-
PDF
- Split View
-
Views
-
Cite
Cite
Jiaojiao Wu, Chuang Wang, Luqing Zheng, Lu Wang, Yunlong Chen, James Whelan, Huixia Shou, Ethylene is involved in the regulation of iron homeostasis by regulating the expression of iron-acquisition-related genes in Oryza sativa, Journal of Experimental Botany, Volume 62, Issue 2, January 2011, Pages 667–674, https://doi.org/10.1093/jxb/erq301
Close - Share Icon Share
Abstract
Plants employ two distinct strategies to obtain iron (Fe) from the soil. In Strategy I but not Strategy II plants, Fe limitation invokes ethylene production which regulates Fe deficiency responses. Oryza sativa (rice) is the only graminaceous plant described that possesses a Strategy I-like system for iron uptake as well as the classic Strategy II system. Ethylene production of rice roots was significantly increased when grown under Fe-depleted conditions. Moreover, 1-aminocyclopropane-1-carboxylic acid (ACC) treatment, a precursor of ethylene, conferred tolerance to Fe deficiency in rice by increasing internal Fe availability. Gene expression analysis of rice iron-regulated bHLH transcription factor OsIRO2, nicotianamine synthases 1 and 2 (NAS1 and NAS2), yellow-stripe like transporter 15 (YSL15) and iron-regulated transporter (IRT1) indicated that ethylene caused an increase in transcript abundance of both Fe (II) and Fe (III)-phytosiderophore uptake systems. RNA interference of OsIRO2 in transgenic rice showed that ethylene acted via this transcription factor to induce the expression of OsNAS1, OsNAS2, OsYSL15, and OsIRT1. By contrast, in Hordeum vulgare L. (barley), no ethylene production or ethylene-mediated effects of Fe response could be detected. In conclusion, Fe-limiting conditions increased ethylene production and signalling in rice, which is novel in Strategy II plant species.
Introduction
Iron (Fe) is predominantly present as oxidized Fe (III) compounds in soils, which are poorly soluble under high pH conditions and not readily available to plants (Kim and Guerinot, 2007). Under Fe-deficiency conditions, plants induce morphological and physiological changes to maximize iron uptake and utilization. Higher plants have evolved two strategies for Fe acquisition. The reduction strategy, Strategy I, is employed by all plant species except the grasses. The Strategy I approach involves pumping protons by H+-ATPases to acidify the rhizosphere and increase Fe solubility in the soil. A ferric chelate reductase (FRO) reduces Fe (III) to Fe (II), and Fe (II) transporters (IRTs) transport Fe into cells (Kim and Guerinot, 2007; Walker and Connolly, 2008; Giehl et al., 2009). Grass plants use the chelation strategy (Strategy II) (Kim and Guerinot, 2007; Walker and Connolly, 2008). In this approach, grass plants synthesize and release mugineic acid family phytosiderophores (MAs) from their roots to chelate Fe (III). The Fe (III)–MA complexes are then taken up through Fe (III)–MA transporters (Curie et al., 2001; Murata et al., 2006; Lee et al., 2009; Inoue et al., 2009).
Rice is one of the most important grasses, responsible for approximately 21% of the calorific supply of the world's population, which reaches almost 80% in some South-East Asian countries. As in other graminaceous plants, rice employs a Strategy II system for Fe uptake through the Fe (III)–MAs system. MAs are synthesized from methionine via nicotianamine (NA). The expression of genes that encode key enzymes of this pathway are induced by Fe deficiency in rice and barley with a significant increase in MAs synthesis (Inoue et al., 2003; Kobayashi et al., 2005; Bashir et al., 2006; Nagasaka et al., 2009). The levels of transcripts that encode Fe (III)–MAs transporters, ZmYS1, OsYSL15, and HvYS1 in maize, rice, and barley, respectively, are also increased under Fe-deficient conditions (Curie et al., 2001; Lee et al., 2009; Ueno et al., 2009). Mutation in the rice nicotianamine aminotransferase gene (naat1) diminished the production of 2′-deoxymugineic acid (DMA) and Fe (III) uptake (Cheng et al., 2007). However, naat1 rice plants grow normally when Fe (II)-EDTA was supplied, indicating that naat1 rice plants can use Fe (II) as an iron source. It has been shown that rice can directly absorb Fe (II) using a Strategy I-like system (Ishimaru et al., 2006, 2007; Cheng et al., 2007). Two Fe-deficiency induced genes from rice, OsIRT1 and OsIRT2, have been identified as Fe (II) transporters (Ishimaru et al., 2006).
