-
PDF
- Split View
-
Views
-
Cite
Cite
Sean M Stuart, Megan L Bohan, Emily E Friedrich, Speed, Skill Retention, and End User Perceptions of iTClamp Application by Navy Corpsmen on a Manikin Model of Femoral Hemorrhage, Military Medicine, Volume 188, Issue 7-8, July/August 2023, Pages e2496–e2501, https://doi.org/10.1093/milmed/usac355
Close - Share Icon Share
ABSTRACT
Tactical Combat Casualty Care guidelines recommend packing junctional wounds with gauze, applying direct pressure for 3 minutes, and then securing with an external pressure dressing. This method is time-consuming, which can be problematic in a combat environment. Alternatively, the iTClamp has documented efficacy and rapid application. However, no studies have evaluated device application by military prehospital medical providers, such as Navy corpsmen, or their user experience with the device.
Research data derived from a protocol were approved by the Naval Medical Center Portsmouth’s Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects. Navy corpsmen with the current Tactical Combat Casualty Care certification applied the iTClamp or standard pressure dressing on a manikin model of femoral hemorrhage in a crossover study design. Each participant used both devices in a randomized fashion. Time to application was recorded, and participants completed Likert scale surveys to evaluate both devices for preference, ease of use, and physical assessment. A repeat assessment was performed 1 month later to assess skill atrophy. Repeated-measures ANOVA was used to compare application time. Likert scale survey data were analyzed using Mann–Whitney and Wilcoxon tests to compare survey data within and between time points, respectively.
The application of the iTClamp was more than twice as fast as the application of pressure dressings at both the initial and follow-up evaluations. There was no statistically significant difference in application times between the first evaluation and the 30-day assessment of either device, indicating no atrophy in skill. While 65% and 52% of the participants expressed preference in for the iTClamp in their surveys during the initial and follow-up respective visits, the difference in preference was not statistically significant for either the initial or the follow-up survey. Open-ended survey responses yielded both perceived advantages and disadvantages for each treatment option.
In austere or hostile environments, speed of treatment and extrication can have significant implications for the safety of both the patient and the medical providers. Hemorrhage control interventions must be both effective and easy to use for a prehospital provider to ensure its efficacy in a live battlefield situation. The iTClamp is small, simple, and fast to use, but its wide adoption in the field may be based on limitations perceived by participants, including narrow indications for use. However, based on our findings, it is reasonable to field the iTClamp depending on provider preference.
INTRODUCTION
Exsanguinating hemorrhage remains the leading cause of preventable death in military trauma care.1 Rapid transport from a prehospital to a hospital setting has been associated with a reduction in death from traumatic injuries.2,3 Rapid hemorrhage control and patient stabilization are required to achieve rapid prehospital transport. Consequently, continued improvement of early hemorrhage control interventions nearest to the point of injury is critical in the reduction of prehospital mortality. Although increased use of traditional tourniquets has improved outcomes for patients with extremity hemorrhage, wound patterns such as junctional injuries require more complex care in a prehospital arena.2,4
The Committee on Tactical Combat Casualty Care (CoTCCC) currently recommends packing an actively hemorrhaging junctional wound with a gauze product and then applying and holding external pressure. Junctional tourniquets are also indicated, but these methods demand costly time, do not prioritize wound closure, and have high failure rates.5 One alternative is the iTClamp Hemorrhage Control System (Innovative Trauma Care, Atlanta, GA, USA).
The iTClamp is a spring-loaded, mechanical wound closure device approved for the control of severe bleeding in the extremities, axilla, and inguinal areas.6 Measuring 7 cm × 7 cm × 4 cm, the iTClamp’s small size and the light weight of 37 g make the device logistically suited for fielding in combat scenarios. By providing direct mechanical pressure at the site of injury, the iTClamp assists in the initiation and formation of a stable clot.7 Currently, CoTCCC has recommended the iTClamp as a primary treatment modality, along with a CoTCCC-recommended hemostatic dressing and direct manual pressure, for hemorrhage control of craniomaxillofacial injuries and penetrating neck injuries with external hemorrhage.5,8
Despite the iTClamp’s documented efficacy in treating head and penetrating neck injuries and junctional hemorrhage,7,9–12 few studies to date have evaluated the ability of non-physician, prehospital providers, such as emergency medical service providers or military corpsmen and medics, to successfully apply the iTClamp when given appropriate training. In battlefield conditions, it is imperative that hemorrhage control measures take place nearest to the point of injury to stabilize the wounded for evacuation to definitive care. Prehospital providers, such as corpsmen, are most frequently the first to respond to and treat patients with exsanguinating hemorrhage and thus require the most effective and practical hemorrhage control tools to carry in-field.13,14
With time being a crucial factor in the outcome of an actively hemorrhaging patient, a hemorrhage control technique that is both quickly applied and easy for prehospital providers to administer can mean the difference between life and death for the wounded. The practicality of fielding the iTClamp with Navy corpsmen was evaluated by their speed in iTClamp or pressure dressing application after initial training and 30 days later and by end user survey ratings of comfort with and confidence in either device.
