-
PDF
- Split View
-
Views
-
Cite
Cite
Kathleen M Sarber, Peter O’Connor, Erik K Weitzel, Jayne Stevens, James K Aden, John Breeze, Local Effect of Ballistic Fragments Embedded Along the Carotid Sheath of a Porcine Animal Model, Military Medicine, Volume 188, Issue 7-8, July/August 2023, Pages e1774–e1780, https://doi.org/10.1093/milmed/usac276
Close - Share Icon Share
ABSTRACT
Energized ballistic fragments from improvised explosive devices were the most common cause of injury to coalition service personnel during conflicts in Iraq and Afghanistan. Surgical excision of retained fragments is not routinely performed unless there is a concern for injury to vital structures. However, no clear guidelines dictate when or if a fragment should be removed, reflecting a lack of objective evidence of their long-term effects. Using a porcine model, we aimed to evaluate changes to the carotid artery produced by retained fragments over time.
Institutional Animal Care and Use Committee approval for all experiments was obtained before commencement of the study. Eighteen female swine (mean mass 62.0 ± 3.4 kg) were randomized into three study groups corresponding to the time of survival after implantation of ballistic fragments: 1, 6, and 12 weeks. Two animals from each group were randomly assigned to have one of the three different fragments implanted within the right carotid sheath in zones 1-3 of the neck. The left carotid served as the control. The vascular flow rate and arterial diameter were measured at each level before implantation and again after the survival interval. Baseline and interval angiograms were performed to identify gross vascular changes.
No abnormalities were identified on baseline or interval angiograms. No significant difference was found when the baseline was compared to interval measurements or when compared to the control side for all gross and physiological measures at 1 and 6 weeks (P = .053-.855). After 12 weeks, the flow and diameter changed significantly (P < .001-.03), but this significant change was found in both the control and affected carotid.
The lack of significant gross anatomical and physiological changes at 6 weeks postimplantation lends evidence toward the current policy that early removal of retained ballistic fragments around cervical vessels is not required. Changes were significant after 12 weeks which suggest that surveillance may be required; however, such changes could be explained by physiological animal growth.
INTRODUCTION
The Problem
Energized fragments have been the most common cause of injury to coalition service personnel during the recent conflicts in Iraq and Afghanistan.1,2 Advances in body armor have significantly reduced the immediate mortality from ballistic injury.3 Further improvements in prehospital interventions, evacuation timelines, surgical techniques, and antibiotic coverage have contributed to an approximately 95% overall resultant survivability.4 As a consequence, the incidence of wounds in the neck (where body armor is limited due to ergonomic considerations) has increased to between 9% and 29% of all injuries5–8 in U.S. service members wounded in these conflicts. Explosive devices, such as improvised explosive devices, mortars, rockets, and grenades, are the primary mechanism for most of these injuries to the neck.9,10 Energized fragments with enough velocity at impact will penetrate the neck, but most lack the kinetic energy to exit and thus become retained. The majority of retained fragments from modern antipersonnel munitions, such as grenades, are preformed and weigh only a few hundred milligrams,11 with diameters in the range of 2-5 mm.12,13 Such fragments are often spherical in shape in order to reduce drag in air and thereby improve range.13
Potential Adverse Health Effects of Retained Fragments
While some retained fragments may be extruded from the skin quickly,14 much of this foreign material remains embedded. Retained fragments in neck wounds are frequently not palpable and often widely dispersed.15 Over the past several decades, there has been controversy about whether retained embedded fragments adversely impact health and warrant removal.16 In the past, it was thought that retained fragments did not pose a significant health risk and, therefore, were rarely surgically removed.
There are a small number of case series and case reports discussing the effect of retained foreign bodies on soft tissues17 and major vascular structures.18 Large intracranial aneurysms that developed as a result of retained shrapnel after 25 years have been reported.19 Many service personnel who are injured have large numbers of retained fragments,15 and the reported incidence of physical complications is low.15,17 When considering this, one may extrapolate that retained fragments represent a low risk. However, the objective evidence to justify such an approach is lacking. The wounding pattern and the pathophysiology of small energized fragments have been evaluated in animal models.20 Animal models have also been used to examine systemic metal levels derived from retained fragments.21 However, the anatomic effect that retained fragments themselves exert on tissues also remains unclear in regard to the possible long-term sequelae, including inflammation, aneurysms, or oncogenesis. Better insight into the pathology incurred over time may help determine if and when intervention may be needed.
