-
PDF
- Split View
-
Views
-
Cite
Cite
Augustine H Chuang, Justin Bordlemay, Jeremy L Goodin, James C McPherson, Effect of Sodium Lauryl Sulfate (SLS) on Primary Human Gingival Fibroblasts in an In Vitro Wound Healing Model, Military Medicine, Volume 184, Issue Supplement_1, March-April 2019, Pages 97–101, https://doi.org/10.1093/milmed/usy332
Close - Share Icon Share
Abstract
Sodium lauryl sulfate (SLS) is a surfactant used to decrease the surface tension of water. Most commercially available dentifrices contain 0.5–2.0% SLS. This study investigated the potential effect of SLS on oral wound healing using primary human gingival fibroblasts (HGFs).
HGFs cells were grown in12-well culture plates in DMEM medium. A 3 mm wound was created on confluent HGFs. The cells were challenged with 0 (the control group), 0.01, 0.02, 0.03, 0.04, or 0.05% SLS-containing media once daily for 2 minutes. The cells were stained on day 0, 2, 4, 6 and 8. The percent of wound fill area was measured.
On day 2, 4, 6, and 8, the wound fill of the control group (0% SLS) was 15, 35, 67 and 98%, respectively; at 0.01% SLS, it was 10, 20, 65 and 84%; at 0.02%, it was 7, 10, 15 and 25%; at 0.03% SLS, it was only 5% and 8% on day 2 and 4.
Our results showed a dose- and time-dependent inhibition on HGFs wound fill by SLS; however, future in vivo studies are needed to validate if our in vitro findings using SLS-free dentifrices to avoid the potential delay of wound healing.
INTRODUCTION
Maintaining good oral hygiene practice is essential for every day health and is a vitally important issue as an integral part of combat readiness for service members. It is achieved by brushing teeth with dentifrices. Sodium lauryl sulfate (SLS) is an inexpensive anionic surfactant functioning as a foaming agent to decrease the surface tension of water. It has been widely used in numerous personal hygiene products, laundry, and household cleaning agents as well as in most commercially available dentifrices at 0.5–2.0% concentration.
Surgical procedures are an integral part of periodontic treatment. A surgical incision is made and two soft tissue wound margins are created. At the completion of the procedure, the two margins are approximated and sutured. A fibrin clot soon forms. Stability of the clot allows for a good matrix and source of chemical mediators for cellular repopulation into the wound. The adjacent connective tissue to the wound margin contains mesenchymal cells which emigrate then proliferate, ultimately producing collagen and extracellular matrix components. These collagen producing mesenchymal cells are fibroblasts. The collagen within the wound is the backbone of the extracellular matrix and provides the framework for tissue maturation. Thus, an important part of wound healing is the ability of fibroblast to repopulate a wound and produce collagen.
Various negative intraoral side effects of SLS have been reported in the literature. These include: oral epithelial sloughing; ulcerations; inflammation; increased permeability to non-electrolytes; widening of the stratum corneum due to separation and loss of surface epithelial layers; protein denaturation; and membrane expansion.1–5 Rubright et al were one of the first to report a negative effect of SLS in oral healthcare.1 A case report established a cause and effect relationship between SLS and desquamation in a patient taking drugs with anti-sialic activity. The amount of sloughing was found to be directly related to the use of the SLS-containing toothpaste. When the use of the toothpaste was discontinued, the desquamation was reversed within 24 hours. As a result of the case report, Rubright, Searls and Berg2 performed a pilot study using healthy female dental hygiene students to further investigate the contributory effects of SLS on oral sloughing. Thirty students were divided into two groups. Two dentifrices were formulated. The control dentifrice contained no detergents, preservatives, fluorides or other therapeutic agents. The test dentifrice contained the same ingredients as the control but with the addition of 2.4 grams of SLS. The results showed an increase in epithelial cell count following expiration of saliva after brushing with an SLS-containing dentifrice for 1 week, compared with the control. Herlofson and Barkvoll compared the topical effect of a test dentifrice with different concentrations of SLS to a placebo dentifrice on visible oral desquamation and epithelial flakes in saliva.6 Five dentifrices were used varying only in different concentration of SLS (0.0, 0.25, 0.5, 1.0, and 1.5%). They demonstrated a dose–response effect with increasing concentrations of SLS, to the increased incidence of mucosal desquamation. These authors also observed the denaturing effect of SLS on the oral mucin layer, with exposure of underlying epithelium, induced incidence of recurrent aphthous ulcers (RAU).7 The results of their clinical double-blind crossover study on 30 patients with frequent occurrences of RAU suggested that an SLS-free toothpaste may be recommended for patents with RAU.8