It has been shown that ethylene production is induced in roots by Fe deficiency in Strategy I plants (Romera et al., 1999; Romera and Alcantara, 2004). Treatment of Arabidopsis, tomato, and cucumber plants with 1-aminocyclopropane-1-carboxylic acid (ACC), the immediate precursor of ethylene, induced the expression of ferric reductase (AtFRO2, LeFRO1, CsFRO1), iron transporter (AtIRT1, LeIRT1, CsIRT1), H+-ATPases (CsHA1), and transcription factors (LeFER, AtFIT), that regulate the iron deficiency response. Conversely, the addition of ethylene inhibitors, such as silver thiosulphate (STS), Co2+, aminoethoxyvinylglycine (AVG), and aminooxylacetic acid (AOA), markedly repressed the expression of these genes (Romera and Alcantara, 1994; Lucena et al., 2006; Waters et al., 2007). Thus, Fe limitation enhanced the ethylene production that, in turn, increases Fe acquisition mediated via LeFER (or AtFIT) transcriptional regulation (Lucena et al., 2006; Giehl et al., 2009).
In contrast to Strategy I plants, research indicates that ethylene is not involved in the regulation of Fe-deficiency stress responses by Strategy II plants (Welch et al., 1997; Romera et al., 1999). As rice has both Strategy I and II systems for Fe uptake, ethylene may be involved in regulating the Fe-deficiency responses. The expression of several rice ACC synthases (ACS) and ACC oxidases (ACO) were up-regulated under Fe-deficient conditions as determined from studies using microarray analysis (Zheng et al., 2009), suggesting a role for ethylene in signalling under Fe-limited conditions. In this study, it has been demonstrated that ethylene is involved in the Fe-deficiency response in rice, but not in barley.
Materials and methods
Plant materials, growth conditions, and treatments
Rice (Oryza sativa L. cv. Nipponbare) seeds were germinated in tap water for 2 d and transferred into culture solution prepared as previously described by Yoshida et al. (1976). The initial nutrient solution contained 1.425 mM NH4NO3, 0.323 mM NaH2PO4, 0.513 mM K2SO4, 0.998 mM CaCl2, 1.643 mM MgSO4, 0.009 mM MnCl2, 0.075 μM (NH4)6Mo7O24, 0.019 mM H3BO3, 0.155 μM CuSO4, 0.070 mM citric acid, and 0.152 μM ZnSO4 with 0.125 mM EDTA-Fe(II) or no Fe. Seedlings were grown in a growth chamber at 30/22 °C day/night temperatures with a 12/12 h light/dark regime (450 mmol photons m−2 s−1) until they were 3 weeks old. Seedlings were then transplanted to 1.0 l glass vessels with different treatments. Nutrient solution was refreshed daily and the pH of the solution was adjusted to 5.5. 1 μM of ACC, 50 μM STS, or 50 μM CoCl2 were added as indicated.
Barley (Hordeum vulgare L.) genotype CV Zhemai 1 was used in this study. Seeds were surface-sterilized in 2% (v/v) H2O2 for 10 min, rinsed several times with deionized water, and then germinated in hermetically-sealed plastic containers with sterilized moist quartz sand at 20 °C with a 12/12 h light/dark regime (450 mmol photons m−2 s−1). Germinated seeds were transferred into a hydroponic solution culture for 5 d. The composition of the basic nutrient solution was 0.365 mM (NH4)2SO4, 0.547 mM MgSO4, 0.091 mM K2SO4, 0.183 mM KNO3, 0.365 mM Ca(NO3)2, 0.182 mM KH2PO4, 0.02 mM Fe-citrate, 4.5 μM MnCl2, 0.38 μM ZnSO4, 0.16 μM CuSO4, 0.047 mM HBO3, and 0.056 μM H2MoO4. The pH of the solution was adjusted to 6.5 every other day. Five-day-old seedlings were transferred into solution cultures with or without Fe for 1 week prior to sampling. All physiological experiments were carried out in triplicate.