Aims
The aim of this study was to test several hypotheses. First, we hypothesized that the different physical and chemical makeup of each fragment would cause varying effects that would evolve over time. Second, we suspected that the sharp fragments from the Vietnam fragment would cause the most damage. To test these hypotheses, we used commonly available tools that are often used in the clinical setting—ultrasound and angiogram.
METHODS
Choice of Animal Model
A porcine model was chosen because of the size comparison between humans and the young adult swine with regard to vasculature (the diameter of the common carotid adult male is 5.5-7.5 mm, similar to a 70-kg swine) and hemodynamics22 (adult swine possess similar heart rate and blood pressure to humans). In Yorkshire swine, the internal carotid artery does not emerge until after the rete mirabile at the skull base.23 Thus, all zones of the neck in the manuscript are measurements of the common carotid. For this study, 18 female Sus scrofa domesticus 6 months of age, with a mean mass of 62.0 kg (SD 3.4 kg), were used for all experiments. Female sex was chosen to limit any possible sex-related variation in measurements. All animals were in good health and were absent of apparent disease or anatomic abnormalities. Because the effect of the fragments on the animals was unknown, we had no other exclusion criteria. All animals underwent the procedures described later. The right common carotid served as the experimental side, and the left common carotid served as the control. A schematic diagram of the study design is illustrated in Figure 1. The protocol and manuscript were prepared using the Animal Research: Reporting of In Vivo Experiments guidelines (ARRIVE version 2.0).24
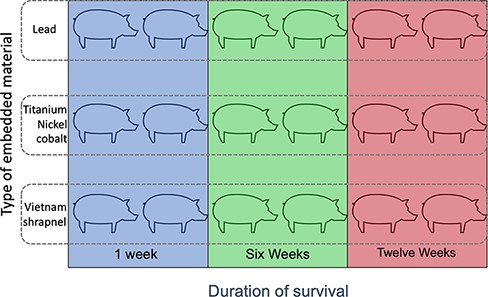
Schematic representation of the 18 swine used in the study. Animals were grouped by the type of ballistic fragment implanted as well as the survival time after surgery to embed the fragment.
Fragment Selection
Our goal was to use fragments that would be chemically and physically similar to those found in domestic and wartime ballistic wounds. Pellets were obtained from the Air Force Institute of Radiobiology. Cylindrical fragment simulating projectiles (FSPs) were chosen as these are the most common shapes identified from computed tomography in the necks of service personnel injured by improvised explosive devices.25 FSPs measured 1 mm in diameter and 2 mm in length. The sharp steel fragments were shaved off an actual shrapnel fragment that had been obtained during the Vietnam War. These were irregular but were 1-2 mm in length. Although “real-world” ballistic fragments are by nature contaminated, our aim was to minimize variables that could confound the effect of the fragment itself; thus, all fragments were sterilized before placement in the animal subject. Swine were randomized to have one of these three fragments implanted:
Fragment 1: A lead FSP. Lead is a common element in bullets and shot, which are the most commonly retained foreign body resulting from civilian gunshot wounds.26
Fragment 2: Tungsten–nickel–cobalt alloy FSP. This simulates the alloy that is most commonly used in modern military armor-penetrating ammunition.27,28
Fragment 3: A random fragment shaved from a piece of sharp steel shrapnel that was obtained from a mortar attack during the Vietnam War.
Experimental Procedures
Anesthesia was induced with ketamine and maintained within a range of 2%-4% isoflurane.
To minimize confounding factors, all experiments were performed in the same order and same manner. The senior author was aware of the group allocations. After the induction of general endotracheal anesthesia, an angiogram was performed via percutaneous access to the femoral artery. Any gross arterial abnormalities were recorded. The access site was then removed, and local pressure was held for 20 minutes. The wounds were then dressed.
Ultrasound was then used to record the vascular flow (time-averaged peak [TAP] velocity) and intra-arterial diameter (mm) in each of the three zones of the neck (zone 1: 2 cm cephalad to the thoracic inlet, zone 2: at the level of the thyroid cartilage, and zone 3: 2 cm caudal to the skull base) on the left and the right.
