-
PDF
- Split View
-
Views
-
Cite
Cite
Wolfram Buss, Brett A Ford, Eloise Foo, Wendelin Schnippenkoetter, Philippa Borrill, Brenton Brooks, Anthony R Ashton, Peter M Chandler, Wolfgang Spielmeyer, Overgrowth mutants determine the causal role of gibberellin GA2oxidaseA13 in Rht12 dwarfism of wheat, Journal of Experimental Botany, Volume 71, Issue 22, 31 December 2020, Pages 7171–7178, https://doi.org/10.1093/jxb/eraa443
Close - Share Icon Share
Abstract
The induced dwarf mutant Rht12 was previously shown to have agronomic potential to replace the conventional DELLA mutants Rht-B1b/Rht-D1b in wheat. The Rht12 dwarfing gene is not associated with reduced coleoptile length (unlike the DELLA mutants) and it is dominant, characteristics which are shared with the previously characterized dwarfing genes Rht18 and Rht14. Using the Rht18/Rht14 model, a gibberellin (GA) 2-oxidase gene was identified in the Rht12 region on chromosome 5A. A screen for suppressor mutants in the Rht12 background identified tall overgrowth individuals that were shown to contain loss-of-function mutations in GA2oxidaseA13, demonstrating the role of this gene in the Rht12 dwarf phenotype. It was concluded that Rht12, Rht18, and Rht14 share the same height-reducing mechanism through the increased expression of GA 2-oxidase genes. Some of the overgrowth mutants generated in this study were semi-dwarf and taller than the original Rht12 dwarf, providing breeders with new sources of agronomically useful dwarfism.
Introduction
During the ‘Green Revolution’ wheat yields increased markedly due to the introduction of mutant Della genes (Rht-B1b and Rht-D1b) that reduced plant growth and allowed more carbon to be partitioned to the grain (Hedden, 2003). Yields also increased because more nitrogen fertilizer and irrigation could be applied without the crop lodging. Rht-B1b and Rht-D1b dwarfing alleles reduce stem growth by stabilizing the encoded DELLA proteins against degradation through the interaction with the growth hormone gibberellin (GA) and its receptor (Peng et al., 1999). In dryland, rain-fed environments, these dwarfing genes constrain genetic progress and longer term capacity to deliver growing global food needs because they also reduce early seedling growth (Laing and Fischer, 1977; Keyes et al., 1989). Reduced early growth and shorter coleoptiles can lead to poor crop establishment and increased water loss from the soil surface. This is particularly relevant for rainfed, semi-arid environments where up to 50% of soil water can be lost from the surface due to evaporation during the early growth phase (Richards, 1992). In these regions, reducing water loss through better crop establishment is a key objective to improve water productivity (Sadras and Rodriguez, 2007).
Several alternative dwarfing genes, at loci unrelated to Rht-1, were generated in wheat following mutagenesis (Konzak, 1982). Generally, these mutant alleles have less effect on early growth and coleoptile length, and are therefore more suitable for rainfed environments with high evaporative demand (Ellis et al., 2004). Agronomic assessments have shown that some of these genes have good potential to replace conventional DELLA dwarfing genes globally (Rebetzke et al., 2012). One of the dwarf mutants (Rht18) limited stem growth by reducing the content of bioactive GA. The mutation, originally generated in tetraploid wheat, increased the expression of a GA 2-oxidase gene (GA2oxA9) on chromosome 6A. This resulted in the removal of GA precursors from the biosynthetic pathway, leading to a reduced content of bioactive GA and reduced growth (Ford et al., 2018). This study also showed that Rht14, an independent mutant that was generated in a different tetraploid background, increased the expression of the same GA 2-oxidase gene member that resulted in a dwarf phenotype (Gale et al., 1985).
Rht12 is another dwarf mutant which was generated in hexaploid wheat following γ-radiation and assessed for its agronomic potential (Konzak, 1982). Rht12 reduced plant height by 40–50% and contributed to increased spikelet fertility, but it also delayed ear emergence. In some genetic backgrounds, the gene was associated with reduced grain size (Worland et al., 1994; Chen et al., 2013). The Rht12 and Rht18 mutants share important characteristics; both were isolated following radiation treatment; they have a relatively strong effect on height, but do not reduce coleoptile length; and both were reported to be dominant (Sutka and Kovacs, 1987; Ellis et al., 2004). However, their genomic locations differ: Rht12 maps to chromosome 5A, and Rht18 to chromosome 6A (Worland et al., 1994; Korzun et al., 1997; Tang, 2015). Nevertheless, given the similarities between these dwarfing genes, and our previously reported characterization of Rht18, we hypothesized that plant height was reduced by the same mechanism involving the overexpression of a GA 2-oxidase gene member on chromosome 5A in the Rht12 mutant (Ford et al., 2018).