Measurement of chlorophyll content
Soil–plant analyser development (SPAD) values were determined on the fully expanded youngest leaves of 3-week-old seedlings with a portable chlorophyll meter (SPAD-502, Minolta Sensing, Japan).
Measurement of ethylene production
Ethylene production in the roots of rice seedling was analysed using intact roots of treated seedlings by placing detached roots into 15 ml glass vials containing 1 ml water and rapidly sealing with a gas-proof septum. The sealed vials were incubated in a dark growth chamber for 4 h at 30 °C. One millilitre of gas was withdrawn from the airspace of each vial using a gas-tight syringe (Focus GC, Thermo, USA) and injected into a gas chromatograph (Focus GC, Thermo, USA) equipped with a capillary column (CP-carboPLOT P7, Varian, CA, USA) and flame-ionization detector for ethylene determination. Ethylene production was calculated on the basis of fresh weight (FW) of root samples.
Measurement of the total and soluble Fe
To determine total Fe roots of seedling plants were washed three times with deionized water and dried at 80 °C. Dried samples were ground to fine powders and digested with 5 ml of 11 M HNO3 for 5 h at 150 °C. Fe concentrations were measured using Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Agilent 7500ce, CA, USA).
Extraction of water-soluble Fe was performed as previously described (Zheng et al., 2009). Briefly, approximately 0.5–1 g of shoot samples were ground in liquid nitrogen and extracted in 5 vols of deionized water at room temperature. After centrifugation, the supernatant was recovered. Fe concentration was measured as described above.
Construction of OsIRO2 RNA interfering (RNAi) rice
The OsIRO2 RNA interfering construct was made using the Gateway™ cloning system according to the manufacturer's recommendations (Invitrogen, Carlsbad, CA, USA). In brief, a fragment of OsIRO2 was amplified from cDNA (the reverse transcript from total RNA as described below) with primers IRO2-F (5′-CACCTCGTGCAACATCAGCTCACT-3′) and IRO2-R (5′-AGCAGAGGCGGATGATCTCCT-3′). The amplified fragment was cloned into pENTR/D-TOPO vector (Invitrogen, Carlsbad, CA, USA) and verified by DNA sequencing. The cDNA insert was introduced into the final vector pH7GWIWG2 (Karimi et al., 2002) in both sense and antisense orientations to construct the binary vector of the OsIRO2 RNA interfering construct. The above construct was used to generate OsIRO2 RNAi transgenic plants via Agrobacterium-mediated transformation as previously described (Chen et al., 2003; Wang et al., 2008).
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted from plant samples using the TRIzol Reagent (Invitrogen, CA, USA) according to the manufacturer's recommendations. First-strand cDNAs were synthesized from total RNA using SuperScript II reverse transcriptase (Invitrogen, CA, USA). Quantitative RT-PCR (qRT-PCR) was performed using SYBR Premix Ex Taq™ (Perfect Real Time) Kit (TaKaRa Biomedicals, Tokyo, Japan) on a LightCycler 480 (Roche Diagnostics, Basel, Switzerland), according to the manufacturer's instructions. Triplicate quantitative assays were performed at each cDNA sample. The housekeeping gene OsACTIN was used as an internal control. The relative level of expression was calculated using the formula 2–ΔΔCt. All the primers and annealing temperatures used for these PCR reactions are given in Supplementary Table S1 at JXB online.
Statistical analysis of data
Data were compared with one-way analysis of variance and differences between groups were compared with LSD t test (P <0.05).