METHODS
Subjects and Setting
The protocol was approved by the Naval Medical Center Portsmouth’s Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects. Research data were derived from that approved protocol. The study was conducted in an indoor classroom setting at Naval Medical Center Portsmouth in Portsmouth, VA.
Participants were volunteer active duty U.S. Navy sailors (N = 26) who had received Tactical Combat Casualty Care (TCCC) training. Participants were educated on the study procedures and signed the informed consent document before study participation. Individuals who had no prior TCCC training, were outside the ages of 18-45, were not able to physically complete the study, or could not complete study surveys were excluded from the study.
Materials
iTClamp is a 7 cm × 7 cm × 4 cm hinged, plastic self-locking wound closure device with stainless steel teeth of suture needle construction designed to grab and pull together and then lock into place opposing tissue edges of a wound (Fig. 1).

Photographs of the iTClamp open, partially closed, and fully closed.
FemoraLineMan (Simulab Corporation, Seattle, WA, USA) is an ultrasound compatible task trainer with central venous or arterial access using the femoral site. Simulated blood is pumped into the manikin’s vessels, in which an arterial pulse can be felt and vein compresses under palpation.
The Emergency Trauma Dressing (North American Rescue, Greer, SC, USA) is a 6” elastic wrap equipped with a sterile non-adherent pad intended to absorb blood and apply direct pressure to a wound.
Methods
In compliance with ethical guidelines, participants provided written informed consent, documenting their agreement to participate in the study and their understanding of their rights as research subjects, including their right to withdraw from the study. Enrolled participants were assigned a subject ID number, and the order in which each participant would apply the two hemostatic agents was randomized. After participants completed a demographic survey, they were given a period of instruction by the investigators on proper hemostatic agent application. This instruction period consisted of a brief lecture covering the hemostatic devices and their application, demonstration, and hands-on practice. The instruction period continued until all participant questions had been answered by the investigators, and the participants verbalized their understanding of each hemostatic agent’s application.
The hemostatic agent assigned by randomized order was placed in their original packaging next to the FemoraLineMan simulator. When subjects indicated that they were ready, artificial blood was pumped into the simulator’s femoral artery tube, in which a biopsy punch was used to place a 4-mm defect, simulating a severe hemorrhagic wound. The measurement by stopwatch of time to application began as soon as the subject picked up the assigned hemostatic agent. During pressure bandage application, the subject packed the wound with gauze and wrapped a pressure bandage around the wound. During iTClamp application, as per manufacturer specification, the subject placed the double row of needles one-half inch on either side of the simulated wound incision. Once the needles were in place, the subject closed and locked the device. The stopwatch measuring time to application was stopped once the subject voiced their completion of application. Correct application and appropriate wound closure were monitored by a physician. The application procedure was repeated for the second randomized hemostatic agent. Time to application for all trials was recorded.
Following completion of both treatments, participants completed a written post-event user survey. To evaluate skill retention after 1 month, participants repeated the two application trials without any refresher training according to the initially assigned randomization order and completed an additional post-event survey.
Subjective variables from post-event user surveys included user perceptions of ease of device use, device stability, and device reliability (each on a 1-5 scale: 1 = strongly disagree, 5 = strongly agree). Participants were also asked to indicate which device they preferred overall. The post-event survey also included open-ended questions, allowing users to identify their major concerns regarding each device and to make suggestions for device improvement.
