake arterial line and right internal jugular central line were obtained with local anesthesia. The cardiothoracic surgeon performed femoral venous and arterial cannulation prior to induction in the event venous-arterial extracorporeal membrane oxygenation (VA-ECMO) was required. General anesthesia was induced with small, titrated doses of propofol, ketamine, and remifentanil. Neuromuscular blockade was avoided to maintain spontaneous ventilation. A size 37 French left-sided, double lumen tube was placed uneventfully using video laryngoscopy and confirmed using a fiberoptic bronchoscope.
The patient was placed in the left lateral decubitus position and the right chest was prepped. Upon entry into the chest cavity, the mass was solid and well circumscribed. Copious material was evacuated that was granular material with hair, bone, and a pungent aroma (Fig. 2). The mass resection was intricate with multiple high risk dissection areas, including the pericardium, aortic arch, distal ascending aorta, and the subclavian artery. The mass was densely adherent to the superior vena cava (SVC) and tedious dissection allowed for separation, despite several entries into the SVC which were easily repaired. The mass was adherent to the medial portion of both lungs and required wedge resections of the medial portion of the left upper lobe, as well as medial portions of the right middle lobe.
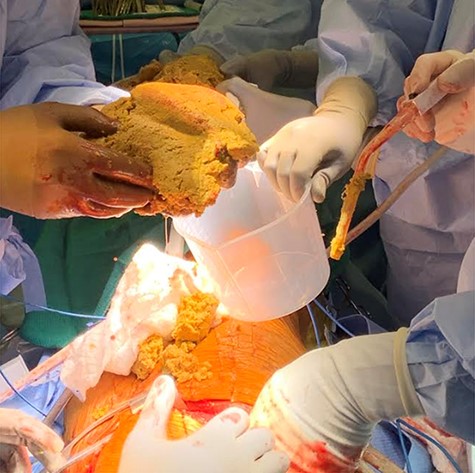
Copious amounts of cheesy, granular material removed with careful dissection.
Anesthetic management included inhalational anesthesia (sevoflurane 1.5%–3%), fentanyl, and remifentanil. Rocuronium was used to maximize surgical exposure once airway compression was ruled out with direct vision and bronchoscopy. Estimated blood loss for the case was 1200cc and three units of packed red blood cells were transfused. Given her low estimated total blood volume due to her small size, as well as periods of bleeding related to the densely adherent mass in proximity to major vessels and extent of dissection, a low transfusion threshold was utilized during periods of bleeding. There were several episodes of bleeding that were quickly managed but not before transient decreases in blood pressure and blood transfusions initiated in response. At the end of the case, while the patient was still intubated, a paravertebral catheter was placed for postoperative pain control. The double lumen tube was exchanged for a single lumen 8.0 endotracheal tube for possible surgical intensive care unit (ICU) care. A fiberoptic bronchoscopy confirmed no airway compression or evidence of tracheomalacia. Therefore, the patient was extubated in the operating room, without difficulty, after neuromuscular reversal with sugammadex. She was taken to the intensive care unit in stable condition. She recovered without incident.
DISCUSSION
Tumors within the mediastinum are often characterized by their location within the thoracic cavity (anterior, posterior, or middle), type (benign or malignant), size, and involvement of local structures. A pre-operative chest CT is the best method for assessing the size and extent of local structure involvement.1 The diagnosis can be clear, but often requires tissue sampling. The most common causes of anterior mediastinal masses include thymoma, teratoma, thyroid disease, and lymphoma. Middle mediastinal masses are often congenital cysts (including foregut and pericardial cysts). Posterior mediastinal masses are often neurogenic tumors.2
Symptoms usually present as a consequence of local invasion or as a sequelae of a systemic secondary syndrome.2,3 Depending on the size, tumors in the mediastinum can range from asymptomatic to life threatening depending on their size and location in the intrathoracic cavity.
Teratomas are the most common mediastinal germ cell tumors. They consist of at least two of the three primitive germ layers including ectodermal tissue (including skin, hair, sweat glands, and teeth), mesodermal tissue (fat, bone, cartilage, and smooth muscle), or endodermal tissue (respiratory and intestinal epithelium).4 Teratomas that consist of intestinal mucosa can secrete digestive enzymes or pancreatic tissue that can lead to rupture of the bronchi, pleura, pericardium, or lung.5 Mature teratomas have the potential to undergo malignant transformation including the development of rhabdomyosarcoma, adenocarcinoma, anaplastic small cell tumors, and leukemia.6
In our patient, the teratoma was found by chest radiograph after the patient experienced dyspnea during the early stages of pregnancy. Close attention to pre-operative symptoms, as well as a detailed history and physical can be utilized to provide insight into the risks associated with mediastinal masses, such as airway compression. Assessing the patient for dyspnea or coughing in the supine position can be an indication of airway compression. Stridor or wheezing can indicate compressive symptoms of the upper or lower airways, respectively. Observing the head and neck for edema, vein enlargement, plethora, headache, or confusion may indicate the presence of SVC syndrome.