The periodontium is the tissues investing and supporting the teeth. The fibroblast is the most abundant cell type of periodontium and has a central role in homeostasis, pathogenesis, and healing. Sixty-five percent of cells in gingival connective tissue are fibroblasts. The role of this cell type is to produce the structural connective tissue proteins, collagen, elastin, glycoproteins, and glycosaminoglycan that comprise the periodontal ligament ground substance.9,10
In the present study, we investigated the dose–response of SLS over a period of 8 days on primary human gingival fibroblasts (HGFs) using an in vitro wound healing model.11,12
METHODS
HGFs were obtained from the Augusta University, Augusta, GA, USA. The cells were cultured from gingival biopsies obtained from systemically healthy and non-smoker patients, in accordance with an approved Institutional Review Board Protocol (# DE 05-17) Eisenhower Army Medical Center, US Army. Under sterile conditions, clinically healthy marginal gingival tissues were excised from the interproximal premolar and molar papillae of patients without periodontal disease. The gingival tissues were minced into 2 × 2 × 3 mm2 pieces and placed in six-well culture plates in Dulbecco’s Modified Eagles Medium (DMEM) containing 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100 μg/mL streptomycin, and 50 μg/mL amphotericin B. Explants were incubated at 37° C in an atmosphere of 5% CO2 and 95% air. The DMEM was replaced after one week and every 3 days thereafter until confluency was observed. Cells migrating from tissue explants were released from the tissue culture surface using 0.25% trypsin-EDTA and re-plated into 75 cm2 flasks in the same DMEM but without amphotericin B. These cells were designated passage#1, maintained in culture medium, and sub-cultured weekly or as needed for experimentation. All cells were utilized between passages four and eight. Cell cultures were monitored for confluency using phase contrast microscopy.
Culture Preparation
HGFs were seeded into 12-well culture plates. The HGFs were seeded at a density of 2.5 × 104 cells per well in plating medium, consisting of DMEM with phenol red, 10% FBS and the appropriate antibiotics/antifungals listed above. The seeded cells were incubated in a humidified atmosphere of 5% CO2 and 95% air until cultures reached 90% confluency. The culture medium was then replaced with phenol red-free medium containing 1.5% FBS for 24 hours to establish a quiescent state and cell cycle synchronization.
In Vitro Wound Protocol
A modification of the in vitro wound healing model described by Lackler et al and Dinos et al was used in this study.11,12 A 3-mm wide wound (a void in the cell monolayer) was created in each well by dragging a rubber tip instrument (rubber policeman), fixed on a 5 mL pipette tip, across the surface of the tissue culture well. This action, coupled with the suction from the pipette, disrupted a consistent portion of the confluent monolayer and removed the cellular material while exerting physical damage upon the cells and creating the wound area. The wound was made centrally on the well and was extended throughout entire the length of the well. After the wound site was created, each well was rinsed twice with phenol red-free Hank’s balanced salt solution (HBSS) to eliminate any residual cells before the next experimental step. The wound sites were examined under a phase contrast light microscope to ensure that all cellular and extracellular material was removed.
SLS Exposure
Wounded HGFs cultures received one of the following SLS treatments: 0.00% (control), 0.01, 0.02, 0.03, 0.04, or 0.05% of SLS (weight/volume) in media containing 5% FBS, for two minutes. At the end of the two minute incubation period, the SLS was removed and the cells rinsed twice with HBSS, and fresh media containing 5% FBS added to the cultures. The cultures were exposed to SLS once each day. Cultures were terminated on days 0, 2, 4, 6, and 8.
Quantification of Wound Repopulation
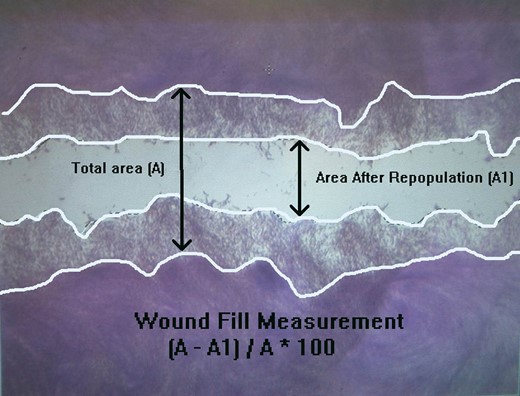
The total wounded area (A) and the area after wound repopulated (A1) of HGFs.