We used the previously published genetic map position of Rht12 and sequences of GA 2-oxidase gene members to identify a candidate gene for Rht12 dwarfism (Korzun et al., 1997; Pearce et al., 2015). This same GA 2-oxidase gene was recently reported as a candidate gene for Rht12 based on mapping studies, its expression profile, and GA content analysis in wheat (Sun et al., 2019). Here we report the isolation of overgrowth (ovg) mutants in a Rht12 background. These mutants contain second site mutations within this locus that generate a range of intermediate height phenotypes, as well as presumed loss-of-function derivatives that are taller than the original tall parental line. Through mutation analysis, our study provides the functional proof that a GA 2-oxidase gene member on chromosome 5A is responsible for Rht12 dwarfism.
Materials and methods
Plant material
The Rht12 mutant was isolated in winter wheat ‘Karcagi’ following γ-irradiation, and was first reported as Karcagi 522M 7K (Viglasi, 1968). Rht12 was backcrossed into UK wheat ‘Mercia’ by Worland et al. (1994) and then used as donor to transfer the gene into the Australian breeding line ‘Vigour18’ before backcrossing into the spring cultivar ‘Halberd’. Halberd Rht12 was developed after four backcrosses of a Vigour18 (Rht12) line into the Halberd genetic background (G. Rebetzke, personal communication).
Mutagenesis
Grain of Halberd Rht12 was imbibed in water at 4 °C overnight and transferred to fresh water the next day before aerating for 8 h at room temperature with one change of water after 4 h. The water was removed and grain was incubated for 2 h in freshly prepared 1 mM sodium azide dissolved in 0.1 M phosphate (K+) buffer (pH 3.0), and then washed in running water for 2 h before the grain was dried overnight and sown the following day.
Suppressor screen to isolate tall mutants
Sodium azide-treated Halberd Rht12 grain was sown in rows in the birdcage in Canberra (Australia) during the 2017 growing season, and M2 seed was harvested as bulk rows. M2 plots were sown in the field at Yanco in NSW (Australia) in 2018. Plants that were at least 5 cm taller than the plot average were harvested individually. Putative ovg mutants which were identified in plots that originated from separate bulk rows in 2017 represented independent events.
Putative mutants (129 lines) were grown in the glasshouse at CSIRO Black Mountain (Canberra, ACT, Australia) during autumn 2019 at 21 °C day/18 °C night temperatures with daylength extended to 16 h. Four M3 grains from each ovg mutant were sown in 20 cm pots containing a soil/compost mix with slow-release fertilizer. A subset of 27 mutant lines was selected based on their uniformity in height within several different major height classes. One M3 family (9-4) was selected that segregated for height. Plant height was measured at maturity from the soil level to the base of the spike. Leaf tissue was harvested for DNA extraction and sequencing
Molecular analysis
The target gene (TraesCS5A02G543100) was identified using the GA2oxA9 gene sequence in Blast searches of the Chinese Spring reference genome (Appels et al., 2018) in the region of chromosome 5AL where Rht12 was previously mapped (Korzun et al., 1997).
TraesCS5A02G543100 was amplified with the following primers which were flanking the gene: AATCTCTCGATCGACCATGC (forward) and AAGGAATTTTCCTCCGATGC (reverse). Standard PCR conditions were used to generate the expected size amplicon. Enzymatic clean-up with FastAP (alkaline phosphatase) and exonuclease 1 was followed by Sanger sequencing.
Expression analysis
The basal 50% of elongating peduncles from the main stem were harvested at 50% final length and immediately frozen in liquid nitrogen. RNA was extracted separately from at least four individual plants of each of Halberd Rht12, Halberd, and the 18-6 mutant line. Extractions were performed using the Maxwell® RSC Plant RNA Kit and RSC Instrument from Promega™. RNA samples were subjected to an additional DNase treatment using the TURBO DNA-free™ Kit from Invitrogen™. cDNA was generated from 2 µg of RNA for each sample using the SuperScript® III First-Strand Synthesis System for RT-PCR kit from Invitrogen™. A 3 µl aliquot of 1:10 dilutions of the cDNA mixes was subjected to quantitative real-time PCR (qRT-PCR) using the iTaq Universal SYBR Green Supermix and the CFX96 Touch Real-Time PCR Detection System from Biorad™. Reaction conditions included an initial denaturization at 95 °C for 3 min, 40 cycles of denaturization at 95 °C for 10 s, and annealing/elongation at 60 °C for 30 s, followed by a melt step range of 65–95 °C with increments of 0.5 °C. GA2ox-A13 gene relative expression experiments included three technical replicates for each of at least four biological replicates using primers GA2ox-A13-F AATCTCTCGATCGACCATGC and GA2ox-A13-R GTGGCTTCCGAAATAGAGTAG. Means of the ΔCq values were calculated, relative gene expression values were log(base 2) transformed, and SEs were determined for the data using the expression of wheat glyceraldehyde phosphate dehydrogenase (GADPH) as reference gene with primers GADPH-RT-F TTAGACTTGCGAAGCCAGCA and GADPH-RT-R AAATGCCCTTGAGGTTTCCC.
