-
PDF
- Split View
-
Views
-
Cite
Cite
Sergio M. de Faria, Abdala G. Diedhiou, Haroldo C. de Lima, Robson D. Ribeiro, Antoine Galiana, Alexandre F. Castilho, João C. Henriques, Evaluating the nodulation status of leguminous species from the Amazonian forest of Brazil, Journal of Experimental Botany, Volume 61, Issue 11, June 2010, Pages 3119–3127, https://doi.org/10.1093/jxb/erq142
Close - Share Icon Share
Abstract
Numerous leguminous species are used or have potential uses for timber production, pharmacological products, or land reclamation. Through N2-fixation, many leguminous trees contribute to the N-balance of tropical wetlands and rainforests. Therefore, studies of the N2-fixation ability of leguminous species appear to be crucial for the better use and conservation of these resources. The global nodulation inventory in the Leguminosae family is constantly being enriched with new records, suggesting the existence of undiscovered nodulated species, especially in tropical natural ecosystems and other hot spots of biodiversity. In this respect, the nodulation of leguminous species from the Amazonian forest of Porto Trombetas (Brazil) was surveyed. Overall, 199 leguminous species from flooded and non-flooded areas, were examined for their nodulation status by combining field observations, seedling inoculations, and screening of N2-fixing bacterial strains from the collected nodules. The results revealed a tendency for a higher relative frequency of nodulation in the species from the flooded areas (74%) compared with those from the non-flooded areas (67%). Nodulation was observed in the Caesalpinioideae, Mimosoideae, and Papilionoideae, with 25, 88, and 84% of the examined species in each subfamily, respectively. Of the 137 nodulated leguminous species, 32 including three Caesalpinoideae, 19 Mimosoideae, and 10 Papilionoideae are new records. One new nodulated genus (Cymbosema) was found in the Papilionoideae. Twelve non-nodulating leguminous species were also observed for the first time. The results are discussed based on the systematics of the Leguminosae family and the influence of available nutrients to the legume–bacteria symbiosis.
Introduction
The Leguminoseae family is one of the most represented in terms of number and frequency of species in the Amazonian region of Brazil (Ducke, 1949; Prance et al., 1976). Leguminous tree species have been estimated to reach about 40% of the total phytomass in the Amazonian region of French Guyana (Puig et al., 1990). Out of the Amazonian basin, the legume family has also been reported as the most represented in terms of specific diversity among trees of the West African natural rainforest (Diabate et al., 2005). In addition, numerous leguminous species are used or have potential uses for timber production, firewood, pharmacological products, and ornamental and other beneficial usages. Moreover, through their N2-fixation ability, many leguminous trees contribute significantly to the N-balance of tropical wetlands and rainforests (Martinelli et al., 1992; Barrios and Herrera, 1993; Roggy et al., 1999,a, b; Pons et al., 2007), and thus play an important role in the function and ecology of these tropical forests. The capacity of leguminous species to fix nitrogen is also regarded as an important feature of their establishment on nutrient-deficient soils (Moreira et al., 1992) and has, therefore, been exploited for reforestation and land reclamation of waste substrates like iron and gold mining among other substrates devoid of nutrients (Franco and de Faria, 1997).
Nevertheless, despite the substantial importance of known leguminous species both to agriculture and the function of natural ecosystems (Rogers et al., 2009), only 20% have been examined for nodulation, and this feature is more frequent in the subfamilies of the Papilionoideae (∼97%) and Mimosoideae (∼90%) compared to the Caesapinioideae with only about 23% of species examined (de Faria et al., 1989; Sprent, 1995). This, together with the beneficial roles of leguminous species mentioned above, has induced numerous studies about their nodulation status from different forest ecosystems (de Faria and de Lima, 1998; Roggy and Prévost, 1999; James et al., 2001; Perreijn, 2002; Diabate et al., 2005; Ng and Haug, 2009). Although, new records of nodulation in groups where it is uncommon need to be confirmed (Sprent, 2005), most of these studies have reported new nodulated leguminous species. For instance, Diabate et al. (2005) reported, for the first time, the nodulation ability of 31 species and four genera of leguminous trees in the West African tropical rainforest. Among these four new nodulated genera one has been confirmed, while the others are from tribes where nodulation is rare or absent (Sprent, 2005). Ng and Hau (2009) had also contributed to the world nodulation inventory with a new record of five nodulated leguminous species in Hong Kong (China). Previously, Moreira et al. (1992) surveying the nodulation status of 172 leguminous species from different ecosystems within the Amazon region of Brazil had found that 98 nodulated species were new records. Hence, the increase in new records of nodulated tree species in the Leguminosae suggests that this family may include potential nodulated species that have not been investigated yet, particularly in densely colonized areas with high biodiversity, such as the natural rainforests.
In this context, the nodulation status of leguminous species in the district of Porto Trombetas (Central Amazonia, Brazil) was evaluated with the aim of inventorying new nodulated species. The results here presented were obtained after more than 10 years of field and laboratory investigations.
Materials and methods
Studied areas
The survey of the nodulation status of leguminous species in natural conditions took place in the district of Porto Trombetas, Oriximiná Council located in the state of Pará, central Amazonia, Brazil (01° 27' S and 56° 22' W). The areas studied were located 100 km west of the confluence of Rio Trombetas with the Amazon River, and 450 km east from Manaus (Fig. 1). The climate is classified as AF1 with two distinct seasons: winter from December to May and summer from June to November with a long period without rain. The annual precipitation varies from 2500 mm to 3000 mm, and the average temperature is 26 °C. The region is also characterized by a wide distribution of bauxite mining sites, and water courses with flooded areas.
The field investigations were made in the forests located in non-flooded areas (Terra Firme), small rivers (Igapós), and areas in the margins of rivers subjected to annual floods (Várzeas). In the non-flooded areas (Terra Firme), the majority of the expeditions were made in the adjacent forests of bauxite mining sites (Papagaio, Almeidas), reforested areas of the Mineração Rio do Norte, and forests surrounding the districts of Terra Santa and Faro. The district of Campinas Arenosas was also investigated (Fig. 1). The expeditions, when in the flooded areas, were made in the margins of the Trombetas rivers, from Oriximiná to Cachoeira Porteira, including Erepecuru, Mapuera, Nhamundá, Matió, and the margins of numerous lakes (Moura, Batata, Vapor, Ajudante, Jaraoacá, Mãe Cué, do Espelho). In Igapós, the main areas investigated were Almeidas, Nhamundá Araticum, Madeireiro, das Pedras, do Sindicato, Urupanã, and do Cachimbo.
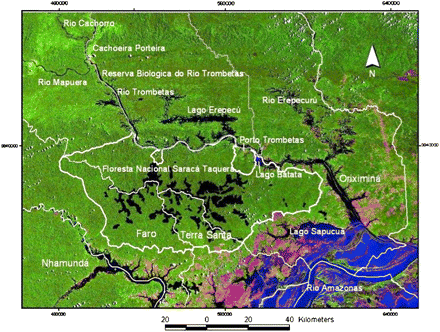
Map of the surveyed areas for nodulation status. (This figure is available in colour at JXB online.)