Statistical Analysis
Data generated were continuous, numerical values. The percent of wound repopulation was calculated for each group examined. A two-way analysis of variance was used to evaluate the data. Each pair-wise comparison was compared to a Bonferroni-adjusted a level of 0.05/80 + 0.0004.
A p-value ≤0.05 is statistically different on wound repopulation inhibition.
RESULTS
Light microscopic examination of HGFs from days 2 to 8 of treatment with 0 (control), 0.01, 0.02, 0.03, or 0.04% SLS in media demonstrated a significant dose- and time-dependent decrease in the percent wound fill with increasing SLS concentration or time as shown in Figure 2. At 0.05% SLS, little wound fill was evident at day 2, and by day 4, a large percent of confluent cells had detached.
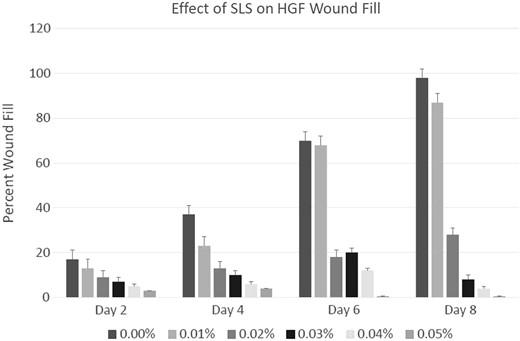
Percent wound filled on day 2, 4, 6, and 8 after a 3 mm wound was created on a confluent and synchronized layer of HGF in media containing 0, 0.01, 0.02, 0.03, 0.04, and 0.05% SLS. Significant inhibitions of wound fills were observed at concentrations at 0.025 and higher from Day 4 (p ≤ 0.05).
Figure 3 shows the dose-dependent effect of SLS on HGFs repopulation on the time course. The repopulation of the wound by HGFs in the control well is clearly distinct from the sparsely repopulated wound in the 0.03% SLS treated culture at day 8. The wound fills in the 0.01% SLS treated cultures are not significantly different from the control at any time period, but the wound fill at all SLS treatments of 0.02% or greater are significantly decreased compared to either the control or the 0.01% SLS treatment (p ≤ 0.001).
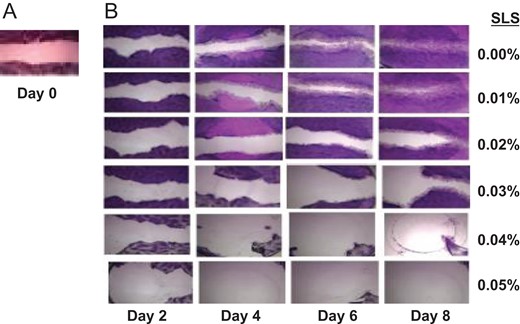
(A) A presentative of day 0 wound on the confluent layer of HGFs. (B) The presentative of progressive wound fills of HGFs on day 2, 4, 6 and 8 in media containing 0.00, 0.01, 0.02, 0.03, 0.04, and 0.05% of SLS 0n row 1, 2, 3, 4, 5, and 6, respectively.