Protein structure modelling
The predicted Rht12 protein structure was modelled on the GA2oxA13 homologue 2BRT:A (anthocyanidin synthase). The amino acid sequences were aligned and the Rht12 mutated amino acids were assigned as follows. Rht12 G129D to 2BRT G126, A133V to A130, E143K to D141, S164F to P162, D234N to D234, D244N to N244, Q276H to E276, Q276Q to E276, A302T to A302, V334M to K341, G346E to E353, G346V to E353, and L347F to K354. The Rht12 sequence was also modelled using Swiss Model and 2BRT:A as a template.
RasMol version 2.7.1 (Herbert J. Bernstein) was used for protein visualization.
GA content analysis
To quantify GA1 content, the basal 25% of elongating peduncles (including the node) were harvested when the peduncle had achieved 50% of its final length and were immediately frozen in liquid nitrogen. Each biological replicate consisted of ~2 g FW of pooled peduncles from at least four individual plants. GAs were extracted as detailed by Ford et al. (2018). Tissue was homogenized, covered in 80% MeOH overnight, and [2H2]GA1 was added as an internal standard. Samples were extracted by passing though C18 Sep-Pak (Waters Pty Ltd, Australia) followed by SAX (Maxi-Clean™; Grace Davison Discovery Sciences, USA) column as previously described. Samples were then derivatized with EDC [N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride; Sigma Aldrich, Australia]. Samples were then analysed using a Waters Acquity H-Class UPLC instrument coupled to a Waters Xevo triple quadrupole mass spectrometer. A Waters Acquity UPLC BEH C18 column (2.1 mm×100 mm×1.7 µm) was used. The instrument was operated and multiple reaction monitoring (MRM) transitions were monitored. For all samples, peak areas for endogenous and labelled GAs were compared and combined with the fresh weight of the sample to calculate ng g FW–1.
Results
Overgrowth mutants in the Rht12 background
Rht12 dwarfism is dominant, a characteristic that is shared with the previously described Rht18 dwarfing gene in wheat. Due to its similar phenotype, it is possible that the dwarfism in Rht12 and Rht18 involves the same mechanism (Ford et al., 2018). If the overexpression of a GA 2-oxidase gene reduces bioactive GA content in Rht12, it was hypothesized that ovg mutants could be generated in a Rht12 background, as they were for Rht18. Therefore, a large M2 population derived from treated Halberd Rht12 was screened in the field and putative ovg mutants were identified that were clearly taller than Halberd Rht12 and, in some cases, taller than the original tall parent Halberd. Overall, 129 ovg mutants were harvested and progeny tested, and a subset of 28 lines representing different classes and homogeneity for height was selected for further analysis.
The tall, Halberd control had an average height of 81.9±0.3 cm (SE) and the dwarf Halberd Rht12 line a height of 33.8 ±1.9 cm (SE). Halberd does not contain any known dwarfing genes but is considered a relatively short ‘tall’ variety. The introduction of Rht12 resulted in a height reduction of 59%, which is comparable with the results of Worland et al. (1994) who observed an average reduction in height of 52% across four wheat varieties. The average heights of 28 ovg mutants ranged between 53.3 cm and 118.5 cm, with some lines significantly exceeding the height of Halberd. Final heights of a subset of mutants are shown in Figs 1 and 2 and listed in Table 1.
