-
PDF
- Split View
-
Views
-
Cite
Cite
Sean M Stuart, Gregory Zarow, Alexandra Walchak, Julie McLean, Paul Roszko, Pilot Study of a Novel Swine Model for Controlling Junctional Hemorrhage Using the iTClamp in Conjunction With Hemostatic Agents, Military Medicine, Volume 184, Issue Supplement_1, March-April 2019, Pages 367–373, https://doi.org/10.1093/milmed/usy337
Close - Share Icon Share
Abstract
Exsanguinating hemorrhage is a primary cause of battlefield death. The iTClamp is a relatively new device (FDA approval in 2013) that takes a different approach to hemorrhage control by applying mechanism wound closure. However, no previous studies have explored the feasibility of utilizing the iTClamp in conjunction with hemostatic packing. To fill this important gap in the literature, a novel swine model was developed, and a total of 12 trials were performed using QuikClot Combat Gauze or XSTAT sponges in conjunction with the iTClamp to treat arterial injuries through 5 cm or 10 cm skin incisions in the groin, axilla, or neck. First-attempt application success rate, application time, and blood loss were recorded. Hemostasis was achieved on all wounds, though reapplication was required in one Combat Gauze and three XSTAT applications. Application averaged ~50% slower for Combat Gauze (M = 41 seconds, 95%CI: 22–32 seconds) than for XSTAT (M = 27 seconds, 95%CI: 35–47 seconds). XSTAT application was faster than Combat Gauze for each wound location and size. The 10 cm wounds took ~10 seconds (36%) longer to close (M = 27 seconds, 95%CI: 35–47 seconds) than the 5 cm wounds (M = 27 seconds, 95%CI: 35–47 seconds). Blood loss was similar for Combat Gauze (M = 51 mL, 95%CI: 25–76 mL) and XSTAT (M = 60 mL, 95%CI: 30–90 mL). Blood loss was roughly twice as great for 10 cm wounds (M = 73 mL, 95%CI: 47–100 mL) than for 5 cm wounds (M = 38 mL, 95%CI: 18–57 mL). This pilot study supports the feasibility of a novel model for testing the iTClamp in conjunction with hemostatic packing towards controlling junctional hemorrhage.
INTRODUCTION
Exsanguinating hemorrhage is a primary cause of death on the battlefield. Up to 90% of all preventable causes of death on the battlefield are caused by hemorrhage and over one-third of those cases are due to extremity or junctional injuries.1,2 While tourniquets have been shown to be both safe and effective on extremity wounds that are amenable to placement,2 treating junctional wounds remains challenging. It is therefore not surprising that, in its Combat Trauma Lessons Learned from Military Operations 2001–2013, the Defense Health Board specifically identified the need for continued research in the areas of non-compressible hemorrhage, hemostatic dressings, and junctional hemorrhage control.
Current guidelines recommend packing junctional wounds with gauze or hemostatic agents, applying pressure, then covering with a pressure dressing.1,3–5 However, while there have been advances in hemostatic agents, ranging from impregnated gauze products to injectable hemostatic sponges,5 the standard guideline methods are time consuming, effectiveness varies, and maintaining hemostasis during patient transport is difficult.5–7 To bridge this gap, junctional tourniquets have been developed,. However, broad application of current junctional tourniquets is limited by bulky materials, few case reports on real-world effectiveness, and simulation studies that demonstrated only moderate effectiveness or poor efficacy in transport.7–9 Further, with the exception of one device, present junctional tourniquets are designed only for groin injuries.10–13 Towards preserving life on the battlefield, a better approach is needed.
The iTClamp is a mechanical device that takes a novel approach to hemorrhage control. The iTClamp is a compact (2.3” × 2.6” × 1.6”; 2.0 oz.), hinged, medical grade polycarbonate device featuring two rows of needle-like stainless steel teeth (Fig. 1). The teeth are aligned with the wound margin and the hinge mechanism clamps closed and locks into place, sealing the wound and creating a tamponade effect to control the hemorrhage.