Design and Data Analysis
Sample size was determined using an a priori power analysis. Briefly, assuming a 95% CI (alpha = .05) and an effect of 0.6,15 G*Power software16 revealed that statistically significant results would be conferred 80% of the time (power = 0.80; beta = 0.20) with as few as 24 participants in the within-subject design. Application times were normally distributed, continuous data and were therefore described as a mean and SD. Application time data were analyzed using repeated-measures ANOVA followed by Sidak’s multiple comparison test. The Likert scale survey data were described by median and interquartile range (IQR). Likert scale survey data were analyzed using Mann–Whitney and Wilcoxon tests to compare survey data within and between time points, respectively. All differences were considered statistically significant at the P < .05 threshold.
RESULTS
Most participants were males (85%), and most of the participants had been serving in the military for 3 or more years (92%) (Table I). In their pre-event surveys, all subjects confirmed having previous experience using pressure dressings in training and in active care situations, yet only one subject indicated that they had previous exposure to the iTClamp during training. Participants reported that they were either confident (25%) or very confident (75%) in their ability to successfully stop bleeding from a hemorrhagic wound before the study.
| Characteristic . | Count (%) . |
|---|---|
| Sex | |
| Female | 4 (15%) |
| Male | 22 (85%) |
| Rank | |
| E-3, Seaman | 7 (27%) |
| E-4, Petty officer third class | 10 (38%) |
| E-5, Petty officer second class | 8 (31%) |
| E-6, Petty officer first class | 1 (4%) |
| Time in service | |
| <3 years | 2 (8%) |
| 3-4 years | 19 (73%) |
| 5-6 years | 5 (19%) |
| Time since TCCC training | |
| 1-3 months | 4 (15%) |
| 3-6 months | 4 (15%) |
| 6-12 months | 6 (23%) |
| >12 months | 10 (38%) |
| No response | 2 (8%) |
| Pressure dressing use in the last year | |
| 0 times | 8 (31%) |
| 1-3 times | 5 (19%) |
| 4-7 times | 6 (23%) |
| ≥8 times | 7 (27%) |
| Characteristic . | Count (%) . |
|---|---|
| Sex | |
| Female | 4 (15%) |
| Male | 22 (85%) |
| Rank | |
| E-3, Seaman | 7 (27%) |
| E-4, Petty officer third class | 10 (38%) |
| E-5, Petty officer second class | 8 (31%) |
| E-6, Petty officer first class | 1 (4%) |
| Time in service | |
| <3 years | 2 (8%) |
| 3-4 years | 19 (73%) |
| 5-6 years | 5 (19%) |
| Time since TCCC training | |
| 1-3 months | 4 (15%) |
| 3-6 months | 4 (15%) |
| 6-12 months | 6 (23%) |
| >12 months | 10 (38%) |
| No response | 2 (8%) |
| Pressure dressing use in the last year | |
| 0 times | 8 (31%) |
| 1-3 times | 5 (19%) |
| 4-7 times | 6 (23%) |
| ≥8 times | 7 (27%) |
| Characteristic . | Count (%) . |
|---|---|
| Sex | |
| Female | 4 (15%) |
| Male | 22 (85%) |
| Rank | |
| E-3, Seaman | 7 (27%) |
| E-4, Petty officer third class | 10 (38%) |
| E-5, Petty officer second class | 8 (31%) |
| E-6, Petty officer first class | 1 (4%) |
| Time in service | |
| <3 years | 2 (8%) |
| 3-4 years | 19 (73%) |
| 5-6 years | 5 (19%) |
| Time since TCCC training | |
| 1-3 months | 4 (15%) |
| 3-6 months | 4 (15%) |
| 6-12 months | 6 (23%) |
| >12 months | 10 (38%) |
| No response | 2 (8%) |
| Pressure dressing use in the last year | |
| 0 times | 8 (31%) |
| 1-3 times | 5 (19%) |
| 4-7 times | 6 (23%) |
| ≥8 times | 7 (27%) |
| Characteristic . | Count (%) . |
|---|---|
| Sex | |
| Female | 4 (15%) |
| Male | 22 (85%) |
| Rank | |
| E-3, Seaman | 7 (27%) |
| E-4, Petty officer third class | 10 (38%) |
| E-5, Petty officer second class | 8 (31%) |
| E-6, Petty officer first class | 1 (4%) |
| Time in service | |
| <3 years | 2 (8%) |
| 3-4 years | 19 (73%) |
| 5-6 years | 5 (19%) |
| Time since TCCC training | |
| 1-3 months | 4 (15%) |
| 3-6 months | 4 (15%) |
| 6-12 months | 6 (23%) |
| >12 months | 10 (38%) |
| No response | 2 (8%) |
| Pressure dressing use in the last year | |
| 0 times | 8 (31%) |
| 1-3 times | 5 (19%) |
| 4-7 times | 6 (23%) |
| ≥8 times | 7 (27%) |
The average speed of iTClamp application (mean 17.6, SD 11.1 s) was faster than the average speed of the pressure dressing application (mean 42.5, SD 17.4 s) (Fig. 2). This was statistically significant (P < .0001) for both the initial evaluation and the follow-up evaluation, in which the average speed of iTClamp and pressure dressing applications was 14.6 (SD 7.5) sec and 38.0 (SD 11.3) s, respectively. There was no statistically significant difference in iTClamp application time between the first evaluation and 30 days after participants received their initial training on proper application (P = .07). Pressure dressing application results also indicated no statistically significant difference in application time between the two time points.