| Line . | Mean . | SE . | n . | Plant height class . | Mutation position . | Change . |
|---|---|---|---|---|---|---|
| Halberd | 81.9 | 0.3 | 7 | Intermediate | ||
| Halberd Rht12 | 33.8 | 1.9 | 6 | Dwarf | ||
| 4-2 | 53.3** | 1.5 | 3 | Semi-dwarf | 552 | Intron |
| 11-7 | 56.5** | 3.7 | 8 | Semi-dwarf | 491 | S164F |
| 6-ex | 67.0* | 8.0 | 3 | Semi-dwarf | 1127 | A302T |
| 4-6 | 74.6* | 2.0 | 5 | Semi-dwarf | 386 | G129D |
| 1-4 | 78.3 | 3.0 | 4 | Intermediate | 1220 | V334M |
| 10-6 | 87.6 | 1.8 | 10 | Xtall | 800 | D244N |
| 6-2 | 88.3 | 1.8 | 8 | Xtall | 1259 | G346E |
| 15-3 | 91.0 | 4.0 | 3 | Xtall | 398 | A133V |
| 15-8 | 91.4 | 1.8 | 8 | Xtall | 427 | E143K |
| 1-3 | 94.0 | 1.4 | 8 | Xtall | 1261 | L347F |
| 19-1 | 97.0 | 1.5 | 3 | Xtall | 770 | D234N |
| 5-1 | 81.1 | 0.9 | 8 | Intermediate | 898 | Splice site |
| 14-7 | 86.7 | 2.3 | 3 | Xtall | 898 | Splice site |
| 4-5 | 88.3 | 1.7 | 3 | Xtall | 573 | Splice site |
| 3-2 | 101.0 | 0.6 | 3 | Xtall | 899 | Splice site |
| 3-8 | 91.8 | 1.7 | 8 | Xtall | 384 | W128* |
| 7-4 | 97.6 | 1.6 | 8 | Xtall | 249 | W83* |
| 13-7 | 101.1 | 1.2 | 8 | Xtall | 384 | W128* |
| 18-6 | 109.3 | 1.5 | 4 | Xtall | 1240 | Q340* |
| 9-4a | 56.1 | 2.8 | 7 | Semi-dwarf | 504 | Splice site |
| Line . | Mean . | SE . | n . | Plant height class . | Mutation position . | Change . |
|---|---|---|---|---|---|---|
| Halberd | 81.9 | 0.3 | 7 | Intermediate | ||
| Halberd Rht12 | 33.8 | 1.9 | 6 | Dwarf | ||
| 4-2 | 53.3** | 1.5 | 3 | Semi-dwarf | 552 | Intron |
| 11-7 | 56.5** | 3.7 | 8 | Semi-dwarf | 491 | S164F |
| 6-ex | 67.0* | 8.0 | 3 | Semi-dwarf | 1127 | A302T |
| 4-6 | 74.6* | 2.0 | 5 | Semi-dwarf | 386 | G129D |
| 1-4 | 78.3 | 3.0 | 4 | Intermediate | 1220 | V334M |
| 10-6 | 87.6 | 1.8 | 10 | Xtall | 800 | D244N |
| 6-2 | 88.3 | 1.8 | 8 | Xtall | 1259 | G346E |
| 15-3 | 91.0 | 4.0 | 3 | Xtall | 398 | A133V |
| 15-8 | 91.4 | 1.8 | 8 | Xtall | 427 | E143K |
| 1-3 | 94.0 | 1.4 | 8 | Xtall | 1261 | L347F |
| 19-1 | 97.0 | 1.5 | 3 | Xtall | 770 | D234N |
| 5-1 | 81.1 | 0.9 | 8 | Intermediate | 898 | Splice site |
| 14-7 | 86.7 | 2.3 | 3 | Xtall | 898 | Splice site |
| 4-5 | 88.3 | 1.7 | 3 | Xtall | 573 | Splice site |
| 3-2 | 101.0 | 0.6 | 3 | Xtall | 899 | Splice site |
| 3-8 | 91.8 | 1.7 | 8 | Xtall | 384 | W128* |
| 7-4 | 97.6 | 1.6 | 8 | Xtall | 249 | W83* |
| 13-7 | 101.1 | 1.2 | 8 | Xtall | 384 | W128* |
| 18-6 | 109.3 | 1.5 | 4 | Xtall | 1240 | Q340* |
| 9-4a | 56.1 | 2.8 | 7 | Semi-dwarf | 504 | Splice site |
Type of mutation event and position in GA2oxA13 listed for overgrowth mutants. Height classes defined as Dwarf (34 cm, =Halberd Rht12); Semi-dwarf (40–75 cm); Intermediate (75–82 cm—height of Halberd); Xtall (87–110 cm, taller than Halberd). Mutation in 9-4 was isolated at a heterozygous level. Lines that were significantly shorter than Halberd are indicated as follows (t-test): *significance level <0.05; **significance level <0.01.
a Line 9-4 was isolated as a heterozygous mutant.