Survey of the nodulation status
The study of nodulation was carried out following two procedures: firstly through direct field observations, by removing the soil carefully around seedlings, saplings, and mature trees of leguminous species, according to de Faria et al. (1984). Nodules, when found, were collected and kept in a flask containing anhydrous calcium chloride for the preservation of bacterial viability. Nodule morphology (determinate or indeterminate) was noted in all the leguminous species examined. Secondly, the nodulation capacity was examined in greenhouse conditions at Embrapa Agrobiologia (Seropédica, Brazil), using seeds collected from the sampled leguminous trees. This latter procedure was applied (i) to confirm the nodulation ability of leguminous tree species observed in the field conditions and (ii) to examine the nodulation status of leguminous trees, whose roots were not accessible due to floods, particularly in the Igapós and Várzeas areas.
When the seeds were found and harvested, they were planted in plastic bags (300 cm3) containing a vermiculite and sand mixture (1:1 v/v), with the addition of a nitrogen-free nutrient solution (Norris and Date, 1976) every 15 d until the end of the experiment. After sowing, the seedlings were inoculated with a mixture of 200–300 bacterial strains isolated from taxonomically related leguminous species. The nodules formed were collected between 2 months and 4 months later, according to the development of leguminous species (de Faria et al., 1984), nodule morphology was noted, and nodules were conserved as described above. The nodulation status of each leguminous species was ultimately assigned using the GRIN database (http://www.ars-grin.gov/∼sbmljw/cgi-bin/taxnodul.pl) which is based entirely on reports in the literature, whether properly authenticated or not.
All plant materials (leaves, roots, and seedlings) collected from the field and nursery beds were pressed, dried, and catalogued at the Jardim Botânico do Rio de Janeiro Herbarium and the Mineração Rio do Norte Herbarium for correct taxonomic identification and reference.
Screening of N2-fixing bacterial strains
From the nodules obtained by the two procedures of the nodulation survey, bacterial strains were isolated and purified in YMA according to Vincent (1970), and then stored at the Embrapa Agrobiologia bacterial collection. The isolated strains were tested in ‘Leonard jars’ (Gibson, 1980) to confirm whether they were N2-fixing bacteria and, whenever possible, according to the availability of legume seeds, experiments were carried out in order to select the most efficient N2-fixing bacterial strains. Whenever there were not enough seeds of the leguminous species observed in field conditions, the selection of efficient N2-fixing bacterial strains was made with leguminous species which were not directly relevant to our analysis of nodulation ability (see Supplementary Table S2 at JXB online). The procedure of selecting N2-fixing bacterial strains was first made in sterile conditions and then in non-sterile soil conditions (Franco and de Faria, 1997). Hence, more than 1000 bacterial strains were isolated from the harvested nodules. Among them, 171 efficient N2-fixing bacterial strains were selected for 86 leguminous species (see Supplementary Table S2 available at JXB online). Moreover, the efficiency of many N2-fixing bacterial strains, which were previously selected by Franco and de Faria (1997) for different leguminous species, was confirmed.
Results and discussion
A total of 657 specimens of the Leguminoseae family were collected. They fell into 234 species (including 21 infra-species), containing 61 Caesalpinioideae, 79 Mimosoideae, and 94 Papilionoideae (see Supplementary Table S1 at JXB online). Among the legume taxa identified, 46% were exclusively from the flooded areas (Igapós and Várzeas), 45% from the non-flooded areas (Terra firme and Campinas), and 7% from both areas. The remaining 2% represented introduced species which were excluded from the analysis of the nodulation ability of the leguminous species from the flooded and the non-flooded areas. For instance, all species examined (eight species comprising 24 individuals) of the genus Abarema were exclusively sampled from the non-flooded areas. Abarema is a neotropical genus comprising about 46 species which display a wide range of habitats including lowland and mountain humid forests, wooded slopes on Table mountain sandstone, coastal scrubs and woodlands, and rarely riparian flooded areas (Barneby and Grimes, 1996). However, species belonging to the genus Inga seemed to be evenly distributed between the flooded and non-flooded areas, with 16 species (comprising 70 individuals) sampled from the non-flooded areas, 18 species (comprising 86 individuals) from the flooded areas, and 11 species (comprising 52 individuals) common to both areas (Table 1; see Supplementary Table S1 at JXB online). The genus Inga contains about 300 species which are common in tropical and subtropical America from Mexico to Brazil. They thrive in rainforests on non-flooded lands, in riparian habitats on periodically flooded lands, along streams, on lowlands and wet highlands, and rarely in seasonally dry areas (Allen and Allen, 1981; Pennington, 1997; Richardson et al., 2001).