Next, a midline neck incision was made from the angle of the mandible to the sternal notch. The carotid sheath was exposed on the right from 2 cm superior to the thoracic inlet to the skull base. Metzenbaum scissors were then used to create a small pocket within the carotid sheath in zones 1-3 to expose the carotid. The randomly chosen fragments (three FSPs of lead or tungsten–nickel–cobalt or three shards of Vietnam shrapnel) were then placed against the carotid. The pocket was then closed with 4-0 silk to prevent fragment migration. The same procedure was performed on the left side, with pockets being developed, and then sewn closed to serve as a control to the contralateral side. The incision was closed in layers. The animals were housed in isolation for 1 week after surgery to allow the incision to heal and then were placed with other swine in an interactive and enriched environment.
Period of Study
The time of survival after implantation of ballistic fragments was 1, 6, or 12 weeks. These timings were based on likely common time points that a stably wounded soldier may be evaluated. It is assumed that trauma resulting in unstable wounds would be immediately evaluated in theater. For stable but injured patients who require evacuation from theater, we chose a time point of 1 week. Fox et al. evaluated patients with stable penetrating cervical injuries at the Walter Reed Army Medical Center from 2005 to 2007.29 The median time for evaluation was 8.5 days. Our second time point was at week 6, corresponding to a reasonable follow-up time for a stable injury. Twelve weeks was chosen as our endpoint of the study as an intermediate-term follow-up time point that was possible within the constraints of the protocol.
Imaging
At the animal’s designated interval (1, 6, or 12 weeks) after implantation, the animals were placed under general endotracheal anesthesia. Ultrasound was again used to record the vascular flow and intra-arterial diameter directly adjacent to the fragments, which were identified via ultrasound. The volume flow rate was calculated as equal to (π × radius2 × TAP velocity × 60) mL min−1. All measurements were taken by the first author. An experienced vascular technician (human vascular technician with further experience in other porcine studies) trained the first author on the first six animals at the baseline, and then, the first author took the measurements solo for the remainder of the study. TAP measurements were taken as close to the middle of the artery as possible, and the probe was held as perpendicular as possible to the plane of the artery. The neck was marked, and these measurements were taken at the same level on the left (control) side. An angiogram was also performed before euthanasia in an identical fashion to the baseline procedure in order to evaluate for any gross abnormalities, such as pseudoaneurysm, dissection, or stenosis. The first author performed the angiograms. Because only gross changes were being evaluated, no measurements were taken from the angiograms. They were downloaded, and the ‘baseline and interval images’ were compared. The interpretations were agreed upon by the senior author and two other authors (E.W. and P.C.).
Data Analysis
Our primary outcome was a change in intraluminal diameter. Based on an a priori power analysis, a sample size of six per group provided 80% power to detect a moderate effect of 0.38 (0.76 SD) difference among means for the main effect of cohort, a moderate effect of 0.4 (or approximately 0.8 SD) among means for the main effect of group, and a moderate effect of 0.34 (or approximately 0.68 SD) difference among means for the main effect of time, with interaction effects ranging from 0.38 for cohort × time, 0.40 for group × time, and 0.46 for cohort × group, when testing with a repeated measures mixed model analysis of variance (RM ANOVA) at the α level of .05 (NCSS PASS 2002).
Our hypothesis was tested using the following four variables: (1) fragment type, (2) survival time (baseline/presurgical and 1, 6, or 12 weeks after surgery), (3) carotid artery laterality (left was the control side and right was the experimental side), and (4) neck zone where the FSP was embedded (three zones). Factors that were determined to be significant were then analyzed using the RM ANOVA with two repeated measures over time, four test groups (three FSP types and one control), and three cohorts (1 week, 6 weeks, and 3 months).
Institutional Approval
Institutional Animal Care and Use Committee approval for all experiments was obtained before commencement of the described study. The approved protocol is registered as protocol FWH20120044A with the 59th Clinical Research Division. Experiments were carried out in an Association for the Assessment and Accreditation of Laboratory Animal Care accredited facility at the Wilford Hall Ambulatory Surgical Center, 59th Medical Wing, Joint Base San Antonio-Lackland, TX, USA. Animals were used in accordance with the Guide for the Care and Use of Laboratory Animals.30 The funders of the study played no role in the design or analysis of the study. Data are available with the author and are available by request.