| Line . | Mean . | SE . | n . | Plant height class . | Mutation position . | Change . |
|---|---|---|---|---|---|---|
| Halberd | 81.9 | 0.3 | 7 | Intermediate | ||
| Halberd Rht12 | 33.8 | 1.9 | 6 | Dwarf | ||
| 4-2 | 53.3** | 1.5 | 3 | Semi-dwarf | 552 | Intron |
| 11-7 | 56.5** | 3.7 | 8 | Semi-dwarf | 491 | S164F |
| 6-ex | 67.0* | 8.0 | 3 | Semi-dwarf | 1127 | A302T |
| 4-6 | 74.6* | 2.0 | 5 | Semi-dwarf | 386 | G129D |
| 1-4 | 78.3 | 3.0 | 4 | Intermediate | 1220 | V334M |
| 10-6 | 87.6 | 1.8 | 10 | Xtall | 800 | D244N |
| 6-2 | 88.3 | 1.8 | 8 | Xtall | 1259 | G346E |
| 15-3 | 91.0 | 4.0 | 3 | Xtall | 398 | A133V |
| 15-8 | 91.4 | 1.8 | 8 | Xtall | 427 | E143K |
| 1-3 | 94.0 | 1.4 | 8 | Xtall | 1261 | L347F |
| 19-1 | 97.0 | 1.5 | 3 | Xtall | 770 | D234N |
| 5-1 | 81.1 | 0.9 | 8 | Intermediate | 898 | Splice site |
| 14-7 | 86.7 | 2.3 | 3 | Xtall | 898 | Splice site |
| 4-5 | 88.3 | 1.7 | 3 | Xtall | 573 | Splice site |
| 3-2 | 101.0 | 0.6 | 3 | Xtall | 899 | Splice site |
| 3-8 | 91.8 | 1.7 | 8 | Xtall | 384 | W128* |
| 7-4 | 97.6 | 1.6 | 8 | Xtall | 249 | W83* |
| 13-7 | 101.1 | 1.2 | 8 | Xtall | 384 | W128* |
| 18-6 | 109.3 | 1.5 | 4 | Xtall | 1240 | Q340* |
| 9-4a | 56.1 | 2.8 | 7 | Semi-dwarf | 504 | Splice site |
| Line . | Mean . | SE . | n . | Plant height class . | Mutation position . | Change . |
|---|---|---|---|---|---|---|
| Halberd | 81.9 | 0.3 | 7 | Intermediate | ||
| Halberd Rht12 | 33.8 | 1.9 | 6 | Dwarf | ||
| 4-2 | 53.3** | 1.5 | 3 | Semi-dwarf | 552 | Intron |
| 11-7 | 56.5** | 3.7 | 8 | Semi-dwarf | 491 | S164F |
| 6-ex | 67.0* | 8.0 | 3 | Semi-dwarf | 1127 | A302T |
| 4-6 | 74.6* | 2.0 | 5 | Semi-dwarf | 386 | G129D |
| 1-4 | 78.3 | 3.0 | 4 | Intermediate | 1220 | V334M |
| 10-6 | 87.6 | 1.8 | 10 | Xtall | 800 | D244N |
| 6-2 | 88.3 | 1.8 | 8 | Xtall | 1259 | G346E |
| 15-3 | 91.0 | 4.0 | 3 | Xtall | 398 | A133V |
| 15-8 | 91.4 | 1.8 | 8 | Xtall | 427 | E143K |
| 1-3 | 94.0 | 1.4 | 8 | Xtall | 1261 | L347F |
| 19-1 | 97.0 | 1.5 | 3 | Xtall | 770 | D234N |
| 5-1 | 81.1 | 0.9 | 8 | Intermediate | 898 | Splice site |
| 14-7 | 86.7 | 2.3 | 3 | Xtall | 898 | Splice site |
| 4-5 | 88.3 | 1.7 | 3 | Xtall | 573 | Splice site |
| 3-2 | 101.0 | 0.6 | 3 | Xtall | 899 | Splice site |
| 3-8 | 91.8 | 1.7 | 8 | Xtall | 384 | W128* |
| 7-4 | 97.6 | 1.6 | 8 | Xtall | 249 | W83* |
| 13-7 | 101.1 | 1.2 | 8 | Xtall | 384 | W128* |
| 18-6 | 109.3 | 1.5 | 4 | Xtall | 1240 | Q340* |
| 9-4a | 56.1 | 2.8 | 7 | Semi-dwarf | 504 | Splice site |
Type of mutation event and position in GA2oxA13 listed for overgrowth mutants. Height classes defined as Dwarf (34 cm, =Halberd Rht12); Semi-dwarf (40–75 cm); Intermediate (75–82 cm—height of Halberd); Xtall (87–110 cm, taller than Halberd). Mutation in 9-4 was isolated at a heterozygous level. Lines that were significantly shorter than Halberd are indicated as follows (t-test): *significance level <0.05; **significance level <0.01.
a Line 9-4 was isolated as a heterozygous mutant.

Final plant height of selected overgrowth mutants that were generated in the Halberd Rht12 background. (This figure is available in color at JXB online.)
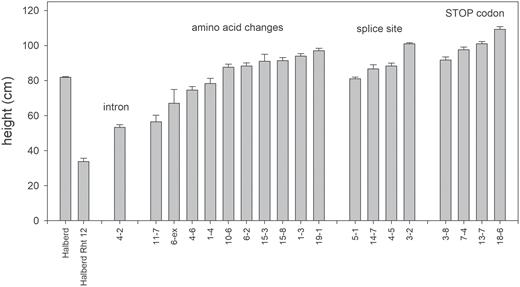
Means of plant height (cm) of Halberd, Halberd Rht12, and selected mutant lines at maturity including standard errors. Mutants are grouped according to mutation events: first intron, predicted amino acid changes, splice acceptor site mutations, and the introduction of early termination codons.
