-
PDF
- Split View
-
Views
-
Cite
Cite
Petra Stamm, Prakash P. Kumar, The phytohormone signal network regulating elongation growth during shade avoidance, Journal of Experimental Botany, Volume 61, Issue 11, June 2010, Pages 2889–2903, https://doi.org/10.1093/jxb/erq147
Close - Share Icon Share
Abstract
In contrast to animals, plants maintain highly plastic growth and development throughout their life, which enables them to adapt to environmental fluctuations. Phytohormones coordinately regulate these adaptations by integrating environmental inputs into a complex signalling network. In this review, the focus is on the rapid elongation that occurs in response to canopy shading or submergence, and current knowledge and recent advances in deciphering the network of phytohormone signalling that regulates this response are explored. The review concentrates on the involvement of the phytohormones auxins, gibberellins, cytokinins, and ethylene. Despite the occurrence of considerable gaps in current understanding of the underlying molecular mechanisms, it was possible to identify a network of phytohormone signalling intermediates at multiple levels that regulates elongation growth in response to canopy shade or submergence. Based on the observations that there are spatial and temporal differences in the interactions of phytohormones, the importance of more integrative approaches for future studies is highlighted.
Introduction
Plants retain a high level of growth plasticity throughout their life in order to adapt to and survive unfavourable and unexpected fluctuations in their environment. This adaptability is achieved by integrating various complex environmental as well as developmental signals. Plant hormones, or phytohormones, which are a collection of structurally unrelated small molecules, serve as integrators of those exogenous (environmental) and endogenous (developmental) cues. The main classes of phytohormones are auxins, cytokinins, gibberellins, ethylene, (+)-abscisic acid, brassinosteroids, and jasmonates, with more substances being added to the list periodically.
Phytohormones regulate every aspect of plant growth and development—from the determination of the stem cell niches during embryogenesis (reviewed in Wolters and Jürgens, 2009) to organogenesis and growth during post-embryonic development. During the past two decades various genes encoding key players for biosynthesis, perception, and signalling of phytohormones have been identified for most classical phytohormones (e.g. Teale et al., 2006; Hirayama and Shinozaki, 2007; To and Kieber, 2008; Schwechheimer and Willige, 2009; Yoo et al., 2009). Also, interesting similarities between the signalling cascades of different phytohormones have been identified (McSteen and Zhao, 2008). The ubiquitin–proteasome pathway, in particular, seems to play a key role in many phytohormone signalling pathways (reviewed in Santner and Estelle, 2009). Furthermore, several signalling intermediates have been identified as common to the signalling pathways of at least two different phytohormones, which has fuelled considerable research interest in phytohormone interactions (e.g. Benková and Hejátko, 2009; Santner and Estelle, 2009; Wolters and Jürgens, 2009; Yoo et al., 2009).
The notion that phytohormones interact at some level in order to integrate the vast number of external as well as internal inputs into a growth response is not new. Phytohormones, in contrast to the animal hormones, are known to have pleiotropic effects, which is mirrored by the fact that many mutations affecting the level or signalling of phytohormones produce overlapping phenotypes. In fact, many phytohormone signalling mutants show a defect in the response to at least one other phytohormone (a list for auxin mutants is given in Swarup et al., 2002).
Comparing changes in the transcriptome of Arabidopsis seedlings upon treatment with various phytohormones, Nemhauser et al. (2006) identified a surprisingly small number of genes that appear to be coordinately regulated by more than one phytohormone. This led the authors to reject the idea of the occurrence of a common set of genes acting as a central plant growth module. The phytohormones tested in this study, namely abscisic acid, gibberellin, auxin, ethylene, cytokinin, brassinosteroid, and jasmonate, thus appear to regulate similar processes through the activation of different sets of genes. This corresponds to observations of certain phytohormone responses being independent of other phytohormones, and the fact that a total deficiency in one phytohormone cannot be fully rescued by the application of any other phytohormone.