Extracorporeal membrane oxygenation (ECMO) has emerged as a management option for patients with extrinsic or fixed airway compression during induction of chemotherapy and with surgical manipulation.7–11 The timing of cannulation can be a point of contention among providers. There are cases of mortality in patients with airway compression leading to hypoxia when ECMO access was unable to be obtained in a timely manner.7 Proper planning and a lower threshold for initiating ECMO in patients with an anterior mediastinal mass can help mitigate poor outcomes when applied early in the proper setting. High risk patients to consider pre-induction cannulation include, but are not limited to, those with SVC syndrome, pulmonary artery or right ventricular outflow tract compression, compression of the bronchus, or great vessel involvement.7 The benefit of VA-ECMO over venous-venous ECMO (VV-ECMO) lies in its ability to provide both respiratory and hemodynamic support, whereas VV-ECMO provides respiratory support, but requires maintenance of cardiac blood flow to function and does not provide cardiac support to assist systemic circulation.12 VA-ECMO is often selected for patients with extrinsic compression on the airways and vasculature or for use in the peri-arrest setting, while VV-ECMO is often utilized in patients with tracheal or bronchial tumors with fixed airway obstruction.7 In this patient, the risks associated with tumor size and the adjacency of the mass to airway and cardiac structures prompted preinduction cannulation of the femoral vessels in the operating room, with a perfusionist and cardiopulmonary bypass circuit on standby throughout the case. If compressive airway collapse occurred during this case, either VV or VA- ECMO likely would have been sufficient, but given the size and extent of the teratoma and its close proximity to the pericardium, the risk of cardiovascular compromise remained high, thus, VA-ECMO was prepared as a precaution.
Airway patency must be reassessed frequently. The presence of anterior mediastinal masses in combination with muscle relaxation can increase the chance of airway obstruction. Maintaining spontaneous ventilation is often recommended in these patients to ensure airway patency and to maximize recovery of airway patency if collapse begins. Neuromuscular blockade was withheld early in our case until airway patency and hemodynamic stability was confirmed via bronchoscopy during positive pressure ventilation. Confirming the ability to provide positive pressure ventilation, prior to neuromuscular blockade minimizes the risks of inadequate ventilation, as once pharmacologic paralysis is induced, positive pressure ventilation is the only method to ventilate.13 Additionally, some clinicians recommend more caution by preserving spontaneous respiration until sternotomy in order to maintain a normal transpulmonary pressure gradient.14 This avoids the use of positive pressure ventilation which can cause increased intrathoracic pressure, decreased preload, decreased right ventricular output, and decreased pulmonary blood flow resulting in cardiopulmonary compromise. Clinicians should be prepared for immediate sternotomy to elevate the mass off the great vessels, should compression occur.15
The effects of neuromuscular blockade on patients with neuromuscular diseases are variable. Data remains limited to small studies and appropriate management is highly individualized. Individuals with neuromuscular disease are at an increased risk of weakness after receiving neuromuscular blockers, and it is known that succinylcholine can lead to hyperkalemia in neuromuscular disease.16 Thus, with neuromuscular illness, efforts to mitigate postsurgical weakness are necessary and may involve avoiding neuromuscular blockade. If neuromuscular blockade is required, establishing a functional baseline may be of benefit to confirm postoperative return of function.17
The development of post-polio syndrome (PPS) occurs with the paralytic form of poliomyelitis, and usually many years later.18 Therefore, individuals without a known diagnosis of PPS, but a history of poliomyelitis, may be at risk of developing PPS. The implications for long-term outcomes after neuromuscular blockade in PPS patients is unknown and may never be fully understood. Some literature suggests limiting the amount of muscle relaxant if their use is necessary.17,19 Exercising caution in patients with or at risk for PPS can prevent unwanted sequelae. Our patient was noted to have minor residual gastrocnemius weakness with atrophy, and did not appear to manifest a full recovery from childhood poliomyelitis. While she did not have a formal diagnosis of PPS, the anesthetic implications of caring for these patients were considered.
Studies are inconclusive regarding neuromuscular blockade in patients with poliomyelitis, a past history of poliomyelitis, or PPS. Although research is limited, any neuromuscular disease has the potential to have sensitivity to neuromuscular blockade resulting in prolonged postoperative weakness or, rarely, permanent neuromuscular dysfunction. A small study in pediatric patients with a history of poliomyelitis showed increased sensitivity to nondepolarizing muscular blockers.19 Postoperative respiratory failure, associated with weakness, over-sedation, or both is described in patients with a history of poliomyelitis. Vigilant monitoring is recommended to avoid complications. Utilizing shorter acting agents such as rocuronium or mivacurium can reduce muscle weakness after surgery.17 Some authors suggest starting with half the normal dose of neuromuscular blocking agents.17,19 Succinylcholine has been used successfully in some patients with PPS without resultant hyperkalemia,20,21 but caution is recommended given multiple case reports showing patients with poliomyelitis, PPS, or pathology similar to PPS developing succinylcholine-induced hyperkalemia and circulatory arrest.22–24 Rocuronium was used to maximize surgical exposure, accepting the risk of prolonged neuromuscular blockade which would likely be completely reversed with sugammadex, a direct competitive inhibitor, if needed.
A paravertebral catheter was utilized for pain control. Anesthesiologists have questioned whether the use of regional anesthesia could exacerbate existing neuromuscular disease or mask disease progression and complications. While animal studies have determined specific intrathecal concentrations of local anesthetics are toxic for neurons,25 it is difficult to know if a difference exists for patients with damaged motor neurons. Logically, the lethal dose for neurons would be decreased in patients with prior neuronal injury, but there is no data to confirm this hypothesis.17 Ropivacaine 0.2% was infused post-operatively without any complications.
SUMMARY
Removal of a mediastinal mass requires a multifaceted approach. A carefully planned induction can reduce the risk of airway collapse. Preinduction preparation for ECMO provides an added layer of safety. Maintaining spontaneous ventilation can provide maximum airway patency until definitive airway decompression is established. Careful use of neuromuscular blocking agents is advocated to minimize cardiopulmonary compromise. In addition to these considerations, a history of poliomyelitis presented additional considerations for neuromuscular blockade and pain management. This case report demonstrates techniques to safely manage a patient with a large symptomatic mediastinal mass and potential neuromuscular disease.
FUNDING
None declared.
CONFLICT OF INTEREST STATEMENT
None declared.