Identification of a candidate gene and functional proof
To identify a candidate gene for Rht12, we used the GA2oxA9 sequence to search the Chinese Spring wheat reference genome for a GA 2-oxidase gene member in the Rht12 region on chromosome 5AL (Sutka and Kovacs, 1987; Worland et al., 1994; Korzun et al., 1997). Blast searches identified the candidate gene TraesCS5A02G543100 within the Rht12 region, which was closely related to the previously described GA2ox13 in the homoeologous position on chromosome 4BL and related (63% amino acid identity) to the GA2oxA9 gene member at the Rht18 locus (Pearce et al., 2015). The predicted ORF had 357 amino acids spanning three exons (Fig. 3). We adopted the nomenclature published in Pearce et al. (2015) and named the gene GA2oxA13. The same gene was previously named GA2oxA14 and reported as a candidate gene for Rht12 dwarfism without functional proof using transgenic studies and/or characterization of mutants (Sun et al., 2019).
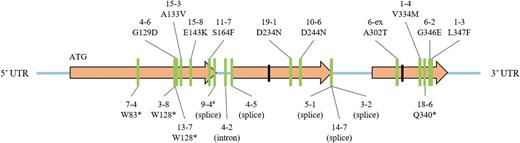
Graphical representation of gene structure and the positions of mutations in the GA2oxA13 gene (ORF 1596 bp). The area between black bars is highly conserved in the GA 2-ox gene family. The line marked with # was isolated as a heterozygous mutant. (This figure is available in color at JXB online.)
A single genetic change in GA2-oxA13 was identified in each one of the 28 ovg lines, including single nucleotide changes in 20 lines (Table 1; Supplementary Table S1 at JXB online) and putative deletion events in an additional eight lines (Supplementary Fig. S1). The results in Table 1 and Fig. 3 show lines with predicted amino acid changes, splice site mutations, and early termination codons, and one line with a mutation in the first intron. Based on these results, we conclude that mutations in GA2oxA13 were responsible for the increased height of ovg mutants. The predicted ORF of GA2oxA13 was identical in both tall and dwarf (Rht12) Halberd lines, suggesting that increased expression of GA2oxA13 was responsible for Rht12 dwarfism, analogous to the previously reported mechanism for Rht18 (Ford et al., 2018). The expression analysis of GA2oxA13 confirmed that the transcript level was higher in the Rht12 dwarf and derived tall ovg mutant 18-6 compared with Halberd (Fig. 4). To confirm that Rht12 dwarfism was due to a lower GA content, bioactive GA1 was quantified in the same lines. As predicted, the GA1 content was lower in the Rht12 dwarf compared with the very tall ovg line, while the GA1 content in Halberd was intermediate, consistent with its height phenotype (Fig. 4). Based on amino acid sequence comparisons with GA2ox-A9, which was shown to catabolize the GA precursor GA12, the predicted GA2oxA13 protein also belongs to the C20 GA 2-oxidase class that removes GA precursors early in the biosynthetic pathway (Fig. 5). We therefore predict that the content of the GA12 substrate would be lower in Rht12 dwarf than in the tall, consistent with results of Sun et al. (2019), and that the content of the GA110 product would be increased in the dwarf, although this GA metabolite was not measured in the previous study.
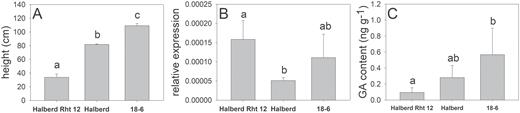
(A) Final plant height, (B) relative expression of GA2oxA13, and (C) GA1 content (ng g–1 FW) in elongating peduncles of Halberd Rht12, Halberd, and ovg mutant 18-6. Significance level <0.05.
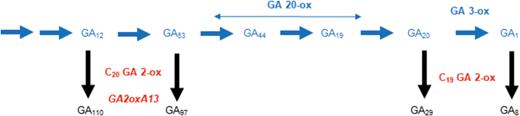
GA biosynthetic pathway showing GA precursors that are being converted by GA 20-oxidase and GA 3-oxidase enzymes to bioactive GA1. C20 GA 2-oxidase and C19 GA 2-oxidase are the main catabolic enzymes converting GA precursors into inactive forms. We predicted that Rht12 increased expression of GA2oxA13, lowering GA12 content and thereby reducing the biosynthetic flux through the pathway and lowering GA1 content. Mutations within GA2oxA13 knock out or reduce the function of predicted proteins which increased GA1 content in overgrowth mutants. (This figure is available in color at JXB online.)