Number of examined leguminous species and their nodulation status (+, nodulated; –, non-nodulated)
| Leguminous subfamily/genus | No. of species examined | No. of species in flooded areas | No. of species in non-flooded areas | No. of species common to both areas | Nodulation status of species |
| Caesalpinioideae | |||||
| Apuleia | 1 | 0 | 1 | 0 | – |
| Batesia | 1 | 0 | 1 | 0 | – |
| Bauhinia | 4 | 3 | 1 | 0 | – |
| Campsiandra | 1 | 1 | 0 | 0 | + |
| Cassia | 3 | 2 | 1 | 0 | – |
| Chamaecrista | 5 | 3 | 2 | 0 | + |
| Crudia | 2 | 2 | 0 | 0 | – |
| Cynometra | 1 | 1 | 0 | 0 | – |
| Dialium | 1 | 1 | 0 | 0 | – |
| Dimorphandra | 3 | 1 | 3 | 1 | + |
| Eperua | 3 | 1 | 2 | 0 | – |
| Heterostemon | 1 | 1 | 0 | 0 | – |
| Hymenaea | 3 | 0 | 3 | 0 | – |
| Macrolobium | 9 | 8 | 1 | 0 | – |
| Martiodendron | 1 | 0 | 1 | 0 | – |
| Peltogyne | 2 | 1 | 1 | 0 | – |
| Senna | 9 | 2 | 7 | 0 | – |
| Tachigali | 5 | 1 | 4 | 0 | + |
| Vouacapoua | 1 | 0 | 1 | 0 | – |
| Mimosoideae | |||||
| Abarema | 8 | 0 | 8 | 0 | + |
| Albizia | 3 | 2 | 1 | 0 | + |
| Balizia | 2 | 0 | 2 | 0 | + |
| Calliandra | 4 | 2 | 2 | 0 | + |
| Dinizia | 1 | 0 | 1 | 0 | – |
| Entada | 1 | 1 | 0 | 0 | + |
| Enterolobium | 2 | 0 | 2 | 0 | + |
| Hydrochorea | 3 | 3 | 0 | 0 | + |
| Inga | 23 | 18 | 16 | 11 | + |
| Macrosamanea | 2 | 2 | 0 | 0 | + |
| Mimosa | 7 | 4 | 3 | 0 | + |
| Parkia | 7 | 1 | 6 | 0 | – |
| Pseudopiptadenia | 1 | 0 | 1 | 0 | + |
| Senegalia | 1 | 1 | 0 | 0 | – |
| Stryphnodendron | 3 | 0 | 3 | 0 | + |
| Zygia | 5 | 4 | 1 | 0 | + |
| Papilionoideae | |||||
| Acosmium | 1 | 1 | 0 | 0 | + |
| Aeschynomene | 4 | 4 | 0 | 0 | + |
| Alexa | 1 | 1 | 1 | 1 | – |
| Andira | 2 | 1 | 1 | 0 | + |
| Bowdichia | 1 | 0 | 1 | 0 | + |
| Centrosema | 4 | 2 | 2 | 0 | + |
| Clathrotropis | 1 | 1 | 0 | 0 | + |
| Clitoria | 2 | 2 | 0 | 0 | + |
| Cymbosema | 1 | 1 | 0 | 0 | + |
| Dalbergia | 4 | 3 | 1 | 0 | + |
| Desmodium | 2 | 1 | 1 | 0 | + |
| Dioclea | 2 | 1 | 1 | 0 | + |
| Diplotropis | 3 | 0 | 3 | 0 | + |
| Dipteryx | 3 | 0 | 3 | 0 | – |
| Hymenolobium | 4 | 0 | 4 | 0 | + |
| Indigofera | 1 | 1 | 0 | 0 | + |
| Lecointea | 1 | 0 | 1 | 0 | – |
| Lonchocarpus | 1 | 0 | 1 | 0 | + |
| Machaerium | 5 | 4 | 1 | 0 | + |
| Mucuna | 1 | 1 | 0 | 0 | + |
| Ormosia | 5 | 2 | 3 | 0 | + |
| Platymiscium | 3 | 2 | 1 | 0 | + |
| Poecilanthe | 1 | 1 | 0 | 0 | + |
| Pterocarpus | 2 | 1 | 1 | 0 | – |
| Rhynchosia | 1 | 1 | 0 | 0 | + |
| Sesbania | 1 | 1 | 0 | 0 | + |
| Swartzia | 7 | 5 | 2 | 0 | + |
| Taralea | 2 | 1 | 1 | 0 | – |
| Tephrosia | 1 | 1 | 0 | 0 | + |
| Trischidium | 1 | 0 | 1 | 0 | – |
| Vatairea | 1 | 1 | 0 | 0 | – |
| Vigna | 1 | 1 | 0 | 0 | + |
| Leguminous subfamily/genus | No. of species examined | No. of species in flooded areas | No. of species in non-flooded areas | No. of species common to both areas | Nodulation status of species |
| Caesalpinioideae | |||||
| Apuleia | 1 | 0 | 1 | 0 | – |
| Batesia | 1 | 0 | 1 | 0 | – |
| Bauhinia | 4 | 3 | 1 | 0 | – |
| Campsiandra | 1 | 1 | 0 | 0 | + |
| Cassia | 3 | 2 | 1 | 0 | – |
| Chamaecrista | 5 | 3 | 2 | 0 | + |
| Crudia | 2 | 2 | 0 | 0 | – |
| Cynometra | 1 | 1 | 0 | 0 | – |
| Dialium | 1 | 1 | 0 | 0 | – |
| Dimorphandra | 3 | 1 | 3 | 1 | + |
| Eperua | 3 | 1 | 2 | 0 | – |
| Heterostemon | 1 | 1 | 0 | 0 | – |
| Hymenaea | 3 | 0 | 3 | 0 | – |
| Macrolobium | 9 | 8 | 1 | 0 | – |
| Martiodendron | 1 | 0 | 1 | 0 | – |
| Peltogyne | 2 | 1 | 1 | 0 | – |
| Senna | 9 | 2 | 7 | 0 | – |
| Tachigali | 5 | 1 | 4 | 0 | + |
| Vouacapoua | 1 | 0 | 1 | 0 | – |
| Mimosoideae | |||||
| Abarema | 8 | 0 | 8 | 0 | + |
| Albizia | 3 | 2 | 1 | 0 | + |
| Balizia | 2 | 0 | 2 | 0 | + |
| Calliandra | 4 | 2 | 2 | 0 | + |
| Dinizia | 1 | 0 | 1 | 0 | – |
| Entada | 1 | 1 | 0 | 0 | + |
| Enterolobium | 2 | 0 | 2 | 0 | + |
| Hydrochorea | 3 | 3 | 0 | 0 | + |
| Inga | 23 | 18 | 16 | 11 | + |
| Macrosamanea | 2 | 2 | 0 | 0 | + |
| Mimosa | 7 | 4 | 3 | 0 | + |
| Parkia | 7 | 1 | 6 | 0 | – |
| Pseudopiptadenia | 1 | 0 | 1 | 0 | + |
| Senegalia | 1 | 1 | 0 | 0 | – |
| Stryphnodendron | 3 | 0 | 3 | 0 | + |
| Zygia | 5 | 4 | 1 | 0 | + |
| Papilionoideae | |||||
| Acosmium | 1 | 1 | 0 | 0 | + |
| Aeschynomene | 4 | 4 | 0 | 0 | + |
| Alexa | 1 | 1 | 1 | 1 | – |
| Andira | 2 | 1 | 1 | 0 | + |
| Bowdichia | 1 | 0 | 1 | 0 | + |
| Centrosema | 4 | 2 | 2 | 0 | + |
| Clathrotropis | 1 | 1 | 0 | 0 | + |
| Clitoria | 2 | 2 | 0 | 0 | + |
| Cymbosema | 1 | 1 | 0 | 0 | + |
| Dalbergia | 4 | 3 | 1 | 0 | + |
| Desmodium | 2 | 1 | 1 | 0 | + |
| Dioclea | 2 | 1 | 1 | 0 | + |
| Diplotropis | 3 | 0 | 3 | 0 | + |
| Dipteryx | 3 | 0 | 3 | 0 | – |
| Hymenolobium | 4 | 0 | 4 | 0 | + |