In the attempt also to identify robust target genes of each phytohormone tested, Nemhauser et al. (2006) were able to assemble lists of high confidence, unique targets for each phytohormone. Interestingly, this was not the case for gibberellins; no such robust targets could be identified. This allows the hypothesis that this class of phytohormones might predominantly act through the interaction with other phytohormone pathways. Furthermore, the authors also showed that every phytohormone regulates metabolic genes of at least one other phytohormone. Nevertheless, it has to be taken into consideration that the data set of Nemhauser et al. (2006) was derived from phytohormone-treated whole seedlings. Spatial and temporal specificities of phytohormone action and interaction were thus not factored into this study. Furthermore, target genes of phytohormones at different stages of development, for example during seed germination or flower development, were not captured.
Taken together, it appears that for every phytohormone there are responses which are dependent on, mediated by, as well as independent of other phytohormones, resulting in a complex signalling network, which allows response dynamics and patterns to be fine-tuned in a highly sophisticated way. This also reinforces the hypothesis that the balance of several phytohormones rather than the actual amount of one phytohormone determines each response.
Here, the current understanding of the interaction between phytohormones in relation to enhanced growth as a response to canopy shading is reviewed. In addition, changes in stress-related enhanced growth in response to submergence, which is akin to canopy shading in some ways, are discussed. The recent advances in understanding how the phytohormone balance is altered upon receiving such an environmental stimulus is then examined, with a focus on auxins, gibberellins, cytokinins, and ethylene.
The shade avoidance response
Plants sense changes in light quality due to shading by competing vegetation, using the ratio of red to far-red light (R:FR) with the help of phytochromes. Shade-avoiding species then react by elongating dramatically, altering the whole plant architecture, in order to emerge from the blockage. This elongation response is often linked with reduced leaf development, enhanced apical dominance, and a re-allocation of resources from storage favouring shoot elongation to reproductive growth; a similar elongation response to that which can be observed if a plant is temporarily submerged. If the plant is unsuccessful in outgrowing its competing vegetation, flowering is accelerated (Halliday et al., 1994), a reaction that would be detrimental for the yield of most crop species.
The phytochrome family of photoreceptors in the Arabidopsis genome consists of five members, phyA–phyE. By sensing and reacting to R and FR light, they regulate a variety of developmental processes in response to light (reviewed in Franklin and Quail, 2010). PhyB has been shown to be the key player in shade avoidance (Reed et al., 1993), with additional redundant activities of phyD and phyE (Aukerman et al., 1997; Devlin et al., 1998, 1999; Franklin et al., 2003). On the other hand, phyA, which is the major factor regulating de-etiolation in seedlings, appears to moderate the shade avoidance response in light-grown seedlings (Johnson et al., 1994). Phytochromes directly bind to PHYTOCHROME-INTERACTING FACTORS (PIFs), which are transcription factors involved in light-regulated responses. The model of phytochrome function suggests that the active (FR-absorbing) form of phytochromes (Pfr) interacts with PIFs, leading to their degradation via the ubiquitin–proteasome pathway. Through the absorption of FR light they convert into the red-light absorbing inactive form (Pr), which is unable to interact with PIFs, thus allowing the transcription of light-regulated genes (Smith and Whitelam, 1997; Lorrain et al., 2008; Fig. 2). PIF4 and PIF5 have recently been shown to be positive regulators of shade avoidance responses, participating in the regulation of some key players in these responses, such as ATHB2, a homeodomain leucine-zipper (HD-Zip) protein with a positive role, LONG HYPOCOTYL IN FAR RED1 (HFR1)/SLENDER IN CANOPY SHADE1 (SICS1), a basic helix–loop–helix (bHLH) transcription factor with a major negative regulatory role in shade avoidance, and PIF3-LIKE1 (PIL1), another bHLH transcription factor positively regulating shade avoidance (Salter et al., 2003; Lorrain et al., 2008) (Table 1). Phytochrome-mediated regulation of gene expression thus appears to be one of the main mechanisms of growth regulation in response to light signals (Franklin and Quail, 2010). Furthermore, the shade avoidance response appears to be coupled to the circadian clock, since the rapid elongation response upon a low R:FR signal is strongest at dusk, and requires both PIL1 and TIMING OF CAB EXPRESSION 1 (TOC1), a known circadian clock protein (Salter et al., 2003).