Relationships between type of mutation and final height
The target gene could not be amplified using gene-specific primers (see above) in eight tall ovg lines with heights between 102 cm and 118 cm (Supplementary Fig. S1). Using the same DNA samples, we confirmed the amplification of a control gene. We concluded that GA2oxA13 was deleted, resulting in a tall phenotype. Mutants with early termination mutations in GA2oxA13 that resulted in a truncated protein with predicted loss of function were between 92 cm and 109 cm, similar in height to the deletion mutants and taller than the Halberd control (t-test, P<0.01).
We identified several mutations in predicted splice acceptor sites. Two independent mutations (mutants 5-1 and 14-7) at nucleotide position 898 resulted in intermediate/tall plant heights (81.1 cm and 86.7 cm), suggesting that at least some of the transcripts were correctly spliced; however, a mutation at position 899 in mutant 3-2 resulted in an average height of 101 cm, which was significantly taller than the lines with mutations at position 898 (t-test: P<0.01). In contrast to nucleotide position 898, the ‘G’ residue at position 899 is highly conserved at the core of the acceptor splice site. Mutations at this position are therefore expected to show a strong phenotype, similar to mutants with predicted early termination codons.
Several mutants were identified with predicted amino acid changes in GA2oxA13. Most of these amino acid changes were associated with tall, loss-of-function phenotypes except in three mutants, 11-7, 6-ex, and 4-6, which were semi-dwarfs. The predicted amino acid change in mutant 11-7 (S164F) was mapped to the surface of a predicted 3D structure of a closely related anthocyanidin synthase, consistent with the hypothesis that the mutation did not substantially alter enzyme function because the surface residue was remote from the active site (Fig. 6). It is also possible that random background mutations compromised growth in mutants with semi-dwarf or intermediate height. Mutant 4-2 had a semi-dwarf phenotype which contained a nucleotide change in the first intron. It is possible that the induced variant reduced transcript stability that lowered protein abundance with only a marginal effect on height.
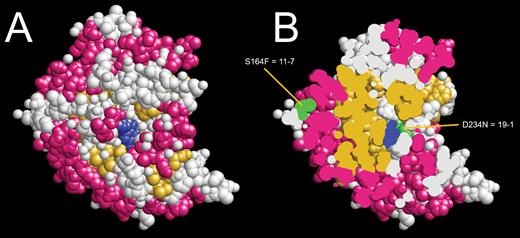
(A) Predicted protein structure of anthocyanidin synthase 2BRT closely related to GA2OXA13. (B) Protein section showing the position of S164F mutation in mutant 11-7 near the surface. (This figure is available in color at JXB online.)
The Rht12 gene is fully dominant
We confirmed the dominant gene action of Rht12 in the absence of potential background effects by measuring the final height in progeny of a mutant 9-4 that was heterozygous for a mutation in GA2oxA13 (splice site mutation) (Sutka and Kovacs, 1987). The average height of heterozygous lines (61.9±3.4 cm SE) was similar to the height of the homozygous dwarf progeny (62.3±6.5 cm SE), indicating that overexpression of one functional gene copy was sufficient for complete dwarfism (Supplementary Table S1). In contrast, the average height of progeny homozygous for the mutation almost doubled, reaching 118.5 cm (±3.5 SE).
Discussion
The results presented here support the hypothesis that Rht12 dwarfism is due to a lower content of bioactive GA1. We are proposing a similar mechanism for height reduction in Rht12 to that previously reported for Rht18 (Ford et al., 2018). An increase in expression of GA2oxA13 reduced the flux through the GA biosynthetic pathway, resulting in a lower content of GA1 and less growth (Fig. 5). Our findings confirm the candidate gene for Rh12 previously reported by Sun et al. (2019) who showed that dwarfism was associated with increased expression of GA2oxA13 and a lower GA content. The current study provides additional evidence by reporting loss-of-function mutations within GA2oxA13 that resulted in tall phenotypes with an associated increase in GA1 content (Fig. 4). The fact that 19 independent ovg lines examined showed a single nucleotide substitution provides proof of function that GA2oxA13 was responsible for regulating height (Figs 2, 3).
Eight of the selected ovg mutants appear to be deletions of the gene. Miraghazadeh et al. (2016) previously reported that approximately half of the ovg mutants isolated in the Rht-B1c dwarfing background were due to deletions with a large range in sizes. It remains unclear whether these deletion events are spontaneous in the population or increased in frequency by the mutagenesis treatment.