| Indigofera | 1 | 1 | 0 | 0 | + |
| Lecointea | 1 | 0 | 1 | 0 | – |
| Lonchocarpus | 1 | 0 | 1 | 0 | + |
| Machaerium | 5 | 4 | 1 | 0 | + |
| Mucuna | 1 | 1 | 0 | 0 | + |
| Ormosia | 5 | 2 | 3 | 0 | + |
| Platymiscium | 3 | 2 | 1 | 0 | + |
| Poecilanthe | 1 | 1 | 0 | 0 | + |
| Pterocarpus | 2 | 1 | 1 | 0 | – |
| Rhynchosia | 1 | 1 | 0 | 0 | + |
| Sesbania | 1 | 1 | 0 | 0 | + |
| Swartzia | 7 | 5 | 2 | 0 | + |
| Taralea | 2 | 1 | 1 | 0 | – |
| Tephrosia | 1 | 1 | 0 | 0 | + |
| Trischidium | 1 | 0 | 1 | 0 | – |
| Vatairea | 1 | 1 | 0 | 0 | – |
| Vigna | 1 | 1 | 0 | 0 | + |
Number of examined leguminous species and their nodulation status (+, nodulated; –, non-nodulated)
| Leguminous subfamily/genus | No. of species examined | No. of species in flooded areas | No. of species in non-flooded areas | No. of species common to both areas | Nodulation status of species |
| Caesalpinioideae | |||||
| Apuleia | 1 | 0 | 1 | 0 | – |
| Batesia | 1 | 0 | 1 | 0 | – |
| Bauhinia | 4 | 3 | 1 | 0 | – |
| Campsiandra | 1 | 1 | 0 | 0 | + |
| Cassia | 3 | 2 | 1 | 0 | – |
| Chamaecrista | 5 | 3 | 2 | 0 | + |
| Crudia | 2 | 2 | 0 | 0 | – |
| Cynometra | 1 | 1 | 0 | 0 | – |
| Dialium | 1 | 1 | 0 | 0 | – |
| Dimorphandra | 3 | 1 | 3 | 1 | + |
| Eperua | 3 | 1 | 2 | 0 | – |
| Heterostemon | 1 | 1 | 0 | 0 | – |
| Hymenaea | 3 | 0 | 3 | 0 | – |
| Macrolobium | 9 | 8 | 1 | 0 | – |
| Martiodendron | 1 | 0 | 1 | 0 | – |
| Peltogyne | 2 | 1 | 1 | 0 | – |
| Senna | 9 | 2 | 7 | 0 | – |
| Tachigali | 5 | 1 | 4 | 0 | + |
| Vouacapoua | 1 | 0 | 1 | 0 | – |
| Mimosoideae | |||||
| Abarema | 8 | 0 | 8 | 0 | + |
| Albizia | 3 | 2 | 1 | 0 | + |
| Balizia | 2 | 0 | 2 | 0 | + |
| Calliandra | 4 | 2 | 2 | 0 | + |
| Dinizia | 1 | 0 | 1 | 0 | – |
| Entada | 1 | 1 | 0 | 0 | + |
| Enterolobium | 2 | 0 | 2 | 0 | + |
| Hydrochorea | 3 | 3 | 0 | 0 | + |
| Inga | 23 | 18 | 16 | 11 | + |
| Macrosamanea | 2 | 2 | 0 | 0 | + |
| Mimosa | 7 | 4 | 3 | 0 | + |
| Parkia | 7 | 1 | 6 | 0 | – |
| Pseudopiptadenia | 1 | 0 | 1 | 0 | + |
| Senegalia | 1 | 1 | 0 | 0 | – |
| Stryphnodendron | 3 | 0 | 3 | 0 | + |
| Zygia | 5 | 4 | 1 | 0 | + |
| Papilionoideae | |||||
| Acosmium | 1 | 1 | 0 | 0 | + |
| Aeschynomene | 4 | 4 | 0 | 0 | + |
| Alexa | 1 | 1 | 1 | 1 | – |
| Andira | 2 | 1 | 1 | 0 | + |
| Bowdichia | 1 | 0 | 1 | 0 | + |
| Centrosema | 4 | 2 | 2 | 0 | + |
| Clathrotropis | 1 | 1 | 0 | 0 | + |
| Clitoria | 2 | 2 | 0 | 0 | + |
| Cymbosema | 1 | 1 | 0 | 0 | + |
| Dalbergia | 4 | 3 | 1 | 0 | + |
| Desmodium | 2 | 1 | 1 | 0 | + |
| Dioclea | 2 | 1 | 1 | 0 | + |
| Diplotropis | 3 | 0 | 3 | 0 | + |
| Dipteryx | 3 | 0 | 3 | 0 | – |
| Hymenolobium | 4 | 0 | 4 | 0 | + |
| Indigofera | 1 | 1 | 0 | 0 | + |
| Lecointea | 1 | 0 | 1 | 0 | – |
| Lonchocarpus | 1 | 0 | 1 | 0 | + |
| Machaerium | 5 | 4 | 1 | 0 | + |
| Mucuna | 1 | 1 | 0 | 0 | + |
| Ormosia | 5 | 2 | 3 | 0 | + |
| Platymiscium | 3 | 2 | 1 | 0 | + |
| Poecilanthe | 1 | 1 | 0 | 0 | + |
| Pterocarpus | 2 | 1 | 1 | 0 | – |
| Rhynchosia | 1 | 1 | 0 | 0 | + |
| Sesbania | 1 | 1 | 0 | 0 | + |
| Swartzia | 7 | 5 | 2 | 0 | + |
| Taralea | 2 | 1 | 1 | 0 | – |
| Tephrosia | 1 | 1 | 0 | 0 | + |
| Trischidium | 1 | 0 | 1 | 0 | – |
| Vatairea | 1 | 1 | 0 | 0 | – |
| Vigna | 1 | 1 | 0 | 0 | + |
| Leguminous subfamily/genus | No. of species examined | No. of species in flooded areas | No. of species in non-flooded areas | No. of species common to both areas | Nodulation status of species |
| Caesalpinioideae | |||||
| Apuleia | 1 | 0 | 1 | 0 | – |
| Batesia | 1 | 0 | 1 | 0 | – |
| Bauhinia | 4 | 3 | 1 | 0 | – |
| Campsiandra | 1 | 1 | 0 | 0 | + |
| Cassia | 3 | 2 | 1 | 0 | – |
| Chamaecrista | 5 | 3 | 2 | 0 | + |
| Crudia | 2 | 2 | 0 | 0 | – |
| Cynometra | 1 | 1 | 0 | 0 | – |
| Dialium | 1 | 1 | 0 | 0 | – |
| Dimorphandra | 3 | 1 | 3 | 1 | + |
| Eperua | 3 | 1 | 2 | 0 | – |
| Heterostemon | 1 | 1 | 0 | 0 | – |
| Hymenaea | 3 | 0 | 3 | 0 | – |
| Macrolobium | 9 | 8 | 1 | 0 | – |
| Martiodendron | 1 | 0 | 1 | 0 | – |
| Peltogyne | 2 | 1 | 1 | 0 | – |
| Senna | 9 | 2 | 7 | 0 | – |
| Tachigali | 5 | 1 | 4 | 0 | + |
| Vouacapoua | 1 | 0 | 1 | 0 | – |
| Mimosoideae | |||||
| Abarema | 8 | 0 | 8 | 0 | + |
| Albizia | 3 | 2 | 1 | 0 | + |
| Balizia | 2 | 0 | 2 | 0 | + |
| Calliandra | 4 | 2 | 2 | 0 | + |
| Dinizia | 1 | 0 | 1 | 0 | – |
| Entada | 1 | 1 | 0 | 0 | + |
| Enterolobium | 2 | 0 | 2 | 0 | + |
| Hydrochorea | 3 | 3 | 0 | 0 | + |
| Inga | 23 | 18 | 16 | 11 | + |
| Macrosamanea | 2 | 2 | 0 | 0 | + |
| Mimosa | 7 | 4 | 3 | 0 | + |
| Parkia | 7 | 1 | 6 | 0 | – |
| Pseudopiptadenia | 1 | 0 | 1 | 0 | + |
| Senegalia | 1 | 1 | 0 | 0 | – |
| Stryphnodendron | 3 | 0 | 3 | 0 | + |
| Zygia | 5 | 4 | 1 | 0 | + |
| Papilionoideae | |||||
| Acosmium | 1 | 1 | 0 | 0 | + |
| Aeschynomene | 4 | 4 | 0 | 0 | + |
| Alexa | 1 | 1 | 1 | 1 | – |
| Andira | 2 | 1 | 1 | 0 | + |
| Bowdichia | 1 | 0 | 1 | 0 | + |
| Centrosema | 4 | 2 | 2 | 0 | + |
| Clathrotropis | 1 | 1 | 0 | 0 | + |
| Clitoria | 2 | 2 | 0 | 0 | + |
| Cymbosema | 1 | 1 | 0 | 0 | + |