Approved by the FDA in 2013, manufacturer’s guidelines state that the iTClamp was designed for control of hemorrhage in the junctional areas of the neck, axilla and groin, as well as scalp and extremity wounds. The iTClamp has been successfully used in cadaver and swine studies,14–17 in prehospital and paramedic care,18,19 and on the battlefield.20 The majority of these studies have utilized a single iTClamp, although there are single reports of using two iTClamps for longer wounds.19 One study used a single iTClamp in conjunction with plain gauze.17 However, no studies to date have sought to combine iTClamp with packing of advanced hemostatic agents on multiple challenging wound sites or with wounds of differing sizes. What was needed was a swine model for testing iTClamp in conjunction with kaolin-impregnated Combat Gauze or rapidly expanding XSTAT sponges on groin, axilla, and neck wounds of different sizes, Further, there was no model for testing the use of iTClamp in conjunction with hemostatic agents in a manner that conserves animals. The present feasibility study was specifically designed to fill these gaps in the literature. The aim of this pilot was to evaluate the feasibility of utilizing the iTClamp in conjunction with hemostatic packing to determine its potential role for use with other hemorrhage control products in the management of junctional hemorrhages.
METHODS
Design
All research was conducted in compliance with the Animal Welfare Act and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals.21 The study was funded by a 2015 Navy Surgeon General CIP Grant and approved by the Naval Medical Center Portsmouth IACUC as protocol # NMCP.2016.0012. Animals were maintained in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Veterinary staff evaluated each animal prior to enrollment and monitored them throughout the experiment. Two 35–45 kg Landrace/Yorkshire cross swine were utilized.
Each wound was closed via iTClamp (Fig. 1) after being packed with either Combat Gauze (Fig. 2) or XSTAT sponges (Fig. 3). QuikClot Combat Gauze (Z-medica, Wallingford, Connecticut) is a 3” × 48” nonwoven gauze impregnated with the hemostatic agent kaolin. The XSTAT30 (RevMedx, Wilsonville, Oregon) is a 30 mm diameter syringe-like applicator filled with rapidly expanding cellulose sponges coated in the hemostatic agent chitosan. This device is designed to inject the sponges into a wound, where they expand and exert their hemostatic effect.

Skin incisions of either 5 or 10 cm were made over the locations of the carotid, brachial, or femoral arteries. The size of skin incisions was based on 5 cm being the wound size a single iTClamp could close. Ten-centimeter incisions were made to assess if two iTClamps could be placed concurrently to seal larger wounds. Vascular injuries were made in the femoral, brachial, or carotid arteries. Treatment arms are outlined in Figure 4. Wounds were then immediately packed with either Combat Gauze or XSTAT sponges and closed with either 1 or 2 iTClamps, depending upon the size of the skin incision. For consistency, all wounds were packed and closed by the primary author, a physician with combat experience. Following hemostasis, joints were repeatedly flexed to simulate patient movement and evaluate device stability. Devices were deemed stable if during and after joint manipulation there was no observable shift in the device placement and no blood loss from the wound. The primary outcome was the ability to obtain hemostasis. Application times (inclusive of packing and iTClamp closure), the number of attempts needed to obtain hemostasis, and total blood loss were recorded as secondary end points.
Procedure
Subjects were fasted at least 12 hours prior to induction of anesthesia with free access to water. They were premedicated with a combination of ketamine (20 mg/kg IM) and butorphanol (0.2 mg/kg). After supine placement on the surgery table, they were orotracheally intubated and general anesthesia was achieved with isoflurane. Animals were placed on mechanical ventilation and gas anesthesia was adjusted throughout the procedure to maintain the desired level of anesthesia.
We did not tabulate vital signs or acquire blood biochemical variables as this pilot focused on the feasibility of application. Additionally, the use of bilateral neck and groin wounds, precluded installing lines continuous invasive monitoring. However, Veterinary staff monitored vital signs (HR, RR, and O2) and animals were monitored for pain/distress continuously throughout the lab, in compliance with ethical guidelines.
To isolate the target vessels, a scalpel was utilized to make either a 5 cm or 10 cm linear incision in the skin overlying the inguinal, axillary, and carotid vessels. The target artery was then exposed and dissected out from the surrounding fascia. A 6 mm punch-biopsy device was utilized to create an arteriotomy. The randomly assigned treatment modality was then applied. For arms utilizing Combat Gauze, two-handed packing was utilized to fill the wound cavity followed immediately by application of the iTClamp. Likewise, for XSTAT treatment arms, the sponges were injected into the wound, followed by immediate iTClamp application (Fig. 4). The wound was then observed to ensure external hemorrhage had stopped. If any bleeding continued the iTClamp was removed and reapplied until hemostasis was obtained.