RESULTS
All animals tolerated the procedure with no adverse events. There were no perioperative infections. One animal in the sixth week and Vietnam fragment group suffered bilateral pneumonia 4 weeks and 3 days after surgery. It was determined that pneumonia was not related to the surgery. The animal was stable to undergo anesthesia, so the experiments were carried out early. The animal was then humanely euthanized. The results from this animal were still included, reflecting an intention-to-treat analysis in order to reduce bias. Our statistician did the analyses with and without this animal, and it did not have any statistical effect on the results.
No gross abnormalities were noted on the baseline angiogram for any animal. There were no gross abnormalities noted on repeat angiograms at 1, 6, or 12 weeks for any animal.
At the baseline, the mean (SD) carotid flow (n = 18) on the right was 164 (39) mL min−1, 146 (33) mL min−1, and 147 (28) mL min−1 in zones 1, 2, and 3, respectively. The mean (SD) carotid diameter (n = 18) was 4.64 (0.03) mm, 4.33 (0.04) mm, and 4.41 (0.05) mm in zones 1, 2, and 3, respectively. There were no significant differences between the left and right sides nor between the planned fragment types at the baseline (P = .078-.674) (Supplementary Table S1; Fig. 3).
Pairwise comparisons for flow and diameter measurements for the right vs. left side showed no significant difference at any level (P = .281-.855). Comparing the baseline vs. interval data, there was no significant change in the flow or diameter at 1 and 6 weeks (P = .053-.368). Compared to the baseline, the diameter increased significantly at 12 weeks (P < .001), and the flow decreased significantly at 12 weeks (P = .03). However, these changes were significant for both the right and left sides (Table I). Figures 2 and 3 graph the flow and diameter measured in each zone of the neck to illustrate the changes over the survival intervals (1, 6, and 12 weeks).
Pairwise Comparisons for Flow (mL min−1) and Carotid Artery Diameter (mm) for the Animals Grouped by Survival Duration
| . | Paired diff . | ||||
|---|---|---|---|---|---|
| Mean diff . | SD diff . | P-value . | |||
| 1 week | Left vs. right | Flow | 1.56 | 35.56 | .855 |
| Diameter | −0.10 | 0.46 | .368 | ||
| Baseline vs. interval | Flow | −13.33 | 28.4 | .063 | |
| Diameter | −0.09 | 0.39 | .324 | ||
| 6 weeks | Left vs. right | Flow | 5.28 | 39.91 | .582 |
| Diameter | −0.11 | 0.54 | .417 | ||
| Baseline vs. interval | Flow | 15.44 | 31.49 | .053 | |
| Diameter | −0.19 | 0.71 | .259 | ||
| 12 weeks | Left vs. right | Flow | 6.89 | 27.93 | .31 |
| Diameter | 0.18 | 0.68 | .281 | ||
| Baseline vs. interval | Flow | 28.22 | 50.64 | .03* | |
| Diameter | −0.52 | 0.49 | <.001* | ||
| . | Paired diff . | ||||
|---|---|---|---|---|---|
| Mean diff . | SD diff . | P-value . | |||
| 1 week | Left vs. right | Flow | 1.56 | 35.56 | .855 |
| Diameter | −0.10 | 0.46 | .368 | ||
| Baseline vs. interval | Flow | −13.33 | 28.4 | .063 | |
| Diameter | −0.09 | 0.39 | .324 | ||
| 6 weeks | Left vs. right | Flow | 5.28 | 39.91 | .582 |
| Diameter | −0.11 | 0.54 | .417 | ||
| Baseline vs. interval | Flow | 15.44 | 31.49 | .053 | |
| Diameter | −0.19 | 0.71 | .259 | ||
| 12 weeks | Left vs. right | Flow | 6.89 | 27.93 | .31 |
| Diameter | 0.18 | 0.68 | .281 | ||
| Baseline vs. interval | Flow | 28.22 | 50.64 | .03* | |
| Diameter | −0.52 | 0.49 | <.001* | ||
Pairwise comparisons are made between the left (control) and right (implanted) sides as well as between the time (survival interval) and baseline data measurements. diff, difference.
Statistically significant results.