| Dalbergia | 4 | 3 | 1 | 0 | + |
| Desmodium | 2 | 1 | 1 | 0 | + |
| Dioclea | 2 | 1 | 1 | 0 | + |
| Diplotropis | 3 | 0 | 3 | 0 | + |
| Dipteryx | 3 | 0 | 3 | 0 | – |
| Hymenolobium | 4 | 0 | 4 | 0 | + |
| Indigofera | 1 | 1 | 0 | 0 | + |
| Lecointea | 1 | 0 | 1 | 0 | – |
| Lonchocarpus | 1 | 0 | 1 | 0 | + |
| Machaerium | 5 | 4 | 1 | 0 | + |
| Mucuna | 1 | 1 | 0 | 0 | + |
| Ormosia | 5 | 2 | 3 | 0 | + |
| Platymiscium | 3 | 2 | 1 | 0 | + |
| Poecilanthe | 1 | 1 | 0 | 0 | + |
| Pterocarpus | 2 | 1 | 1 | 0 | – |
| Rhynchosia | 1 | 1 | 0 | 0 | + |
| Sesbania | 1 | 1 | 0 | 0 | + |
| Swartzia | 7 | 5 | 2 | 0 | + |
| Taralea | 2 | 1 | 1 | 0 | – |
| Tephrosia | 1 | 1 | 0 | 0 | + |
| Trischidium | 1 | 0 | 1 | 0 | – |
| Vatairea | 1 | 1 | 0 | 0 | – |
| Vigna | 1 | 1 | 0 | 0 | + |
It was not possible to survey the nodulation status of 30 out of the 229 identified native leguminous species, due to the unavailability of seeds or their non-germination after sampling. These 30 species were also excluded from the analysis of the nodulation ability of the leguminous species; however, their nodulation status was verified using the GRIN database http://www.ars-grin.gov/∼sbmljw/cgi-bin/taxnodul.pl (see Supplementary Table S1 at JXB online). Indeed, as discussed by Sprent (2005) the study of nodulation and nitrogen fixation requires much careful work from field observations to laboratory tests. The observation of nodulated roots attached to the putative hosts, the examination of nodule structure, and the re-inoculation of isolated bacterial strains on to their putative hosts are among the recommended methods to follow in order to avoid false reports. Therefore, a suite of approaches to survey the nodulation status of leguminous species (even those from flooded areas) by combining field observations, seedling inoculation, and screening of N2-fixing bacterial strains from the collected nodules is presented here.
Overall, 199 leguminous species from both flooded and non-flooded areas were examined for their nodulation (Table 1). The relative frequency of nodulation tended to be higher in the species from the flooded areas (74% of the examined species for both Igapós and Várzeas) compared with those from the non-flooded areas (67% for both Terra Firme and Campinas). Moreira et al. (1992) had also noted that the frequency of nodulation among tribes and genera was higher in flooded areas than in non-flooded areas, and more plants had nodulated in nursery bed experiments when grown on soil from flooded areas compared with those grown on soil from non-flooded areas. Thus they pointed out the influence of nutrient balance in nodulation. The shortage of available N due to high leaching, a decrease in organic matter mineralization, and an increase of denitrification in flooded areas, favour the legume–bacteria symbiosis, and therefore may drive plant selection pressure (Moreira et al., 1992; Barrios and Herrera, 1993; Loureiro et al., 1998; Sprent, 1999; James et al., 2001). Moreover, the beneficial effect of nodulation on N acquisition and plant growth under flooding conditions has recently been shown (Fougnies et al., 2007).
Regardless of the sampling areas, nodulation was observed in 137 leguminous species (69% of the examined species), 32 of which for the first time, including three Caesalpinoideae, 19 Mimosoideae, and 10 Papilionoideae (Tables 1, 2). In the subfamily Papilionoideae, the genus Cymbosema was found for the first time to contain a nodulating species (Cymbosema roseum). For the non-nodulating species, 12 out of the 62 identified species are new records. Among them, 10 species belonged to the Caesalpinioideae, and two to Papilionoideae subfamilies. Moreover, from 12 leguminous species of which the nodulation status was uncertain according to the GRIN database, new evidences about their nodulation status is provided here (see Supplementary Table S1 at JXB online). The large number of new records obtained reflects the fact that only a few (about 20%) of the known leguminous species have been examined for nodulation (de Faria et al., 1989; Sprent, 1995). The difficulties of identifying and collecting nodules on adult trees, particularly in dense forests and flooded areas, and the frequent lack of nodules in equilibrium forests where nitrogen is not usually the growth-limiting factor, are among the reasons which explain this situation (Bonnier and Brakel, 1969; Norris, 1969; de Faria et al., 1984, 1989; Moreira et al., 1992).
New records of non-nodulated and nodulated leguminous species, nodule morphology and habitat (sampling areas)
| Leguminous species | Nodulation status of speciesa | Morphology of nodulesa | Habitat |
| Caesalpinioideae | |||
| Bauhinia cupreonitens Ducke | – | – | Ig/V (F) |
| Chamaecrista viscosa (Kunth) Irwin and Barneby | + | I | C (NF) |
| Dimorphandra campinarum Ducke | + | I | C (NF) |
| Eperua rubiginosa Miq. | – | – | TF (NF) |
| Hymenaea intermedia Ducke | – | – | TF (NF) |