External blood loss was measured by a combination of suctioning blood and weighing blood-soaked absorbent pads and gauze and subtracting the dry weight of the pads and gauze. At necropsy, the iTClamps, gauze, blood clots, and/or remaining blood were removed from the wounds, weighed and added to the total blood loss.
Once external hemorrhage was controlled, the wound was manipulated to test device stability. For wounds in the groin and axilla, the hip or shoulder, respectively, were fully extended and flexed five times. For neck wounds, the head was rotated left and right five times to approximately 45 degrees. Validation of hemorrhage control was determined by two observers, the Primary Investigator and a trained assistant. Control of hemorrhage was defined as the lack of any bleeding from the incision site. At the completion of the experiment, animals were euthanized with euthasol (1 mL/5 kg). Death was defined as a MAP below 20 and end tidal CO2 below 15 mm Hg for 2 continuous minutes.
Data Analysis
As this was a pilot study to assess model feasibility, results are presented as descriptive statistics rather than as inferential null hypothesis statistical testing statistics using p-values.22 Hemostasis data are expressed as frequencies and percentages. Application time and blood loss data are expressed as means (M) and 95% confidence intervals (95%CI).
RESULTS
Hemostasis was achieved on all wounds. For Combat Gauze, hemostasis was achieved on the first attempt on five of six wounds (83%), with two attempts needed for the 10 cm neck wound. For XSTAT, hemostasis was achieved on the first attempt on three of six wounds (50%), with two attempts needed for the 5 cm groin wound and three attempts needed for the 5 cm and 10 cm neck wounds.
Figure 5 shows that time to application averaged ~50% slower for Combat Gauze (M = 41 seconds, 95%CI: 22–32 seconds) than for XSTAT (M = 27 sec, 95%CI: 35–47 seconds). The 10 cm wounds took ~10 seconds (36%) longer to close (M = 27 sec, 95%CI: 35–47 sec) than the 5 cm wounds (M = 27 sec, 95%CI: 35–47 sec). Table I shows that XSTAT was faster than Combat Gauze for each wound location and size.
| . | Groin . | Axilla . | Neck . | Overall . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | Total . |
| Xstat | 21 | 36 | 21 | 34 | 22 | 29 | 22 | 33 | 27 |
| 95%CI | 21–22 | 28–37 | 22–32 | ||||||
| CG | 30 | 43 | 35 | 41 | 43 | 53 | 36 | 46 | 41 |
| 95%CI | 29–44 | 39–53 | 35–47 | ||||||
| Total | 26 | 39 | 28 | 38 | 33 | 41 | 29 | 39 | 34 |
| 95%CI | 22–36 | 32–46 | 28–40 | ||||||
| . | Groin . | Axilla . | Neck . | Overall . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | Total . |
| Xstat | 21 | 36 | 21 | 34 | 22 | 29 | 22 | 33 | 27 |
| 95%CI | 21–22 | 28–37 | 22–32 | ||||||
| CG | 30 | 43 | 35 | 41 | 43 | 53 | 36 | 46 | 41 |
| 95%CI | 29–44 | 39–53 | 35–47 | ||||||
| Total | 26 | 39 | 28 | 38 | 33 | 41 | 29 | 39 | 34 |
| 95%CI | 22–36 | 32–46 | 28–40 | ||||||
| . | Groin . | Axilla . | Neck . | Overall . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | Total . |
| Xstat | 21 | 36 | 21 | 34 | 22 | 29 | 22 | 33 | 27 |
| 95%CI | 21–22 | 28–37 | 22–32 | ||||||
| CG | 30 | 43 | 35 | 41 | 43 | 53 | 36 | 46 | 41 |
| 95%CI | 29–44 | 39–53 | 35–47 | ||||||
| Total | 26 | 39 | 28 | 38 | 33 | 41 | 29 | 39 | 34 |
| 95%CI | 22–36 | 32–46 | 28–40 | ||||||
| . | Groin . | Axilla . | Neck . | Overall . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | Total . |
| Xstat | 21 | 36 | 21 | 34 | 22 | 29 | 22 | 33 | 27 |
| 95%CI | 21–22 | 28–37 | 22–32 | ||||||
| CG | 30 | 43 | 35 | 41 | 43 | 53 | 36 | 46 | 41 |
| 95%CI | 29–44 | 39–53 | 35–47 | ||||||
| Total | 26 | 39 | 28 | 38 | 33 | 41 | 29 | 39 | 34 |
| 95%CI | 22–36 | 32–46 | 28–40 | ||||||
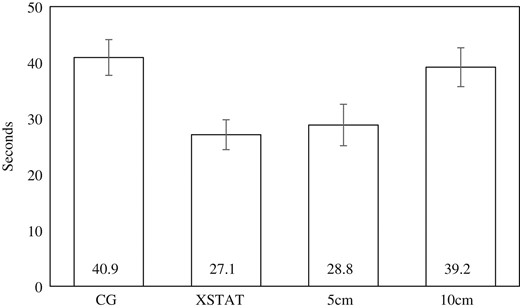
Table II shows that blood loss was similar for Combat Gauze (M = 51 mL, 95%CI: 25–76 mL) and XSTAT (M = 60 mL, 95%CI: 30–90 mL). Blood loss was roughly twice as great for 10 cm wounds (M = 73 mL, 95%CI: 47–100 mL) than for 5 cm wounds (M = 38 mL, 95%CI: 18–57 mL).