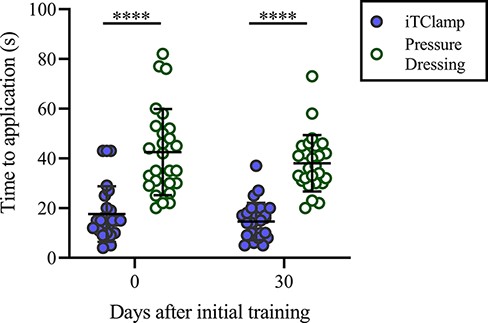
Time to application. Horizontal bars represent mean with SD. ****, P < .0001.
In the initial post-event surveys, participants responded similarly to survey questions for both pressure dressings and the iTClamp (Fig. 3). Participant median (IQR) scores for pressure dressing were 4 (1) for ease of use, 4 (0.75) for effectiveness, and 4 (1) for reliability in construction. Participant median (IQR) scores for the iTClamp were 5 (1) for ease of use, 4 (1.75) for effectiveness, and 4 (2) for reliability in construction. The iTClamp was rated as slightly easier to use; however, this difference did not reach statistical significance (P = .08).
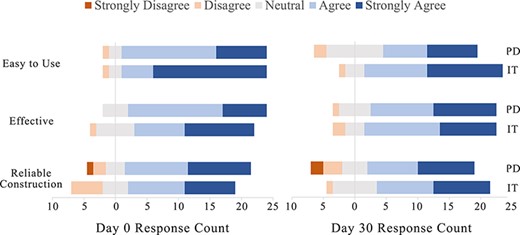
Post-event survey. Responses to Likert scale survey questions displayed as divergent bar graph at day 0 and day 30. N = 26.
The follow-up post-event surveys also indicated similar ratings of ease, effectiveness, and reliability when comparing the two hemostatic agents (Fig. 3). Participant median (IQR) scores for the pressure dressing were 4 (2) for ease of use, 4 (1) for effectiveness, and 4 (2) for reliability in construction. Participant median (IQR) scores for the iTClamp were 4 (1) for ease of use, 4 (1) for effectiveness , and 4 (2) for reliability in construction. Of note, there was no significant change in participant ratings on the ease of use of the iTClamp from the initial evaluation to the 30-day follow-up evaluation.
While 65% and 52% of the participants expressed preference in for the iTClamp in their final surveys during the initial and follow-up visits, respectively, the difference in preference was not statistically significant for either the initial end-survey (P = .12) or the follow-up post-event survey (P = .84). There was no significant change in user preference between the two time points.
DISCUSSION
In a multi-casualty situation, time is critical; a few minutes difference in treatment time can mean the difference between survival and mortality. When used in conjunction with gauze packing, the iTClamp can stop bleeding minutes faster than traditional gauze packing followed by pressure dressing and was associated with decreased blood loss and increased survival.17 The iTClamp can effectively stop hemorrhage when applied by physicians;6–11 however, there are limited data on the military prehospital provider’s capability and preference for applying the device compared to current standard of care. In this study, U.S. Navy corpsmen were able to apply the iTClamp significantly faster than the current standard practice of packing the wound with gauze and then wrapping it with a pressure dressing. The difference in speed would be more pronounced when the additional 3 min of pressure held on the wound is considered. In a simulated hemorrhage model using a manikin, we found that corpsmen could quickly and successfully apply the iTClamp with only a single training session despite the learning curves associated with new tools and techniques.18,19 Importantly, when tested 1 month later with no additional training, the skill was not lost.