| Macrolobium bifolium (Aubl.) Pers. | – | – | Ig (F) |
| Macrolobium multijugum var. multijugum (DC.) Benth. | – | – | Ig (F) |
| Macrolobium pendulum Willd. ex Vogel | – | – | Ig (F) |
| Macrolobium punctatum Spruce ex Benth. | – | – | Ig (F) |
| Macrolobium suaveolens var. suaveolens Spruce ex Benth. | – | – | Ig (F) |
| Peltogyne catingae Ducke | – | – | C (NF) |
| Senna chrysocarpa (Desv.) Irwin & Barneby | – | – | TF (NF) |
| Tachigali macrostachya Hub. | + | I | Ig (F) |
| Mimosoideae | |||
| Abarema campestris (Benth.) Barneby & Grimes | + | I | TF/C (NF) |
| Abarema floribunda (Benth.) Barneby & Grimes | + | I | TF (NF) |
| Abarema piresii Barneby & Grimes | + | I | TF (NF) |
| Balizia elegans (Ducke) Barneby & Grimes | + | I | TF (NF) |
| Calliandra coriacea (Willd.) Benth. | + | I | V (F) |
| Calliandra pittieri Standl. | + | I | TF (NF) |
| Hydrochorea gonggrijpii (Kleinh.) Barneby & Grimes | + | I | Ig (F) |
| Hydrochorea marginata var. marginata (Benth.) Barneby & Grimes | + | I | Ig (F) |
| Inga acuminata Benth. | + | I | Ig (F) |
| Inga duckei Huber | + | I | Ig (F) |
| Inga macrophylla Humb. & Bonpl. ex Willd. | + | I | TF/C (NF) |
| Inga umbratica Poepp. & Endl. | + | I | TF (NF) |
| Inga velutina Willd. | + | I | Ig (F) |
| Macrosamanea duckei (Huber) Barneby & Grimes | + | I | Ig (F) |
| Mimosa myriadenia (Benth.) Benth. | + | I | V (F) |
| Mimosa rufescens Benth. | + | I | TF (NF) |
| Mimosa spruceana Benth. | + | I | TF (NF) |
| Stryphnodendron conicum Scalon | + | I | TF (NF) |
| Zygia ramiflora (F. Muell.) Kosterm. | + | I | Ig (F) |
| Papilionoideae | |||
| Alexa grandiflora Ducke | – | – | TF/Ig (B) |
| Centrosema vexillatum Benth. | + | D | Ig (F) |
| Cymbosema roseum Benth. | + | D | V (F) |
| Diplotropis triloba Gleason | + | I | TF (NF) |
| Machaerium latifolium Rusby | + | A | TF (NF) |
| Poecilanthe amazonica (Ducke) Ducke | + | I | Ig (F) |
| Pterocarpus amazonum (Benth.) Amsh. | – | – | TF (NF) |
| Swartzia corrugata Benth. | + | I | TF (NF) |
| Swartzia grandifolia Bong. | + | I | TF (NF) |
| Swartzia leptopetala Benth. | + | I | Ig (F) |
| Swartzia oriximinaensis Cowan | + | I | Ig (F) |
| Tephrosia nitens Benth. | + | I | Ig (F) |
| Leguminous species | Nodulation status of speciesa | Morphology of nodulesa | Habitat |
| Caesalpinioideae | |||
| Bauhinia cupreonitens Ducke | – | – | Ig/V (F) |
| Chamaecrista viscosa (Kunth) Irwin and Barneby | + | I | C (NF) |
| Dimorphandra campinarum Ducke | + | I | C (NF) |
| Eperua rubiginosa Miq. | – | – | TF (NF) |
| Hymenaea intermedia Ducke | – | – | TF (NF) |
| Macrolobium bifolium (Aubl.) Pers. | – | – | Ig (F) |
| Macrolobium multijugum var. multijugum (DC.) Benth. | – | – | Ig (F) |
| Macrolobium pendulum Willd. ex Vogel | – | – | Ig (F) |
| Macrolobium punctatum Spruce ex Benth. | – | – | Ig (F) |
| Macrolobium suaveolens var. suaveolens Spruce ex Benth. | – | – | Ig (F) |
| Peltogyne catingae Ducke | – | – | C (NF) |
| Senna chrysocarpa (Desv.) Irwin & Barneby | – | – | TF (NF) |
| Tachigali macrostachya Hub. | + | I | Ig (F) |
| Mimosoideae | |||
| Abarema campestris (Benth.) Barneby & Grimes | + | I | TF/C (NF) |
| Abarema floribunda (Benth.) Barneby & Grimes | + | I | TF (NF) |
| Abarema piresii Barneby & Grimes | + | I | TF (NF) |
| Balizia elegans (Ducke) Barneby & Grimes | + | I | TF (NF) |
| Calliandra coriacea (Willd.) Benth. | + | I | V (F) |
| Calliandra pittieri Standl. | + | I | TF (NF) |
| Hydrochorea gonggrijpii (Kleinh.) Barneby & Grimes | + | I | Ig (F) |
| Hydrochorea marginata var. marginata (Benth.) Barneby & Grimes | + | I | Ig (F) |
| Inga acuminata Benth. | + | I | Ig (F) |
| Inga duckei Huber | + | I | Ig (F) |
| Inga macrophylla Humb. & Bonpl. ex Willd. | + | I | TF/C (NF) |
| Inga umbratica Poepp. & Endl. | + | I | TF (NF) |
| Inga velutina Willd. | + | I | Ig (F) |
| Macrosamanea duckei (Huber) Barneby & Grimes | + | I | Ig (F) |
| Mimosa myriadenia (Benth.) Benth. | + | I | V (F) |
| Mimosa rufescens Benth. | + | I | TF (NF) |
| Mimosa spruceana Benth. | + | I | TF (NF) |
| Stryphnodendron conicum Scalon | + | I | TF (NF) |
| Zygia ramiflora (F. Muell.) Kosterm. | + | I | Ig (F) |
| Papilionoideae | |||
| Alexa grandiflora Ducke | – | – | TF/Ig (B) |
| Centrosema vexillatum Benth. | + | D | Ig (F) |
| Cymbosema roseum Benth. | + | D | V (F) |
| Diplotropis triloba Gleason | + | I | TF (NF) |
| Machaerium latifolium Rusby | + | A | TF (NF) |
| Poecilanthe amazonica (Ducke) Ducke | + | I | Ig (F) |
| Pterocarpus amazonum (Benth.) Amsh. | – | – | TF (NF) |
| Swartzia corrugata Benth. | + | I | TF (NF) |
| Swartzia grandifolia Bong. | + | I | TF (NF) |
| Swartzia leptopetala Benth. | + | I | Ig (F) |
| Swartzia oriximinaensis Cowan | + | I | Ig (F) |
| Tephrosia nitens Benth. | + | I | Ig (F) |
+, nodulated; –, non-nodulated; I, indeterminate; D, desmodioid; A, aeschynomenoid; C, Campinas; Ig, Igapós; TF, Terra firme; V, Várzeas; F, flooded areas; NF, non-flooded areas; B, both areas.