| . | Groin . | Axilla . | Neck . | Overall . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | Total . |
| Xstat | 46 | 18 | 78 | 71 | 28 | 120 | 51 | 70 | 60 |
| 95%CI | 22–79 | 12–127 | 30–90 | ||||||
| CG | 18 | 73 | 46 | 91 | 10 | 67 | 25 | 77 | 51 |
| 95%CI | 3–46 | 63–91 | 25–76 | ||||||
| Total | 32 | 46 | 62 | 81 | 19 | 94 | 38 | 73 | 56 |
| 95%CI | 18–57 | 47–100 | 36–75 | ||||||
| . | Groin . | Axilla . | Neck . | Overall . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | Total . |
| Xstat | 46 | 18 | 78 | 71 | 28 | 120 | 51 | 70 | 60 |
| 95%CI | 22–79 | 12–127 | 30–90 | ||||||
| CG | 18 | 73 | 46 | 91 | 10 | 67 | 25 | 77 | 51 |
| 95%CI | 3–46 | 63–91 | 25–76 | ||||||
| Total | 32 | 46 | 62 | 81 | 19 | 94 | 38 | 73 | 56 |
| 95%CI | 18–57 | 47–100 | 36–75 | ||||||
| . | Groin . | Axilla . | Neck . | Overall . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | Total . |
| Xstat | 46 | 18 | 78 | 71 | 28 | 120 | 51 | 70 | 60 |
| 95%CI | 22–79 | 12–127 | 30–90 | ||||||
| CG | 18 | 73 | 46 | 91 | 10 | 67 | 25 | 77 | 51 |
| 95%CI | 3–46 | 63–91 | 25–76 | ||||||
| Total | 32 | 46 | 62 | 81 | 19 | 94 | 38 | 73 | 56 |
| 95%CI | 18–57 | 47–100 | 36–75 | ||||||
| . | Groin . | Axilla . | Neck . | Overall . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Product . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | 5 mm . | 10 mm . | Total . |
| Xstat | 46 | 18 | 78 | 71 | 28 | 120 | 51 | 70 | 60 |
| 95%CI | 22–79 | 12–127 | 30–90 | ||||||
| CG | 18 | 73 | 46 | 91 | 10 | 67 | 25 | 77 | 51 |
| 95%CI | 3–46 | 63–91 | 25–76 | ||||||
| Total | 32 | 46 | 62 | 81 | 19 | 94 | 38 | 73 | 56 |
| 95%CI | 18–57 | 47–100 | 36–75 | ||||||
DISCUSSION
Advances in hemorrhage control and management can save lives on the battlefield as exsanguination remains a leading cause of preventable combat deaths.1,2,11 Of particular interest are junctional hemorrhages, which are challenging to treat and are associated with high rates of potentially preventable combat death.3,13 Given the weaknesses with current treatment methods, continued research should be aimed at not only at new products and methods, but should also explore the potential synergy between different hemorrhage control products.