List of genes involved in the regulation of the shade avoidance and submergence response
| Mediator of response | Genes involved | Nature of action of the protein | Remarks | Reference(s) | |
| Positive | Negative | ||||
| Phytochromes | phyA | Johnson et al. (1994) | |||
| phyB | Reed et al. (1993); Franklin et al. (2003) | ||||
| PhyD | Aukerman et al. (1997); Devlin et al. (1999); Franklin et al. (2003) | ||||
| phyE | Devlin et al. (1998); Franklin et al. (2003) | ||||
| Phytochrome-interacting factors (PIFs) | PIF4 | Transcription factor | Lorrain et al. (2008) | ||
| PIF5 | Transcription factor | Lorrain et al. (2008) | |||
| Key transcription factors | PIL1 | Transcription factor | bHLH type | Lorrain et al. (2008); Salter et al. (2003) | |
| ATHB2 | Transcription factor | HD-Zip type | Lorrain et al. (2008) | ||
| ATHB4 | ATHB4 | Transcription factor | HD-Zip type | Sorin et al., 2009 | |
| HFR1/ SICS1 | Transcription factor | bHLH type | Lorrain et al. (2008) | ||
| Phytohormones | |||||
| Auxins | IAA29 | Signalling intermediate | Sessa et al. (2005) | ||
| PIN3 | Transport of hormone | Devlin et al. (2003) | |||
| PIN7 | Transport of hormone | Devlin et al. (2003) | |||
| TAA1 | Biosynthesis of hormone | Tao et al. (2008) | |||
| IAA19 | Signalling intermediate | Pierik et al. (2009) | |||
| Gibberellins | GA20ox | Biosynthesis of hormone | Devlin et al. (2003) | ||
| GAI | Signalling repressor | DELLA protein | Sessa et al. (2005) | ||
| RGA | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| RGL1 | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| RGL2 | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| Cytokinins | CKX5 | Metabolism of hormone | Sessa et al. (2005) | ||
| CKX6 | Metabolism of hormone | Carabelli et al. (2007) | |||
| Ethylene | ACS8 | Biosynthesis of hormone | Sessa et al. (2005) | ||
| SK1 | Signalling intermediate | Submergence induced | Hattori et al. (2009) | ||
| SK2 | Signalling intermediate | Submergence induced | Hattori et al. (2009) | ||
| Brassinosteroids | BRI1 | Hormone receptor | Devlin et al. (2003) | ||
| Effectors | RpEXPA1 | Cell wall-losening enzyme | From Rumex palustris model | Vreeburg et al. (2005) |
| Mediator of response | Genes involved | Nature of action of the protein | Remarks | Reference(s) | |
| Positive | Negative | ||||
| Phytochromes | phyA | Johnson et al. (1994) | |||
| phyB | Reed et al. (1993); Franklin et al. (2003) | ||||
| PhyD | Aukerman et al. (1997); Devlin et al. (1999); Franklin et al. (2003) | ||||
| phyE | Devlin et al. (1998); Franklin et al. (2003) | ||||
| Phytochrome-interacting factors (PIFs) | PIF4 | Transcription factor | Lorrain et al. (2008) | ||
| PIF5 | Transcription factor | Lorrain et al. (2008) | |||
| Key transcription factors | PIL1 | Transcription factor | bHLH type | Lorrain et al. (2008); Salter et al. (2003) | |
| ATHB2 | Transcription factor | HD-Zip type | Lorrain et al. (2008) | ||
| ATHB4 | ATHB4 | Transcription factor | HD-Zip type | Sorin et al., 2009 | |
| HFR1/ SICS1 | Transcription factor | bHLH type | Lorrain et al. (2008) | ||
| Phytohormones | |||||
| Auxins | IAA29 | Signalling intermediate | Sessa et al. (2005) | ||
| PIN3 | Transport of hormone | Devlin et al. (2003) | |||
| PIN7 | Transport of hormone | Devlin et al. (2003) | |||
| TAA1 | Biosynthesis of hormone | Tao et al. (2008) | |||
| IAA19 | Signalling intermediate | Pierik et al. (2009) | |||
| Gibberellins | GA20ox | Biosynthesis of hormone | Devlin et al. (2003) | ||
| GAI | Signalling repressor | DELLA protein | Sessa et al. (2005) | ||