DISCUSSION
Wound healing is a dynamic and complex biologic process consisting of four continuous overlapping phases: hemostasis, inflammation, proliferation, and remodeling. It is a primary survival mechanism and is one of the central issues of military combat injuries. For a wound to heal successfully, all four phases must occur in a proper sequence and timely frame. There are local factors and general factors that affect wound healing processes. Oxygenation, infection, foreign body, and venous sufficiency are among the local factors, while age, gender, diabetes, obesity, sex hormones, ischemia, hereditary healing disorders, smoking, alcoholism, nutrition, medications, immunocompromised conditions, and stress are among the important general factors.13,14 The present study using HGFs, the most abundant cell type of periodontium connective tissue, investigated the oral wound healing under the influence of SLS in an in vitro healing model.11,12 There are unique aspects in oral wound healing since the process involves less scar formation as that compared to skin after wound healing. Oral wound healing also occurs in a remarkable environment of a warm oral fluid containing millions of microorganisms.14–18
It is important to consider all the various potential factors that SLS may affect oral health and wound healing. As mentioned above, there were negative intraoral side effects of SLS studies on one hand,1–8 while on the other hand some studies showed less harmful effects. For example, the works of Healy et al19 and Shim et al20 on oral ulcers and episodes did not differ significantly between SLS-free and SLS-containing dentifrices. The authors also indicated that although SLS-free did not reduce the number of ulcers and episodes, it affected the ulcer-healing process and reduced pain in the daily lives of patients with RAU. The duration of ulcers and mean pain score were significantly decreased during the period using SLS-free dentifrice compared to two SLS-containing dentifrices at 1.5%. Salzer et al investigated 120 young adults age 18–34 for 8 weeks for bleeding on marginal probing, plaque scores, gingival abrasion, and visual analog scale. The dentifrice without SLS was as effective as regular SLS-containing dentifrice on gingival bleeding scores and plaque score. There was no significant difference in the incidence of gingival abrasion. In patients with RAU, the absence of SLS may even be beneficial.21 Using a double-blind parallel study, they further investigated 90 subjects for the effects of SLS on plaque and gingivitis. No significant difference could be observed with respect to effect on plaque or gingivitis between SLS-containing and SLS-free dentifrice containing enzymes, colostrum and low concentration zinc. Patients enjoyed the duration of taste and the foaming effect of SLS-containing dentifrices.22 While these ulcers represent a different type of healing, as compared with the healing of surgical wounds, they nevertheless present as SLS effect on oral wound healing.
Fakhry-Smith et al determined the amount of SLS retained in the mouth by a healthy population after rinsing or brushing with commercially available products.23 Their results showed, after rinsing, that 96% of available SLS from the rinse was recovered in the collected samples within 2 minutes. Similarly, after brushing, 86% of SLS contained within the dentifrice was recovered from the collected samples within 10 minutes. These results showed that the amount of SLS retained in the oral cavity was minimal and the contact time between SLS and the oral cavity was very short. A second study was conducted to measure the amount of SLS retained in the mouth by a population susceptible to RAU. After rinsing, 97% of available SLS was recovered within first 2 minutes. Following brushing, 89% of the SLS in the dentifrice was recovered within the first 10 minutes. These results were comparable to those observed in a healthy population. Though the retention of SLS in the oral cavity is minimal and brief, there were reports of negative intraoral side effects. Our short exposure time of SLS of 2 minutes is supported by these findings. Even with this short time, we observed significant delay in wound healing.
A study by Cvikl et al utilized nine commercially available toothpastes containing four different detergents.24 Toothpastes were diluted in serum-free medium, centrifuged, filter sterilized, then applied on oral squamous cell carcinoma HSC-2 cells and L929 cells. It was found that toothpastes containing SLS and amine fluoride strongly inhibited cell viability with half-lethal concentration being obtained with conditioned medium prepared with approximately 1% toothpaste. These results demonstrate that the type of detergent in toothpastes can be associated with changes in in vitro cell toxicity. The significantly reduced wound repopulation we observed with increasing concentrations of SLS may be an effect on cell viability as observed in this study.
It is known that wound healing is achieved through a highly complex biological process involving a large number of cell types: platelets, neutrophils, macrophages, lymphocytes, keratinocytes, fibroblasts, and endothelial cells triggering a host of cytokines and growth factors in this event. Multiple factors can cause impaired wound healing by affecting one or more phases of the process. The influences of these factors are not mutually exclusive. Single or multiple factors may play a role in any one or more individual phase contributing to the overall outcome of the subjects predisposed to RAU.
CONCLUSION
In conclusion, the mechanism by which SLS induces oral mucosal desquamation and the delay of wound healing is probably multi-factorial due to the surface active nature of the molecule. This study demonstrated that SLS has a statistically significant inhibition on HGFs wound repopulation in an in vitro wound healing model. These results may suggest that a patient having an intraoral surgical procedure may have prolonged healing time when concurrently using dentifrices containing SLS post surgically. Moreover, the effects of SLS on the various cell types involving in wound healing at different phases as well as on animal and human studies are needed to elucidate the role SLS-containing dentifrices have on wound healing post surgically. To prevent potential negatively modulating the healing response during the early stage of wound healing using an SLS-free dentifrice seems worth considering. One limitation of this study is the effect of SLS on surgical wound healing. These studies are being planned.
Previous Presentation
Presented as a poster at 2017 Military Health System Symposium (Abstract #: MHSRS-17-0157).
Funding
This study was supported by GME Funding of Dwight D. Eisenhower Army Medical Center. This supplement was sponsored by the Office of the Secretary of Defense for Health Affairs.