The iTClamp was approved by the FDA a mere 5 years ago, but this device has shown significant potential. Research reports include the effective iTClamp application in cadaver studies,14,15 securing chest tubes,15,23 and prehospital care.18 Shaw and colleagues found the iTClamp to be effective in treating wounds encountered by paramedics, including cases that required a pair of iTClamps due to the length of the wounds.19 Case studies of successfully treating medial thigh battlefield wounds with iTClamp have also been reported.24 An anonymous worldwide survey indicated that, of 245 cases, iTClamp was attempted only after other techniques were abandoned in 26% of cases, and was ineffective in stopping external bleeding only 8% of cases.20 Relevant to delivering care under fire, marksmanship was superior for iTClamp compared to tourniquet on the dominant arm, and no iTClamp participants withdrew due to pain compared to the majority of tourniquet participants.25 We found only one paper which tested iTClamp in conjunction with a hemostatic agent,17 finding 100% survival. While this study is invaluable towards appreciating the potential value of combining iTClamp in conjunction with other hemostatics, this small-n (eight swine per group) study used one wound size at one anatomical site, and combined iTClamp with plain gauze only, not with XSTAT. What was needed was a model for testing iTClamp with other hemostatic agents using multiple sites of differing sizes on the same animal.
The present study fills this important gap in the literature by demonstrating the feasibility of testing the iTClamp in conjunction with hemostatic agents as hemostasis was achieved for all 12 wounds, whether the wound was 5 cm or 10 cm in length (using dual iTClamps), whether the wound was in the groin, axilla or neck, or whether the iTClamp was using in conjunction with Combat Gauze packing or XSTAT packing. Additionally, we demonstrated a model that can not only potentially speed the testing of iTClamp efficacy across different experimentally-controlled conditions, the number of animals needed can be reduced, saving time, money, and animal resources. Aside from successfully demonstrating the feasibility of this swine model, consistent with our study hypothesis, the results of hemostasis, time to application, and blood loss are also of interest.
However, hemostasis was not always achieved on the first attempt. To achieve hemostasis, iTClamp reapplications were required for one of six in the Combat Gauze condition and in three of six in the XSTAT condition. In each case, the first-attempt failure was caused by extrusion of packing material out from the wound, preventing a tight seal of the wound margins, allowing for continued bleeding (Fig. 6). This phenomenon was more common with XSTAT, which was perhaps not entirely surprising because the XSTAT sponges are specifically designed to impart their hemostatic effect by rapidly expanding when contacted by blood. It is possible that injecting fewer sponges may alleviate this problem, an important area for future research. While extrusion prevented one Combat Gauze first attempt. single ply “wicks” of Combat Gauze did not prevent wound closure (Fig. 7).
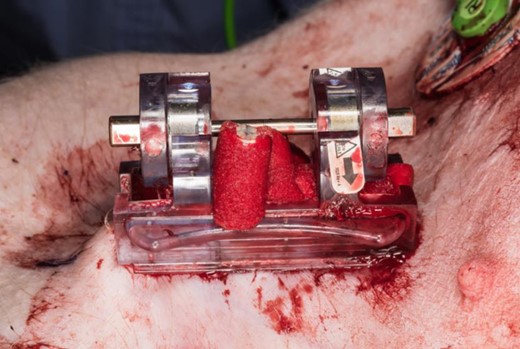
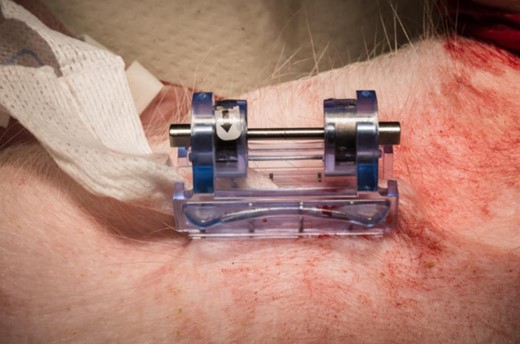
Single ply “wicks” of combat gauze did not prevent wound sealing.
Anatomically, neck wounds had a higher first-attempt failure rate in both 5 cm and 10 cm wounds. This was likely because the target vessels in the neck are more superficial compared to the target vessels in the groin and axilla, resulting in a shallower cavity for packing and greater extrusion of packing material. The implications here include the possibilities that packing may not be efficacious in shallow wounds or that shallow wounds require less packing material, which are open empirical questions for future research. Regardless, operators who choose to seal packed wounds with an iTClamp should ensure that all gauze is packed tightly into the wound and that any excess gauze extruding from the wound is removed prior to application of the iTClamp.