| RGA | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| RGL1 | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| RGL2 | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| Cytokinins | CKX5 | Metabolism of hormone | Sessa et al. (2005) | ||
| CKX6 | Metabolism of hormone | Carabelli et al. (2007) | |||
| Ethylene | ACS8 | Biosynthesis of hormone | Sessa et al. (2005) | ||
| SK1 | Signalling intermediate | Submergence induced | Hattori et al. (2009) | ||
| SK2 | Signalling intermediate | Submergence induced | Hattori et al. (2009) | ||
| Brassinosteroids | BRI1 | Hormone receptor | Devlin et al. (2003) | ||
| Effectors | RpEXPA1 | Cell wall-losening enzyme | From Rumex palustris model | Vreeburg et al. (2005) |
List of genes involved in the regulation of the shade avoidance and submergence response
| Mediator of response | Genes involved | Nature of action of the protein | Remarks | Reference(s) | |
| Positive | Negative | ||||
| Phytochromes | phyA | Johnson et al. (1994) | |||
| phyB | Reed et al. (1993); Franklin et al. (2003) | ||||
| PhyD | Aukerman et al. (1997); Devlin et al. (1999); Franklin et al. (2003) | ||||
| phyE | Devlin et al. (1998); Franklin et al. (2003) | ||||
| Phytochrome-interacting factors (PIFs) | PIF4 | Transcription factor | Lorrain et al. (2008) | ||
| PIF5 | Transcription factor | Lorrain et al. (2008) | |||
| Key transcription factors | PIL1 | Transcription factor | bHLH type | Lorrain et al. (2008); Salter et al. (2003) | |
| ATHB2 | Transcription factor | HD-Zip type | Lorrain et al. (2008) | ||
| ATHB4 | ATHB4 | Transcription factor | HD-Zip type | Sorin et al., 2009 | |
| HFR1/ SICS1 | Transcription factor | bHLH type | Lorrain et al. (2008) | ||
| Phytohormones | |||||
| Auxins | IAA29 | Signalling intermediate | Sessa et al. (2005) | ||
| PIN3 | Transport of hormone | Devlin et al. (2003) | |||
| PIN7 | Transport of hormone | Devlin et al. (2003) | |||
| TAA1 | Biosynthesis of hormone | Tao et al. (2008) | |||
| IAA19 | Signalling intermediate | Pierik et al. (2009) | |||
| Gibberellins | GA20ox | Biosynthesis of hormone | Devlin et al. (2003) | ||
| GAI | Signalling repressor | DELLA protein | Sessa et al. (2005) | ||
| RGA | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| RGL1 | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| RGL2 | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| Cytokinins | CKX5 | Metabolism of hormone | Sessa et al. (2005) | ||
| CKX6 | Metabolism of hormone | Carabelli et al. (2007) | |||
| Ethylene | ACS8 | Biosynthesis of hormone | Sessa et al. (2005) | ||
| SK1 | Signalling intermediate | Submergence induced | Hattori et al. (2009) | ||
| SK2 | Signalling intermediate | Submergence induced | Hattori et al. (2009) | ||
| Brassinosteroids | BRI1 | Hormone receptor | Devlin et al. (2003) | ||
| Effectors | RpEXPA1 | Cell wall-losening enzyme | From Rumex palustris model | Vreeburg et al. (2005) |
| Mediator of response | Genes involved | Nature of action of the protein | Remarks | Reference(s) | |
| Positive | Negative | ||||
| Phytochromes | phyA | Johnson et al. (1994) | |||
| phyB | Reed et al. (1993); Franklin et al. (2003) | ||||
| PhyD | Aukerman et al. (1997); Devlin et al. (1999); Franklin et al. (2003) | ||||
| phyE | Devlin et al. (1998); Franklin et al. (2003) | ||||
| Phytochrome-interacting factors (PIFs) | PIF4 | Transcription factor | Lorrain et al. (2008) | ||
| PIF5 | Transcription factor | Lorrain et al. (2008) | |||
| Key transcription factors | PIL1 | Transcription factor | bHLH type | Lorrain et al. (2008); Salter et al. (2003) | |
| ATHB2 | Transcription factor | HD-Zip type | Lorrain et al. (2008) | ||
| ATHB4 | ATHB4 | Transcription factor | HD-Zip type | Sorin et al., 2009 | |
| HFR1/ SICS1 | Transcription factor | bHLH type | Lorrain et al. (2008) | ||