Results
Iron deficiency induced ethylene production in rice roots
To investigate the effect of Fe supply on ethylene production in rice, 3-week-old rice seedlings were transferred in nutrient solution without Fe for 5 d. A Minolta SPAD-502 meter was used to measure the relative chlorophyll content of leaves. Five days of Fe deprivation (–Fe) resulted in a significant decline of chlorophyll concentration and induced chlorotic symptoms in newly developed leaves (Fig. 1A, B). The Fe concentration in shoots decreased significantly (Fig. 1C). Re-supply of Fe for 2 d restored the Fe concentration to normal levels (Fig. 1C), indicating that the Fe concentrations in leaves could readily be restored following Fe re-supply. The level of ethylene production was inversely correlated with the Fe concentration in plants (Fig. 1D). While removal of Fe from the medium significantly increased the ethylene production from 2.43 to 3.89 nl g−1 FW h−1 (Fig. 1D), re-supply of Fe for 2 d resulted in ethylene production returning to normal levels (Fig. 1D).
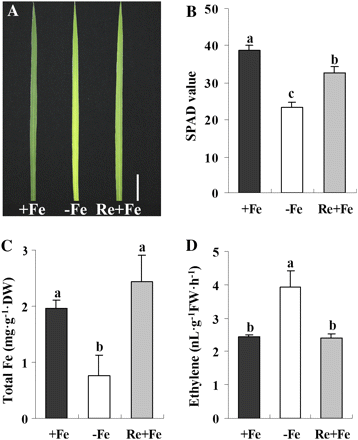
Effects of Fe deficiency and resupply on rice seedlings. Three-week-old seedlings were transferred to Fe-depleted (–Fe) nutrient solution for 5 d. The –Fe treatment caused leaf chlorosis (A) Bar=3 cm), decreased SPAD value (B), decreased total Fe content (C), and increased root ethylene production (D). Two days after Fe was resupplied (Re+Fe) to the seedlings, all the above changes were reversed (A–D). Values are the means ±standard error of three replicates. Points with different letters are significantly different at P <0.05 (LSD test, P <0.05). (This figure is available in colour at JXB online.)
Iron deficiency induced the expression of specific OsACS and OsACO genes
There are six putative ACS genes and seven putative ACO genes in the rice genome (Rzewuski and Sauter, 2008). AtACS10 and AtACS12, which display aminotransferase activity instead of ACS activity (Yamagami et al., 2003). According to phylogenetic analysis of the Arabidopsis and rice ACS gene family, OsACS6 is the closest orthologue of AtACS10 and AtACS12 (Iwai et al., 2006). The gene was excluded from the analysis due to putative function loss of ACS activity. OsACO6 was also excluded because it is a pseudogene (Rzewuski and Sauter, 2008). Our previous microarray data indicated that the transcription of ACS and ACO genes are induced by Fe deficiency (see Supplementary Table S2 at JXB online; Zheng et al., 2009). To determine which of these genes are responsible for the induction of ethylene production by Fe deficiency, the expression of ACS and ACO genes were analysed by qRT-PCR. Five days of Fe deprivation resulted in an increase of expression for OsACS1, OsACS2, OsACS3, OsACO1, OsACO2, OsACO3, and OsACO7 (Fig. 2).
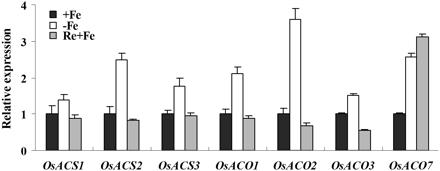
Effect of Fe deficiency and resupply on expression of ethylene biosynthesis enzymes. Three-week-old seedlings were transferred to Fe-depleted (–Fe) nutrient solution for 5 d and were resupplied with Fe for 2 d. After treatment, total root RNA was extracted and used to synthesize cDNA. qRT-PCR was performed using gene-specific primers. Values are the means ±standard error of three replicates.