Application time is also of interest, as faster application of hemostatic treatment is associated with less blood loss.4,26 Current TCCC guidelines recommend packing a junctional wound with a gauze product then applying and holding external pressure for three minutes, presuming this pressure is realized internally at the point of hemorrhage, with no provision for closing the wound.2,11
In our study, regardless of wound location, size, or need for reapplication, the iTClamp with hemostatic packing was able to control hemorrhage significantly faster (M = 36 seconds) than current treatment methods, such a junctional tourniquets (87–124 seconds)7 or the traditional combination of packing and pressure dressing application (87 seconds application time + 3 minutes of required pressure)27 while simultaneously sealing the wound from the external environment. The speed of iTClamp application should not only result in less blood loss but also aid in avoiding the sequelae of prolonged hemorrhage.4 Additionally, rapid application frees the provider to address other casualty management issues, which is critical in prehospital settings in which corpsmen and medics may be operating without assistance. Lengthy application times can potentially be prohibitive or even hazardous to both patients and providers in battlefield or austere settings. Additionally, with the increase in domestic and terrorism-based incidents, rapid and effective hemorrhage control devices can be a force multiplier in mass casualty situations, by allowing a provider to quickly control hemorrhage from multiple sources or patients.
LIMITATIONS
Our study was limited by the sample, which included swine, not humans. This pilot study was narrowly designed to evaluate the feasibility of a model to test iTClamp with hemostatic packing to provide primary control of hemorrhage in three key junctional areas, so we did not include an iTClamp-only condition. As a model feasibility study, we did not seek to evaluate the efficacy or the long-term management, and therefore did not administer tranexamic acid, provide blood products, or take any other resuscitative measures that impact the efficacy of hemorrhage control in trauma patients. Further, in the real world, there is a delay between the onset of a junctional wound and treatment, but we elected to provide immediate treatments to decrease blood loss, allowing multiple application sites to be treated before animal expiration. Only two hemostatic packing materials were included. Lastly, our model was limited to linear injury patterns of two sizes and did not include jagged or irregular wounds.
Future Research
The present pilot study demonstrates the potential utility of a novel model for testing the iTClamp with hemostatic packing to treat junctional wound hemorrhage. This feasibility study sets the stage for larger, appropriately-powered (based on a priori sample size calculations), multi-group studies to formally contrast strategies for treating junctional wounds. An important next-step study will be to determine the relative efficacy iTClamp with Combat Gauze versus XSTAT packing compared to iTClamp-alone as a control condition. Future studies may also include contrasting the efficacy of iTClamp-alone and iTClamp with Combat Gauze or XSTAT sponges (or potentially other hemostatic agents) versus standard care of junctional wound packing and 3-min direct pressure followed by pressure dressing application. These efficacy studies can be conducted using life-challenging protocols, like the frequently used Kherabati swine model that includes a 45 second “free bleed” from a femoral arteriotomy before attempt at hemorrhage control,3,16,17 and can include experimentally-controlled resuscitative treatments, such as tranexamic acid, Terlipressin, or blood products. Efficacy studies should include standard outcomes of survival and post-application blood loss, along with standard animal monitoring of vitals and biochemical measures. It is also important to acquire user feedback and to assess end-user preferences regarding the use of iTClamp compared to other methods for treating junctional wounds. The paucity of first-attempt success closing neck wounds implies that shallow wounds may require less packing material or may potentially have better results without packing, an important area for future research. Determining how to calibrate the optimal about of XSTAT sponges to confer a hemostatic effect without extruding sufficiently to impair iTClamp closure is an area for future research that can benefit from the model described in this paper. It is also important to challenge the model described in the present study by using longer, irregular, or jagged wounds, and to assess possible soft tissue damage from the iTClamp, which was not found in the present study but has been reported elsewhere.28
CONCLUSION
Lethal hemorrhage remains the leading cause of preventable battlefield death and remains a top priority for military medicine. In particular, junctional wounds are difficult to manage. Given its speed, ease of application, and compact size, the iTClamp has the potential to be a useful device for controlling junctional hemorrhage in prehospital and combat settings, but research on combining the iTClamp with hemostatic agents is lacking. Our pilot demonstrates the feasibility of a model for testing the combination of traditional wound packing with wound closure by the iTClamp in three different junctional hemorrhage locations.
Previous Presentations
Presented as a poster at the 2017 Military Health System Research Symposium- MHSRS-17-0745.
Funding
This study was supported by a Navy CIP grant (NMCP.2016.0012). This supplement was sponsored by the Office of the Secretary of Defense for Health Affairs.
References
Author notes
The views expressed in this paper are those of the authors and do not necessarily represent the official position or policy of the U.S. Government, the Department of Defense, or the Department of the Navy.