Pairwise Comparisons for Flow (mL min−1) and Carotid Artery Diameter (mm) for the Animals Grouped by Survival Duration
| . | Paired diff . | ||||
|---|---|---|---|---|---|
| Mean diff . | SD diff . | P-value . | |||
| 1 week | Left vs. right | Flow | 1.56 | 35.56 | .855 |
| Diameter | −0.10 | 0.46 | .368 | ||
| Baseline vs. interval | Flow | −13.33 | 28.4 | .063 | |
| Diameter | −0.09 | 0.39 | .324 | ||
| 6 weeks | Left vs. right | Flow | 5.28 | 39.91 | .582 |
| Diameter | −0.11 | 0.54 | .417 | ||
| Baseline vs. interval | Flow | 15.44 | 31.49 | .053 | |
| Diameter | −0.19 | 0.71 | .259 | ||
| 12 weeks | Left vs. right | Flow | 6.89 | 27.93 | .31 |
| Diameter | 0.18 | 0.68 | .281 | ||
| Baseline vs. interval | Flow | 28.22 | 50.64 | .03* | |
| Diameter | −0.52 | 0.49 | <.001* | ||
| . | Paired diff . | ||||
|---|---|---|---|---|---|
| Mean diff . | SD diff . | P-value . | |||
| 1 week | Left vs. right | Flow | 1.56 | 35.56 | .855 |
| Diameter | −0.10 | 0.46 | .368 | ||
| Baseline vs. interval | Flow | −13.33 | 28.4 | .063 | |
| Diameter | −0.09 | 0.39 | .324 | ||
| 6 weeks | Left vs. right | Flow | 5.28 | 39.91 | .582 |
| Diameter | −0.11 | 0.54 | .417 | ||
| Baseline vs. interval | Flow | 15.44 | 31.49 | .053 | |
| Diameter | −0.19 | 0.71 | .259 | ||
| 12 weeks | Left vs. right | Flow | 6.89 | 27.93 | .31 |
| Diameter | 0.18 | 0.68 | .281 | ||
| Baseline vs. interval | Flow | 28.22 | 50.64 | .03* | |
| Diameter | −0.52 | 0.49 | <.001* | ||
Pairwise comparisons are made between the left (control) and right (implanted) sides as well as between the time (survival interval) and baseline data measurements. diff, difference.
Statistically significant results.
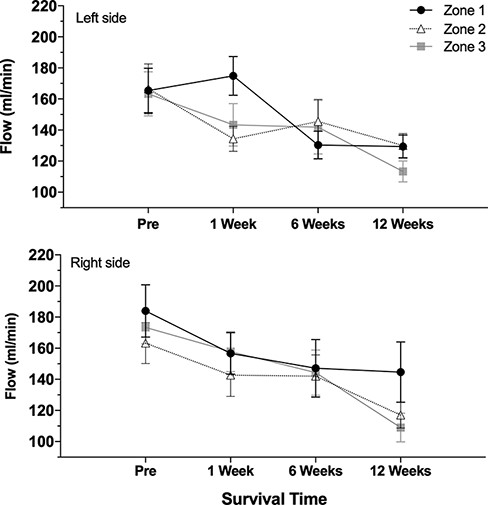
Vascular flow over survival intervals. The top graph illustrates measurements from the left (control) side, and the bottom graph shows measurements from the right side. Each line represents zones 1, 2, and 3, respectively.
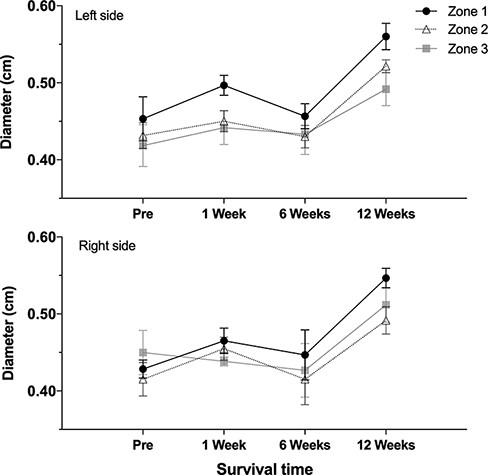
Intracarotid diameters over survival intervals. The top graph illustrates measurements from the left (control) side, and the bottom graph shows measurements from the right side. Each line represents zones 1, 2, and 3, respectively.
Using the RM ANOVA, the fragment type was not significant (P = .1414). Both zones of the neck and survival time were significant at P < .0001. However, we found no significant difference in comparing the interactions between survival time, zones of the neck, or laterality (P = .3876- 0.9703) (Supplementary Table S2).