To test if the induction of ACS and ACO gene expression could be reversed by the re-supply of Fe, seedlings grown in –Fe nutrient solution for 5 d were transferred back to medium with 0.125 mM EDTA-Fe(II) for 2 d. qRT-PCR analysis indicated that the expression of OsACS1, OsACS2, OsACS3, OsACO1, OsACO2, and OsACO3 were restored to the normal (Fig. 2). Strikingly, expression of OsACO7 remained high after Fe re-supply. The marked changes in OsACS2, OsACS3, OsACO1, and OsACO2 expression mirrored changes in ethylene levels, suggesting that these ACS and ACO genes are responsible for the increased of ethylene synthesis in roots under Fe depletion.
Ethylene contributes to iron-deficiency tolerance in rice
To evaluate the role of ethylene on iron homeostasis in rice plants, 3-week-old seedlings were transferred to either a normal or an Fe-deficient nutrient solution supplemented with 1 μM ACC. While 10 d of Fe deprivation resulted in a visible chlorotic leaf phenotype, application of ACC alleviated the symptoms (Fig. 3A–C). Under Fe-sufficient condition, the SPAD values of seedlings with or without ACC supply were similarly high (Fig. 3B). The SPAD value in the seedlings of –Fe+ACC treatment was significantly higher than that of –Fe (Fig. 3B). Analysis of the total and soluble Fe concentrations of seedlings in +Fe, +Fe+ACC, –Fe, and –Fe+ACC showed that the addition of ACC did not change the total Fe concentrations (Fig. 3D), but significantly increased soluble Fe concentration in the +Fe- and –Fe-treated seedlings (Fig. 3E).
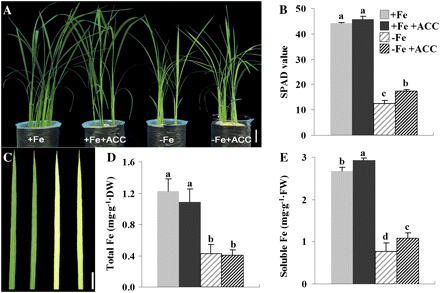
Physiological responses of rice seedlings to ACC treatment under both Fe-sufficient and -deficient conditions. Three-week-old seedlings were transferred to +Fe and –Fe nutrient solution with or without 1 μM ACC for 10 d. (A–E) Growth performance, SPAD values, leaf chlorosis phenotypes, and total and soluble Fe concentrations, respectively. Bar=5 cm (A) and 3 cm (C). Values are the means ±standard error of three replicates. Points with different letters are significantly different at P <0.05 (LSD test, P <0.05).
Regulation of Fe deficiency response genes by ethylene in rice
To determine whether ethylene participates in –Fe responsive signalling, the expression of genes typically induced in response to Fe deficiency, OsIRO2, OsNAS1, OsNAS2, OsYSL15, OsIRT1, and IDEF1 and ethylene responsive element binding protein-encoding genes OsEBP1 (Loc_Os03g64260) and OsEBP89, were analysed by quantitative RT-PCR in roots grown in normal or Fe-deficient medium supplemented with 1 μM ACC. Under Fe-sufficient conditions, the expression of both Fe deficiency and ethylene-responsive genes was strongly induced by ACC treatment (Fig. 4). Although Fe deficiency induced the expression of OsIRO2, OsNAS1, OsNAS2, OsYSL15, OsIRT1, OsEBP1, and IDEF1, addition of ACC further increased these inductions (Fig. 4). Fe deficiency has no effect on the expression of ethylene responsive gene OsEBP89.