End user acceptance of new medical tools and techniques is crucial to their clinical adoption.20 The use of pressure dressings and wound packing for hemorrhage control is widely known to prehospital providers. Despite the comfort and familiarity with the standard of care, participants rated iTClamp similar to traditional pressure dressings in ease of use, effectiveness, and reliability of construction but were evenly split on which treatment they preferred.
We cannot rule out the possibility of bias toward a novel device in these responses. However, only four of the 26 subjects changed their response from preferring the iTClamp to preferring pressure dressing at the 30-day follow-up, while no other subjects changed their answer between dates. For some, 1 month may have been enough to mitigate novelty bias.
The open-ended responses of the post-event surveys provide additional insight into our cohort’s willingness to adopt the iTClamp in practice. Many participants, including those with a preference for pressure dressings, noted that they felt the iTClamp was more quickly applied than the pressure dressing, an expected perception in agreement with our physical data. Participants who indicated a preference for the iTClamp expressed that a faster application time with the iTClamp could expedite hemorrhage control in the field.
In contrast, some participants who indicated a preference for the pressure dressing noted that pressure dressings may confer more direct pressure to a wound than would the application of the iTClamp. Several participants also commented that they viewed pressure dressings as generally more versatile for the treatment of multiple injury patterns. There was a concern among participants that the application of the iTClamp may not maintain stability during transportation of an injured patient, while others noted that it may be more stable in transport than alternatives. Future work should determine if these negative perceptions are true limitations of the device.
We were concerned that the simulated hemorrhage manikin model may produce artificially short application times because of the lack of fidelity to live tissue. In fact, the average time to apply gauze and pressure dressing was over three times faster on the manikin than in our live swine model of hemorrhage.12 However, there was no significant difference in iTClamp application times on manikins or swine between our two studies.
Furthermore, our prior swine study was conducted by a physician, which supports that both physicians and corpsmen can apply the iTClamp with similar speed. The speeds measured in our studies were also consistent with those for emergency physicians and first responders applying the device to a model bleeding limb.21 Taken together, the results of our study should be generalizable to the broader prehospital community.
CONCLUSION
In austere or hostile environments, speed of treatment and extrication can have significant implications for the safety of not only the patient but also the medical providers. Hemorrhage control interventions must be both effective and easy to use for the prehospital provider to ensure its efficacy in a live battlefield situation. The iTClamp can be rapidly applied with minimal training and no significant short-term skill decay. However, the limitations perceived by participants, including narrow indications for use, may hinder widespread adoption in the field.
ACKNOWLEDGMENTS
The authors would like to acknowledge the contributions of their colleagues in the Combat Trauma Research Group at Naval Medical Center Portsmouth and Rodrigo Perez-Segnini for product photography.
FUNDING
This work was supported by funding by the CIP1 Funds from the Navy Surgeon General Grant.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
CLINICAL TRIAL REGISTRATION
Not applicable.
INSTITUTIONAL REVIEW BOARD (HUMAN SUBJECTS)
Research data derived from an approved Naval Medical Center, Portsmouth, VA IRB, protocol.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC)
Not applicable.
INDIVIDUAL AUTHOR CONTRIBUTION STATEMENT
S.M.S. designed and conducted the research. M.L.B. wrote the first draft of the manuscript. E.E.F. provided data analysis, figures, and final editing. All authors read and approved the final manuscript.
INSTITUTIONAL CLEARANCE
Institutional clearance approved.
REFERENCES
Author notes
S.M.S. is a military service member. This work was prepared as part of his official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the U.S. Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or an employee of the U.S. Government as part of that person’s official duties. The authors report no conflicts of interest in this work. The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, DoD, or the U.S. Government.
LCDR Nathan Butler, DO, LCDR Katrina L. Destree, MD, LCDR Sean M. Stuart, DO, Alexandra C. Walchak, MPH. Efficacy and Skill Retention for Application of the iTClamp™ by Navy Corpsmen. Poster Presentation at 2017 Military Health Systems Research Symposium. August 27-30, 2017, Kissimmee, FL, USA.



