-
Views
-
Cite
Cite
Ana-Maria Sevcenco, Martijn W H Pinkse, Emile Bol, Gerard C Krijger, Hubert Th Wolterbeek, Peter D E M Verhaert, Peter-Leon Hagedoorn, Wilfred R Hagen, The tungsten metallome of Pyrococcus furiosus, Metallomics, Volume 1, Issue 5, September 2009, Pages 395–402, https://doi.org/10.1039/b908175e
Close - Share Icon Share
Abstract
The tungsten metallome of the hyperthermophilic archaeon Pyrococcus furiosus has been investigated using electroanalytical metal analysis and native–native 2D-PAGE with the radioactive tungsten isotope 187W (t1/2 = 23.9 h). P. furiosus cells have an intracellular tungsten concentration of 29 μM, of which ca. 30% appears to be free tungsten, probably in the form of tungstate or polytungstates. The remaining 70% is bound by five different tungsten enzymes: formaldehyde ferredoxin oxidoreductase, aldehyde ferredoxin oxidoreductase, glyceraldehyde-3-phosphate ferredoxin oxidoreductase and the tungsten-containing oxidoreductases WOR4 and WOR5. The membrane proteome of P. furiosus is devoid of tungsten. The differential expression, as measured by the tungsten level, of the five soluble tungsten enzymes when the cells are subjected to a cold-shock shows a strong correlation with previously published DNA microarray analyses.
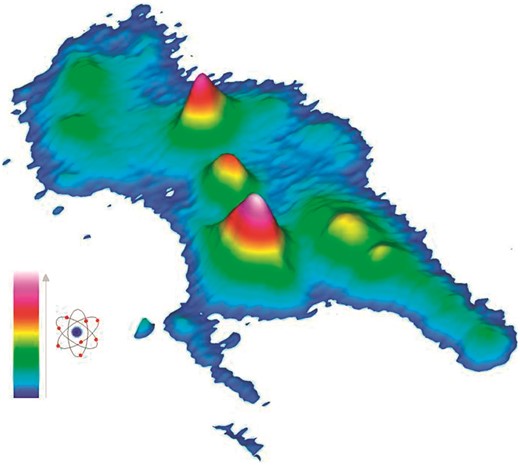
Using native–native 2D-PAGE in combination with the short lived radioisotope 187W, all tungsten containing proteins from Pyrococcus furiosus have been separated, quantified and identified.