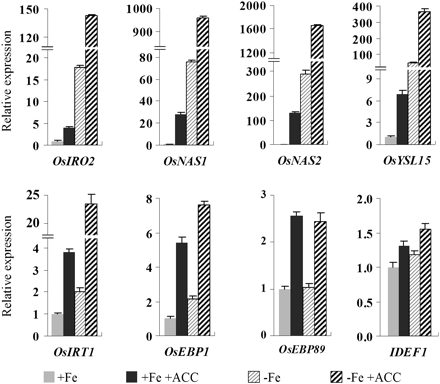
Regulation of OsIRO2, OsNAS1, OsNAS2, OsYSL15, OsIRT1, OsEBP1, OsEBP89, and OsIDEF1 expression by Fe deficiency and precursors of ethylene synthesis in rice seedlings. Three-week-old seedlings were transferred to +Fe and –Fe nutrient solution incubated with or without 1 μM ACC for 5 d. After treatment, total root RNA was extracted and used to synthesize cDNA. qRT-PCR was performed using gene-specific primers. Values are the means ±standard error of three replicates.
To confirm the involvement of ethylene signalling in the induction of these genes, inhibitors of ethylene signalling, STS and CoCl2, were added to the nutrient solution. Addition of both inhibitors repressed the induction of Fe deficiency response genes by –Fe (Fig. 5). Application of the ethylene inhibitors has no significant effect on the total or soluble Fe concentration in rice seedling (data not shown).
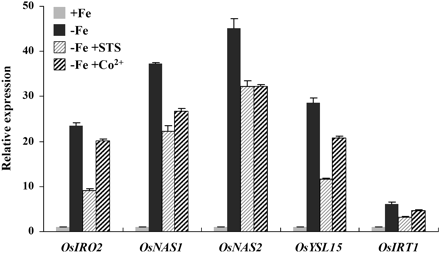
Effect of ethylene inhibitors on the expression of Fe-deficiency responsive genes. Three-week-old seedlings were transferred to +Fe and –Fe nutrient solution for 5 d. Some of the –Fe plants were treated with 50 μM STS or 50 μM CoCl2. After treatment, total root RNA was extracted and used to synthesize cDNA. qRT-PCR was performed using gene-specific primers of OsIRO2, OsNAS1, OsNAS2, OsYSL15, and OsIRT1. Values are the means ±standard error of three replicates.
OsIRO2 is required for the ethylene-mediated Fe regulation
OsIRO2 has been reported to be a novel regulator in Strategy II Fe response signalling (Ogo et al., 2006, 2007). To determine whether OsIRO2 was involved in the ethylene-mediated Fe response, transgenic lines with reduced OsIRO2 transcript levels were produced through RNA interference. Out of the ten independent lines generated, two lines (Ri1 and Ri2) with significantly reduced expression of OsIRO2 were selected for the study (Fig. 6). Under Fe-deficient condition, the expression of OsYSL15, OsNAS1, and OsNAS2 but not OsIRT1 were moderately suppressed in RNAi plants compared with wild-type plants (Fig. 6), which is consistent with a previous report (Ogo et al., 2007). Application of ACC greatly induced the expression of these genes in the wild-type seedlings (Fig. 6), but the extent of the induction was much lower in the Ri1 and Ri2 seedlings. This indicates that OsIRO2 is involved in the ethylene-mediated regulation of these Fe deficiency response genes (Fig. 6).
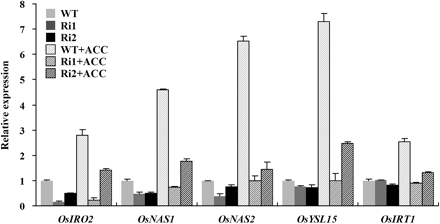
Expression analysis of OsIRO2, OsNAS1, OsNAS2, OsYSL15, and OsIRT1 in OsIRO2 RNAi plants. Three-week-old seedlings were transferred to -Fe nutrient solution with or without 1 μM ACC for 5 d. After treatment, total root RNA was extracted and used to synthesize cDNA. qRT-PCR was performed using gene-specific primers. Values are the means ±standard error of three replicates.
Ethylene is not involved in the Fe deficiency response in barley
To determine whether ethylene production is induced by Fe deficiency in other grass species, 5-d-old barley seedlings were transferred into hydroponic solution cultures with or without Fe. However, ethylene production in barley was not significantly changed by Fe deficiency (Fig. 7A). Application of ACC had no significant effect on the expression of Fe-deficiency response genes in either Fe-sufficient or -deficient conditions (Fig. 7B).