New records of non-nodulated and nodulated leguminous species, nodule morphology and habitat (sampling areas)
| Leguminous species | Nodulation status of speciesa | Morphology of nodulesa | Habitat |
| Caesalpinioideae | |||
| Bauhinia cupreonitens Ducke | – | – | Ig/V (F) |
| Chamaecrista viscosa (Kunth) Irwin and Barneby | + | I | C (NF) |
| Dimorphandra campinarum Ducke | + | I | C (NF) |
| Eperua rubiginosa Miq. | – | – | TF (NF) |
| Hymenaea intermedia Ducke | – | – | TF (NF) |
| Macrolobium bifolium (Aubl.) Pers. | – | – | Ig (F) |
| Macrolobium multijugum var. multijugum (DC.) Benth. | – | – | Ig (F) |
| Macrolobium pendulum Willd. ex Vogel | – | – | Ig (F) |
| Macrolobium punctatum Spruce ex Benth. | – | – | Ig (F) |
| Macrolobium suaveolens var. suaveolens Spruce ex Benth. | – | – | Ig (F) |
| Peltogyne catingae Ducke | – | – | C (NF) |
| Senna chrysocarpa (Desv.) Irwin & Barneby | – | – | TF (NF) |
| Tachigali macrostachya Hub. | + | I | Ig (F) |
| Mimosoideae | |||
| Abarema campestris (Benth.) Barneby & Grimes | + | I | TF/C (NF) |
| Abarema floribunda (Benth.) Barneby & Grimes | + | I | TF (NF) |
| Abarema piresii Barneby & Grimes | + | I | TF (NF) |
| Balizia elegans (Ducke) Barneby & Grimes | + | I | TF (NF) |
| Calliandra coriacea (Willd.) Benth. | + | I | V (F) |
| Calliandra pittieri Standl. | + | I | TF (NF) |
| Hydrochorea gonggrijpii (Kleinh.) Barneby & Grimes | + | I | Ig (F) |
| Hydrochorea marginata var. marginata (Benth.) Barneby & Grimes | + | I | Ig (F) |
| Inga acuminata Benth. | + | I | Ig (F) |
| Inga duckei Huber | + | I | Ig (F) |
| Inga macrophylla Humb. & Bonpl. ex Willd. | + | I | TF/C (NF) |
| Inga umbratica Poepp. & Endl. | + | I | TF (NF) |
| Inga velutina Willd. | + | I | Ig (F) |
| Macrosamanea duckei (Huber) Barneby & Grimes | + | I | Ig (F) |
| Mimosa myriadenia (Benth.) Benth. | + | I | V (F) |
| Mimosa rufescens Benth. | + | I | TF (NF) |
| Mimosa spruceana Benth. | + | I | TF (NF) |
| Stryphnodendron conicum Scalon | + | I | TF (NF) |
| Zygia ramiflora (F. Muell.) Kosterm. | + | I | Ig (F) |
| Papilionoideae | |||
| Alexa grandiflora Ducke | – | – | TF/Ig (B) |
| Centrosema vexillatum Benth. | + | D | Ig (F) |
| Cymbosema roseum Benth. | + | D | V (F) |
| Diplotropis triloba Gleason | + | I | TF (NF) |
| Machaerium latifolium Rusby | + | A | TF (NF) |
| Poecilanthe amazonica (Ducke) Ducke | + | I | Ig (F) |
| Pterocarpus amazonum (Benth.) Amsh. | – | – | TF (NF) |
| Swartzia corrugata Benth. | + | I | TF (NF) |
| Swartzia grandifolia Bong. | + | I | TF (NF) |
| Swartzia leptopetala Benth. | + | I | Ig (F) |
| Swartzia oriximinaensis Cowan | + | I | Ig (F) |
| Tephrosia nitens Benth. | + | I | Ig (F) |
| Leguminous species | Nodulation status of speciesa | Morphology of nodulesa | Habitat |
| Caesalpinioideae | |||
| Bauhinia cupreonitens Ducke | – | – | Ig/V (F) |
| Chamaecrista viscosa (Kunth) Irwin and Barneby | + | I | C (NF) |
| Dimorphandra campinarum Ducke | + | I | C (NF) |
| Eperua rubiginosa Miq. | – | – | TF (NF) |
| Hymenaea intermedia Ducke | – | – | TF (NF) |
| Macrolobium bifolium (Aubl.) Pers. | – | – | Ig (F) |
| Macrolobium multijugum var. multijugum (DC.) Benth. | – | – | Ig (F) |
| Macrolobium pendulum Willd. ex Vogel | – | – | Ig (F) |
| Macrolobium punctatum Spruce ex Benth. | – | – | Ig (F) |
| Macrolobium suaveolens var. suaveolens Spruce ex Benth. | – | – | Ig (F) |
| Peltogyne catingae Ducke | – | – | C (NF) |
| Senna chrysocarpa (Desv.) Irwin & Barneby | – | – | TF (NF) |
| Tachigali macrostachya Hub. | + | I | Ig (F) |
| Mimosoideae | |||
| Abarema campestris (Benth.) Barneby & Grimes | + | I | TF/C (NF) |
| Abarema floribunda (Benth.) Barneby & Grimes | + | I | TF (NF) |
| Abarema piresii Barneby & Grimes | + | I | TF (NF) |
| Balizia elegans (Ducke) Barneby & Grimes | + | I | TF (NF) |
| Calliandra coriacea (Willd.) Benth. | + | I | V (F) |
| Calliandra pittieri Standl. | + | I | TF (NF) |
| Hydrochorea gonggrijpii (Kleinh.) Barneby & Grimes | + | I | Ig (F) |
| Hydrochorea marginata var. marginata (Benth.) Barneby & Grimes | + | I | Ig (F) |
| Inga acuminata Benth. | + | I | Ig (F) |
| Inga duckei Huber | + | I | Ig (F) |
| Inga macrophylla Humb. & Bonpl. ex Willd. | + | I | TF/C (NF) |
| Inga umbratica Poepp. & Endl. | + | I | TF (NF) |
| Inga velutina Willd. | + | I | Ig (F) |
| Macrosamanea duckei (Huber) Barneby & Grimes | + | I | Ig (F) |
| Mimosa myriadenia (Benth.) Benth. | + | I | V (F) |
| Mimosa rufescens Benth. | + | I | TF (NF) |
| Mimosa spruceana Benth. | + | I | TF (NF) |
| Stryphnodendron conicum Scalon | + | I | TF (NF) |
| Zygia ramiflora (F. Muell.) Kosterm. | + | I | Ig (F) |
| Papilionoideae | |||
| Alexa grandiflora Ducke | – | – | TF/Ig (B) |
| Centrosema vexillatum Benth. | + | D | Ig (F) |
| Cymbosema roseum Benth. | + | D | V (F) |
| Diplotropis triloba Gleason | + | I | TF (NF) |
| Machaerium latifolium Rusby | + | A | TF (NF) |
| Poecilanthe amazonica (Ducke) Ducke | + | I | Ig (F) |
| Pterocarpus amazonum (Benth.) Amsh. | – | – | TF (NF) |
| Swartzia corrugata Benth. | + | I | TF (NF) |
| Swartzia grandifolia Bong. | + | I | TF (NF) |
| Swartzia leptopetala Benth. | + | I | Ig (F) |
| Swartzia oriximinaensis Cowan | + | I | Ig (F) |
| Tephrosia nitens Benth. | + | I | Ig (F) |
+, nodulated; –, non-nodulated; I, indeterminate; D, desmodioid; A, aeschynomenoid; C, Campinas; Ig, Igapós; TF, Terra firme; V, Várzeas; F, flooded areas; NF, non-flooded areas; B, both areas.
On the other hand, nodulation in the Cassieae tribe (Caesalpinioideae) has only been confirmed in the Chamaecrista genus. The report of Cassia spruceana as a nodulating species (Roggy and Prévost, 1999) was incorrect (JC Roggy, personal communication). This species was unable to nodulate as confirmed by our observations. The same problem occurred with the Dycorinia reports, since no nodules were found (JC Roggy, personal communication). In the genus Parkia, no species was found to be nodulated, this result confirmed all previous observations (de Faria et al., 1989; Moreira et al., 1992; Roggy and Prévost, 1999). Similar results were observed in the Dipterygeae, the only Papilionoideae tribe where no species was confirmed to be nodulated (de Faria et al., 1989; Sprent, 2001).