DISCUSSION
This study aimed to evaluate the gross and functional changes to the carotid artery produced by retained fragments over time in a porcine model. The findings of this study showed that no fragment produced gross intra-arterial changes. In the short term, there were no significant changes in the vascular flow or diameter. The only significant difference between the baseline and interval measurements was found at the 3-month interval. Both the flow and arterial diameter were significantly different on both the right (fragment) and left (control). The mean mass of the animals increased from a baseline of 67.0 to 96.7 kg at 3 months. This would suggest that the changes in the flow and diameter observed at 3 months were likely due to the change in the overall size of the animal and not from the exposure itself. This conclusion is supported by the findings of the mixed model ANOVA that showed survival duration was significant but no significant difference in comparing the interactions between fragment type, zones of the neck, or laterality. The mixed model also determined that the zone of the neck was a significant main effect. As would be expected, there was a statistically significant relationship between the neck zone and vascular flow, reflecting that the artery in this model, as in humans, decreases in size from caudal (zone 1) to cephalad (zone 3). This served to demonstrate the validity of this measurement method. The key to our experiment was that there was no difference between sides (experimental vs. control) at each of these zones, as illustrated in Figure 3.
No gross angiographic abnormalities were noted at the baseline or at interval survival time.
The lack of significant gross anatomical and physiological changes at 1 and 6 weeks postimplantation may lend evidence toward the current policy that immediate or early removal of embedded ballistic fragments around cervical vessels is not required when a patient is otherwise stable. Changes were significant after 3 months which suggests that surveillance may be required; however, this occurred on both the experimental and control sides, and such changes could potentially be explained by physiological animal growth.
Limitations of this study include the sterility of the fragments. This step was taken to reduce the likelihood of infection, which could compromise the wound and potentially lead to higher morbidity in the animal. While attempting to reduce infection as a confounder, it is well known that injuries from penetrating ballistics are not clean. Retained ballistic fragments in vivo are by their nature contaminated by flora picked up from their storage and passage through air, clothing, and skin. It is likely that flora picked up by the fragment would also become embedded and potentially retained around the fragment, although the effects of these florae have to our knowledge never been determined. Future research is recommended to build upon the model developed for our study; this should comprise a sterilized fragment as a baseline and compare to those fragments soaked in a medium containing representative flora, including invasive fungi such as Aspergillus.31 The study was also limited to clinical outcomes of short duration. It is well known that these retained fragments elute their toxins over time.16,32–34 This is an important problem that the Department of Veterans Affairs is monitoring through Veterans Health Administration Directive 1308 via the Toxic Embedded Fragments registry and Depleted Uranium registry. Future studies could feasibly include urine and blood levels of the metals that are embedded. Another limitation is the small size of the cohort and the smaller size of the different groups. Increasing the size of the cohort and extending the duration of observation in addition to histopathological analysis are recommended to elucidate the effects of retained fragments against major vascular structures in the longer term. Studies that include histopathological examination and longer duration survival of animal models are needed for further clarification.
ACKNOWLEDGMENTS
The authors would like to acknowledge Wensheng Zhang, MD, PhD, Senior Scientist, 59th medical wing/science and technology, Air Force for his timely effort in creating our figures.
SUPPLEMENTARY MATERIAL
SUPPLEMENTARY MATERIAL is available at Military Medicine online.
FUNDING
This manuscript was supported by the U.S. Air Force, 59th Clinical Research Division, protocol number FWH20120044A.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to report.
AUTHOR CONTRIBUTIONS
K.S. participated in the study design, literature search, data collection, writing, and critical revision.
P.O.C. participated in the study design, literature search, data collection, and critical revision.
E.W. participated in the study design, literature search, data collection, and critical revision.
J.S. participated in the literature search, data collection, and critical revision.
J.A. participated in the data analysis, writing, and critical revision.
J.B. participated in the study literature search, writing, the critical revision.
REFERENCES
Author notes
The protocol for this experiment was presented at the Military Health System Research Symposium, Miami, FL, August 2013.
The views expressed are solely those of the authors and do not reflect the official policy or position of the U.S. Army, U.S. Navy, U.S. Air Force, the DoD, or the U.S. Government.