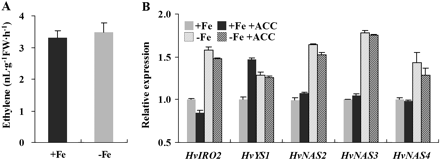
Effect of Fe deficiency on ethylene production (A) and the expression of –Fe responsive genes (B) in barley. Five-day-old seedlings were transferred to +Fe and –Fe nutrient solution with 1 μM ACC for 1 week. After treatment, roots were sampled for ethylene measurements and RNA extraction. qRT-PCR was performed using gene-specific primers. Values are the means ±standard error of three replicates.
Discussion
It has been previously documented that Fe limitation can induce ethylene production and that ethylene signalling can increase Fe acquisition in Strategy I plants but not in Strategy II plants (Romera et al., 1999; Romera and Alcantara, 2004). A similar involvement of ethylene is reported here in the Fe-deficiency response in rice. Fe deficiency induced ethylene production, while a re-supply of Fe reduced ethylene production to normal levels (Fig. 1). The pattern of ethylene production was correlated with the expression pattern of OsACS1, OsACS2, OsACS3, OsACO1, OsACO2, and OsACO3, indicating that these ACS and ACO genes may be responsible for the increase in ethylene synthesis in roots under Fe depletion (Fig. 2). Moreover, ACC treatment induced Fe starvation tolerance in rice seedlings and expression of Fe-deficiency response genes (Figs 3, 4). By contrast, a similar phenomenon was not observed in barley under similar experimental conditions (Fig. 7).
The growth of rice in submerged conditions has resulted in the adoption of a Strategy I-like system to take up Fe (II) directly through Fe (II) transporters (Ishimaru et al., 2006; Cheng et al., 2007). This is in addition to the typical Strategy II iron uptake system through the secretion of mugineic acid family phytosiderophores (MAs) from roots present in grasses (Inoue et al., 2003, 2009; Lee et al., 2009). The genes encoding enzymes that participate in the MAs biosynthetic pathway are highly induced during Fe deficiency (Higuchi et al., 1999; Takahashi et al., 1999; Nakanishi et al., 2000; Kobayashi et al., 2005; Zheng et al., 2009). As both MAs and ethylene are synthesized from the amino acid methionine via the intermediate S-adenosyl-L-methionine (AdoMet), there are potential interactions between MAs secretion and ethylene synthesis. Compared with barley, rice secretes much less MAs from its roots and is more sensitive to iron deficiency (Mori et al., 1991; Ishimaru et al., 2006). This may in part be due to the use of AdoMet in ethylene synthesis. However, both Fe deficiency and ACC treatment strongly induced the expression of OsNAS1 and OsNAS2, which promote MA biosynthesis (Fig. 4). Measurements of the concentration of NA and secreted MA in ACC-treated plants would understand the interactions between ethylene and MA synthesis in rice plants.