The molecular basis of the original Rht12 mutation, which increased expression of GA2oxA13, remains unknown. Similarly, the mutations which caused an increase in expression of GA2oxA9 and result in Rht18 and Rht14 dwarfism have not yet been identified (Ford et al., 2018). The three mutant dwarfing alleles Rht12, Rht14, and Rht18 arose in separate studies in tetraploid and hexaploid wheat following treatment with ionizing radiation (Viglasi, 1968; Konzak, 1982; Gale et al., 1985). These three independent mutational events involve elevated expression of two closely related members of the GA 2-oxidase gene family, one in the telomeric region of chromosome 5A and the other in the centromeric region of chromosome 6A. Their dominant gene action indicates that transcriptional activation of one copy is sufficient to achieve a full reduction in final height. Some molecular models to explain increased expression, for instance the gene now being in a more accessible region of chromatin, might be expected to result in semi-dominance rather than dominance. Other models involving differences in binding of regulatory factors may more easily fit with a dominant phenotype. Since mutation is likely to represent a loss (rather than a gain) of molecular function, we can speculate that in the wild type the expression of these two different GA 2-oxidase genes is maintained at low levels by the binding of transcriptional repressor(s) to regulatory regions of DNA. In the mutants, the transcriptional repressor no longer binds because of DNA sequence changes. It is worth noting that Rht12 and Rht18/14 are semi-dwarfs, indicating that by increasing the expression of GA2oxA13 or GA2oxA9, bioactive GA1 can still accumulate, albeit at a reduced level, to promote growth. Current experimental work focuses on identifying the molecular basis of random mutations that activate transcription of GA 2-oxidase genes.
Some of the ovg mutants with predicted loss-of-function mutations in GA2oxA13 were up to 30% taller than the original Halberd parent, suggesting that the low expression of GA2oxA13 contributed to height reduction in Halberd (Fig. 1). Low expression of GA2oxA13 was also observed in stems of tall Chinese wheat Ningchun45, but the expression was at non-detectable levels in early stems (jointing stage) of Chinese Spring and in peduncles of Azuhurnaya wheat lines (www.wheat-expression.com; Borrill et al., 2016; Ramírez-González et al., 2018; Sun et al., 2019; Chen et al., 2020). The expression in Halberd and Ningchun45 was measured by qRT-PCR, and, in the case of Halberd, in the basal half of the elongating peduncle at 50% of its final length. It is possible that the reported lack of expression in Chinese Spring and Azuhurnaya was due to differences in tissue harvesting, or that the transcriptome was sequenced at insufficient depth. It is also possible that the regulation of this gene varies across wheat genotypes. We previously observed ovg mutants with loss-of-function mutations in GA2oxA9 (chromosome 6A) that were taller than parental lines in Halberd and durum wheat backgrounds (Ford et al., 2018). The expression of GA2oxA9 was low but detectable by qRT-PCR in Halberd and three durum backgrounds, suggesting that this gene may have also contributed to regulating height. The expression of GA2oxA9 was also detectable in stem tissue of Chinese Spring and Azuhurnaya, indicating that the expression level of this gene in general is higher than that of GA2oxA13 (Ramírez-González et al., 2018).
Several alternative dwarfing genes have been described and assessed for their potential to replace the common DELLA genes Rht-B1b and Rht-D1b in wheat. The Rht12 mutant was first reported in the Hungarian winter wheat Karcagi in 1968 and transferred into adapted germplasm before being evaluated for agronomic performance in the UK, China, and Australia (Worland et al., 1994; Rebetzke et al., 2012; Chen et al., 2013). In some of these studies, height reduction associated with Rht12 was too severe and translated into yield penalties. The strong effect of Rht12 on growth was also observed in early seedlings, resulting in plants with reduced leaf area (Rebetzke et al., 2012). However, Rht12 had no effect on coleoptile length, giving the gene a distinct advantage over DELLA mutants, particularly for deployment in dry, rain-fed environments where emergence may be compromised due to the lack of adequate soil moisture. Ovg mutants that were generated in this study represent novel genetic variation and provide breeders with more options to utilize Rht12. Some of the mutants with attenuated function of GA2oxA13 are taller semi-dwarfs than the original Rht12. These semi-dwarfs are expected to also show less reduction in early leaf area, making them more attractive to breeders as a potential replacement for DELLA dwarfing genes in wheat.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Final height of overgrowth mutants with putative deletion events.
Fig. S2. Amino acid sequence alignments of GA2ox proteins.
Table S1. Final height (cm) of progeny derived from mutant 9-4.
Abbreviations
Acknowledgements
We thank Greg Rebetzke for providing germplasm which was used for mutagenesis in this study. We also thank an anonymous reviewer for detailed and constructive comments.
Author contributions
WB contributed to investigation, formal analysis, and writing of the original draft. BF contributed to investigation and formal analysis. EF contributed to investigation, formal analysis, and methodology. WSch contributed to investigation and formal analysis. PB contributed to formal analysis and data curation. BB contributed to investigation. ARA contributed to methodology and formal analysis. PC contributed to conceptualization, writing, review, and editing. WSp contributed to supervision, conceptualization, writing, review, and editing.
Data availability
All data supporting the findings of this study are available within the paper and within its supplementary data published online.




Comments