| Phytohormones | |||||
| Auxins | IAA29 | Signalling intermediate | Sessa et al. (2005) | ||
| PIN3 | Transport of hormone | Devlin et al. (2003) | |||
| PIN7 | Transport of hormone | Devlin et al. (2003) | |||
| TAA1 | Biosynthesis of hormone | Tao et al. (2008) | |||
| IAA19 | Signalling intermediate | Pierik et al. (2009) | |||
| Gibberellins | GA20ox | Biosynthesis of hormone | Devlin et al. (2003) | ||
| GAI | Signalling repressor | DELLA protein | Sessa et al. (2005) | ||
| RGA | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| RGL1 | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| RGL2 | Signalling repressor | DELLA protein | Djakovic-Petrovic et al. (2007); Pierik et al. (2007) | ||
| Cytokinins | CKX5 | Metabolism of hormone | Sessa et al. (2005) | ||
| CKX6 | Metabolism of hormone | Carabelli et al. (2007) | |||
| Ethylene | ACS8 | Biosynthesis of hormone | Sessa et al. (2005) | ||
| SK1 | Signalling intermediate | Submergence induced | Hattori et al. (2009) | ||
| SK2 | Signalling intermediate | Submergence induced | Hattori et al. (2009) | ||
| Brassinosteroids | BRI1 | Hormone receptor | Devlin et al. (2003) | ||
| Effectors | RpEXPA1 | Cell wall-losening enzyme | From Rumex palustris model | Vreeburg et al. (2005) |
The involvement of phytohormones in the shade avoidance response has been shown early and frequently. However, many of the exact molecular mechanisms linking shade-initiated transcriptional changes with phytohormone-related responses are only beginning to be revealed. It has been shown that the phytohormones brassinosteroids, auxins, ethylene, and gibberellins are involved in the shade avoidance response, because mutations in genes involved in their metabolism or signalling lead to a reduced FR light-induced elongation response, and suppression of the constitutive shade-avoiding phyB phenotype, respectively (e.g. Kim et al., 1998; Kanyuka et al., 2003; Pierik et al., 2004; Hisamatsu et al., 2005). In a microarray analysis to identify early and late gene expression changes in FR-enriched light, Devlin et al. (2003) identified several genes involved in phytohormone metabolism and signalling. It was not until Sessa et al. (2005) discovered that HFR1/SICS1 is a major negative regulator of shade avoidance that the first link between perception of canopy shading and phytohormone responses was identified. The bHLH-type transcription factor HFR1 down-regulates ATHB2 (Carabelli et al., 1993; Schena et al., 1993), amongst others. It is thought to possess a fundamental role in plant acclimation by preventing an exaggerated elongation growth, in cases of unsuccessful avoidance of shading. The molecular mechanism of this negative regulation was recently elucidated by Hornitschek et al. (2009). The authors were able to show that PIF4 and PIF5 induce the expression of shade avoidance-related genes by directly binding to G-boxes in their promoters; in prolonged shade HFR1 will accumulate and bind to both PIF4 and PIF5, forming non-DNA-binding heterodimers, thus preventing PIF4- and PIF5-mediated gene expression. In the hfr1 loss-of-function background, several phytohormone-related genes are up-regulated, for example GAI, IAA29, ACS8, or CKX5, linking shading responses with the signalling or metabolism of gibberellins, auxins, ethylene, and cytokinins, respectively (Sessa et al., 2005). This observation is corroborated by the resemblance of the shading-induced elongation phenotype to those phenotypes that are caused by increased levels of auxins, ethylene, or gibberellin, and decreased levels of cytokinin, respectively, further substantiating the involvement of those four phytohormones in the shade avoidance response.