As already known, the ability of Caesalpinioideae species to nodulate is restricted to a few groups of this subfamily, while the majority of Mimosoideae and Papilionoideae species was found to be nodulated (de Faria et al., 1989; Sprent, 1995). The results presented here confirmed this finding with 25, 84, and 88% of nodulation in the Caesalpinioideae, Papilionoideae, and Mimosoideae, respectively. This difference in the nodulation ability of species belonging to the Caesalpinioideae and those of the Mimosoideae and Papilionoideae subfamilies has been also found in leguminous trees native to the West African tropical rainforest (Diabate et al., 2005). There are several possible non-exclusive processes that could generate the pattern of the nodulation ability observed among the legumes. Indeed, it has been suggested that the Caesalpinioideae separately evolved from the Mimosoideae and Papilionoideae in the Leguminosae family (Polhill et al., 1981; Doyle, 1994), and this divergence includes, besides morphological and molecular characters, the capacity to nodulate (Allen and Allen, 1981; Corby et al., 1983; de Faria et al., 1989). Sprent (2001, 2007, 2008) and Sprent and James (2007) based on histological, morphological, molecular, and taxonomical views had substantially discussed the evolution of nodulation in the Leguminosae family. From another point of view, the coevolution of legumes with mycorrhizal fungi, particularly arbuscular mycorrhizal fungi, in relation to the availability and/or demand for carbon and nutrients might contribute to the occurrence of nodulation. It is known that (i) the arbuscular mycorrhizal symbiosis evolved long before legumes (Smith and Read, 2008), (ii) almost all leguminous species form arbuscular mycorrhizas (AM), ectomycorrhizas (ECM) being found mainly in the Caesalpinioideae (Alexander, 1989; Smith and Read, 2008), (iii) arbuscular mycorrhizal fungi displayed different host-plant preferences (Vandenkoornhuyse et al., 2002; Croll et al., 2008), and (iv) the biochemical capabilities for carbon and nutrient acquisition differ substantially between AM and ECM (Read, 1991; Smith and Read, 2008). If all early legumes were associated with AM fungi, it could be speculated that shifts in the availability and/or demand of carbon and nutrients (N, P) during the coevolution of the symbiotic partners (legumes and AM fungi) might induce within the legume subfamilies adaptive mechanisms for the recruitment of ECM fungi and/or N2-fixing bacteria. Indeed, during the coevolution of the symbiotic partners (legumes and AM fungi), inefficient AM fungi might have been reinforced or replaced [in rare cases, e.g. Lupinus the only known nodulated legume genus in which the ability to form mycorrhizal symbioses has been lost (Sprent and James, 2007)] by one or the two other types of symbioses (ECM, and N2-fixing symbiosis) leading to the occurrence of one, two or three types of symbioses (AM, ECM, and N2-fixing symbiosis) on the members of the three legume subfamilies. This speculation may be partially supported by the finding that genetic and environmental factors could influence the formation of AM and ECM on plants that can be colonized by these two mycorrhizal types at the same time (Gehring et al., 2006). Another argument in favour of this speculation comes from some nodulated Australian acacias which can form both types of mycorrhizas. It has been shown from members of these acacias that inoculation with AM fungi promotes the efficiency of N2-fixing symbioses and plant growth, suggesting synergistic functions of these different types of symbioses (Bâ et al., 2010). On the other hand, molecular studies revealed that in legumes such as in Casuarina glauca (actinorhiza-forming plant) the same gene (SymRK) is required in the establishment of both AM and N2-fixing symbioses, suggesting that signalling genes have been recruited from the more ancient AM symbiosis during the coevolution of these symbiotic partners (Gherbi et al., 2008).
Regarding the plant habits in the Caesalpinioideae, trees accounted for 75% of the species examined, the remaining 25% was shared by lianas (12%), herbaceous plants (9%), and shrubs (4%). Among them, nodulation was reported only in trees and herbaceous plants, and all nodulated species formed indeterminate nodules (see Supplementary Table S1 at JXB online). In the Mimosoideae, trees were dominant accounting for 81% of the examined species, followed by shrubs (10%), and lianas and herbaceous plants represented 5% and 4%, respectively. Nodulation was reported in all plant habits, and the nodules formed were indeterminate. In the Papilionoideae, trees were also prevalent (58% of the examined species), while the herbaceous plants were more represented (19% of the examined species) than they were in the Caesalpinioideae and Mimosoideae. The other plant habits, lianas and shrubs, accounted for 14% and 9% of the species examined, respectively. The nodules formed in the tree and shrub categories were mainly indeterminate, while determinate nodules were found in the species belonging to the genera Clitoria and Cymbosema both with the desmodioid form, and the genera Dalbergia and Platymiscium with the aeschynomenoid form. In lianas, the species gathered in the genera Dalbergia and Machaerium formed aeschynomenoid nodules, while those belonging to the genera Dioclea and Mucuna formed desmodioid and mucunoid nodules respectively. In herbaceous plants, the determinate nodules were prevalent in the legumes examined, including eight species gathered from the genera Centrosema, Desmodium, Rhynchosia, and Vigna which displayed the desmodioid form of nodules and four species of Aeschynomene which formed aeschynomenoid nodules; indeterminate nodules being found only in Indigofera suffruticosa. Our results thus confirmed previous findings that the genera of the tribes Aeschynomeneae, Dalbergieae, Desmodieae, and Phaseoleae display different habits and form determinate nodules, while Indigofera, one of the largest genera of the tribe Indigofereae, form indeterminate nodules and tend towards herbaceous plants (Moreira et al., 1992; Lavin et al., 2001; Sprent, 2007). The main features, structures, and forms of nodules, their advantages and disadvantages, and their distribution in the nodulated species of the Leguminoseae family have been well documented by Sprent (1980, 2001, 2007) and Lavin et al. (2001). Indeed, the aeschynomenoid form of nodules well characterizes (synapomorphic) the newly identified dalbergioid clade (Lavin et al., 2001), while the desmodioid nodules are likely to have evolved independently in the Loteae and phaseoloid groups (Sprent, 2001).
Our work has shown that 22% of the examined species are new records on their nodulation status (see Tables 1, 2), and therefore confirmed the fact that numerous nodulated leguminous species remain to be described. It appears that one of the first steps in the attempt to expose ‘the rest of the iceberg’ for our understanding of the interactions between legumes and bacteria as stated by Doyle and Luckow (2003) is to go further in evaluating legume nodulation. Furthermore, owing to their ability to fix nitrogen and their impact on soil N balance, many leguminous species play a key role in the general dynamics and sustainability of rainforests (Roggy and Prévost, 1999), and may constitute potential candidates to facilitate colonization of early successional and infertile habitats (Franco and de Faria, 1997). On the other hand, the nodulation reports from unexplored species may reveal new kinds of efficient nodulating bacteria which could be exploited to improve production of economically important legumes, and other beneficial uses. The study of the genetic diversity of the collected bacterial strains is a priority in our future works in order to reveal the host preference and pattern of coevolution of the collected bacterial strains and their host legumes, and thus to verify if the species richness of legume trees does not necessarily correspond to the diversity of their associated rhizobia, as has been suggested by Moreira et al. (1993).
We are very grateful to Delmo Fonseca da Silva, Sr Pedro Ferreira, Nelson de Araujo Rosa, and José Camarão Pantoja for their huge contribution to the development of this work, in particular for botanical identification, as well as to Glariston Miranda Mello, Jenaldo Carvalho, Adriana Nascimento, Carlos Fernando da Cunha, Carlos Cavalcante, and Telmo Felix for their valued technical assistance. We thank the anonymous referees for their helpful comments on this paper. This work was financially supported by Mineração Rio do Norte and CNPq.




Comments