In Strategy I plants, ethylene regulates an array of Fe deficiency responses mediated via LeFER (or AtFIT1) transcriptional regulation (Lucena et al., 2006). In this study, it was demonstrated that application of ACC greatly induced the expression of Fe response genes, whereas addition of inhibitors of ethylene signalling or synthesis prevented induction of these genes under Fe-deficiency condition (Figs 4, 5). ACC also significantly induces expression of these Fe deficiency response genes even under conditions with sufficient or normal supply of Fe (Fig. 4). These results demonstrate that the ethylene signalling pathway is involved in the regulation of both Fe (III)-MAs and Fe (II) uptake systems in rice. Although STS or CoCl2 repressed the induction of genes induced under Fe-deficient conditions, the expression of these genes under –Fe+STS or –Fe+Co condition was still higher than that in Fe-sufficient condition (Fig. 5), suggesting the existence of other signal pathway(s) independent of ethylene signalling in Fe deficiency responses, as proposed in Strategy I plants (Lucena et al., 2006; Giehl et al., 2009). OsEBP1 and OsEBP89 are AP2/EREBP (Ethylene Response Element Binding Protein) transcription factors involving ethylene signalling (Yang et al., 2002; Mao et al., 2006). ACC treatment induced expression of OsEBP1 and OsEBP89 under Fe-sufficient and -deficient conditions indicating the activation of ethylene signalling (Fig. 4). Consistent with previous studies (Ogo et al., 2007; Zheng et al., 2009), expression of OsEBP1 was also induced by Fe deficiency. The extent of induction of OsEBP89 expression by the addition of ethylene is much lower than that of OsEBP1. Thus, OsEBP89 is less sensitive to ethylene than OsEBP1, which may have accounted for the fact that the expression of OsEBP89 was not significantly induced by Fe deficiency (Fig. 4). It is also possible that Fe deficiency triggers tissue-specific ethylene signalling, which could not induce the expression of OsEB89. The similar expression pattern of OsEBP1 and other Fe-deficiency response genes indicated that OsEBP1, along with other potential ethylene responsive transcription factors, may act as the convergence point that links ethylene and Fe signalling.
In RNAi lines of OsIRO2 plants, the transcript abundance of OsNAS1, OsNAS2, and OsYSL15 were decreased, but not for OsIRT1 (Fig. 6). This was expected because OsIRO2 is the key regulator of the Fe (III)–MAs uptake system and directly regulates the expression of OsNAS1, OsNAS2, and OsYSL15 under Fe deficiency (Ogo et al., 2007). Analysis of RNAi lines of OsIRO2 indicated that ethylene induction of OsNAS1, OsNAS2, and OsYSL15 was under the control of OsIRO2 (Fig. 6). It is interesting that OsIRO2 also mediates ethylene induced expression of OsIRT1. It has been reported that IDEF1 is the central transcription factor upstream of OsIRO2 and OsIRT1 (Kobayashi et al., 2007, 2009). Overexpression of IDEF1 enhanced Fe-deficiency resistance without changing the total Fe content in rice (Kobayashi et al., 2007), similar to what was observed in this study. Although it has been reported that IDEF1 is constitutively expressed (Kobayashi et al., 2010), it was found that expression of IDEF1 is slightly induced by ACC treatment, especially under Fe-deficient conditions (Fig. 4). It needs to be investigated whether the induction of IDEF1 expression by the application of ACC contributes to the increased expression of Fe deficiency responsive genes and tolerance to Fe deficiency. It is also possible that ethylene may directly regulate OsIRO2 and downstream genes by other transcription factors, such as EILs or EREBPs. We have searched the ethylene responsive elements, GCC-box (Broglie et al., 1989), OsEIL-binding sequence (Hiraga et al., 2009), and PERE (Itzhaki et al., 1994) in the promoters (–1500 bp from the start code) of these genes. The results showed that all the promoters of these genes contained at least one ethylene response cis-acting elements (see Supplementary Table S3 at JXB online). Identification of factors that mediate ethylene-regulated Fe starvation signalling would provide a unifying model to explain the physiological response observed in the study.
Abbreviations
- ACC
1-aminocyclopropane-1-carboxylic acid
- ACS
ACC synthase
- ACO
ACC oxidase
- AdoMet
S-adenosyl-L-methionine
- DMA
2'-deoxymugineic acid
- EREBP
ethylene response element binding protein
- FW
fresh weigh
- MA
mugineic acid
- NA
nicotianamine
- RNAi
RNA interfering
- SPAD
soil-plant analyser development
- STS
silver thiosulphate
- qRT-PCR
quantitative real-time PCR
We gratefully acknowledge Dr Feibo Wu who provided us with the barley seeds and the hydroponic culture protocol for barley used in this study. This work was supported by the National Natural Science Foundation (30770191 and 30871585), the Ministry of Agriculture (2009ZX08010-013B and 2009ZX08009-1238), and Outstanding Young Scientist Funds in Zhejing Province (R3090229).
References
Author notes
These authors contributed equally to this work.




Comments