Furthermore, Kurepin et al. (2007) identified significant changes in the content of gibberellins and the auxin indole 3-acetic acid (IAA) in sunflower seedlings in response to changes in light quality. Subsequently, Roig-Villanova et al. (2007) showed that the sensitivity to phytohormones is rapidly changed in response to canopy shading. A second HD-Zip transcription factor, ATHB4, was identified recently as another key player in shade avoidance responses (Sorin et al., 2009). The study revealed that ATHB4 specifically alters the sensitivity of hypocotyls to auxins, brassinosteroids, and gibberellins in FR-enriched light. The authors also showed that it effectively down-regulates subsets of auxin- and/or brassinosteroid-regulated genes. However, it appears to be difficult to categorize ATHB4 as being either a positive or a negative regulator, since both gain and loss of function diminished the hypocotyl elongation response to shading (Sorin et al., 2009).
It thus appears that the perception of canopy shade leads to extensive changes not only in phytohormone content through the regulation of metabolic enzymes, but also in the sensitivity to phytohormones, which might be achieved by the up- or down-regulation of the expression of receptor genes. This in fact was shown to be the case for the brassinosteroid receptor BRI1, whose expression is rapidly up-regulated in FR-supplemented light (Devlin et al., 2003) (Table 1). Loss of function of BRI1 leads to a severely dwarfed stature due to a strong reduction in cell expansion, and dark-grown bri1 seedlings appear de-etiolated (Noguchi et al., 1999), further supporting a key role for BRI1 (and thus brassinosteroids) in elongation growth in response to light signals.
At the same time, intermediates of phytohormone signal transduction pathways appear to be regulated during the shade avoidance response as well. It was therefore suggested by Sorin et al. (2009) that transcriptional networks regulated by light quality changes (low R:FR) intersect with phytohormone-related transcriptional and signalling networks controlling cell proliferation and expansion, as was suggested by Nemhauser (2008) for photomorphogenesis.
Auxin-mediated growth is essential for shade avoidance
Auxins are involved in controlling virtually all aspects of growth and development in plants, including, but not limited to, directional growth responses to external cues (phototropism and gravitropism), de novo organogenesis of leaves, flowers, floral organs, and lateral roots (Benková et al., 2003), the formation of vascular tissue in leaves (Mattsson et al., 2003; Scarpella et al., 2006), and the maintenance of meristem identity in shoot apical meristem (SAM) and root apical mersitem (RAM) (Sabatini et al., 1999; Friml et al., 2003). Auxin responses are mediated by a number of transcriptional regulators named AUXIN RESPONSE FACTORS (ARFs) and corresponding AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) proteins that inhibit ARFs. This inhibition is relieved by the auxin signal triggering the ubiquitination and subsequent degradation of Aux/IAAs (reviewed in Vanneste and Friml, 2009). It thus seems that auxin regulates a wide range of developmental responses by one common mechanism. Vanneste and Friml (2009) therefore suggested that auxins merely act as a trigger for a change that is pre-programmed in the target cell or tissue. In that case, the auxin signal only selects time and space for the change of the developmental programme. Such a genetic framework for auxins in the regulation of lateral root initiation was recently shown by De Smet et al. (2010). The authors elegantly showed that auxins control the initiation of lateral roots through the activation of at least two successive modules, namely the SOLITARY ROOT (SLR)/IAA13-ARF7-ARF19-mediated pericycle cell division, followed by the BODENLOS (BDL)/IAA12-MONOPTEROS/ARF5-mediated lateral root patterning. Interestingly, neither of these modules is able to initiate the formation of lateral roots on its own. The authors speculate, furthermore, that such coordinated activation of multiple modules could be the general mode of action of auxins, providing a possible explanation for the wide variety of responses to the phytohormone.
Interestingly, in the case of auxins, not only the phytohormone itself can serve as a signal, but the actual level of auxins in a specific cell or tissue is able to determine the response. This is reflected in the highly dynamic pattern of differential auxin distribution between cells of a plant tissue, resulting in auxin maxima and gradients. Those gradients are established mostly through local biosynthesis (Cheng et al., 2007; Stepanova et al., 2008; Tao et al., 2008) and polar auxin transport (reviewed in Tanaka et al., 2006). It is well established that both polar auxin transport and the resulting auxin gradient are necessary for plant growth and morphological patterning (Feraru and Friml, 2008). Both auxin biosynthesis and transport are in turn controlled by diverse environmental signals as well as other phytohormones. Interestingly, polar auxin transport appears to be directly influenced by membrane lipid content, since the auxin transport protein PIN1 is redistributed in response to a decrease in sitosterol and an increase in cholesterol (Willemsen et al., 2003) as well as a reduction in very-long-chain fatty acids (Roudier et al., 2010). This reinforces the tight link that phytohormones, in this case auxins, form between exogenous cues and development, since membrane lipid content in turn is subject to environmental control (e.g. Guy et al., 2008; Narise et al., 2010).
Studies have shown that the inhibition of auxin transport alone is sufficient to abolish the hypocotyl elongation response to FR-enriched light (Steindler et al., 1999), and the auxin efflux carriers PIN3 and PIN7 have been identified amongst genes regulated under this condition (Devlin et al., 2003). This led Morelli and Ruberti (2000) to hypothesize that the auxin transport stream is re-directed in response to canopy shading, either by redistribution of specific auxin efflux carrier or activation of regulatory proteins that control those efflux carriers, or both. Furthermore, an enzyme involved in the IAA biosynthetic pathway from L-tryptophan (L-Trp), TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 (TAA1), has been identified recently (Tao et al., 2008). Interestingly, the authors showed that TAA1 is directly involved in de novo auxin biosynthesis in leaves in response to FR-enriched light, and that it is required for hypocotyl and petiole elongation as well as the leaf hyponasty response. These findings suggest that auxin transport to the sites of elongation (the hypocotyl) is required for those responses to occur, a notion that is corroborated by the above-mentioned studies which indicate an important role for auxin transport in shade avoidance responses (Steindler et al., 1999; Devlin et al., 2003).
The actual level of bioactive auxins, however, is equally important for the growth responses to occur, which is represented by the rapid induction of several auxin-responsive genes (IAA genes) upon the perception of FR-enriched light (Devlin et al., 2003). The transcripts of one such auxin-induced gene, IAA19, has been shown to be localized in petioles and hypocotyls (Pierik et al., 2009), further substantiating the need for auxin transport to occur in the shade avoidance response. Despite findings of auxins affecting the stability of DELLA proteins to induce growth in roots (see below; Achard et al., 2003; Fu and Harberd, 2003), Pierik et al. (2009) concluded that the auxin-mediated shade avoidance appears to be independent of the gibberellin signalling pathway. Application of an auxin transport inhibitor abolishes hypocotyl elongation induced by FR-enriched light not only in the wild type, but also in a mutant with increased gibberellin signalling (in a mutant with four out of five DELLA genes knocked out; see below). The auxin pathway thus probably represents an alternative route in the shade avoidance response, functionally parallel to, but not intersecting with the gibberellin route (Pierik et al., 2009). On the other hand, the intersection of the shade-induced auxin pathway with the gibberellin route might also represent a tissue-specific interaction that is necessary for the root elongation growth, but of lesser importance for shade-induced elongation growth.
Gibberellin-induced elongation is necessary, but not sufficient, for the shade avoidance response
According to the classical view, gibberellins are another growth-promoting class of phytohormones, regulating a wide range of growth and developmental processes throughout the life cycle of a plant, including seed germination, leaf expansion, induction of flowering, as well as flower and seed development (Sun and Gubler, 2004; Yamaguchi, 2008). It is therefore not surprising that they frequently appear to work synergistically with auxins. For example, stem elongation has been shown to be coordinately regulated by auxins and gibberellins (Ross et al., 2001). The effects of auxins and gibberellins overlap with respect to cell expansion as well as tissue differentiation. Gibberellin-induced root elongation was also shown to require auxins (Fu and Harberd, 2003).
The level of active gibberellins is controlled by several factors, both external, for example light and temperature (Yamaguchi and Kamiya, 2000; García-Martínez and Gil, 2001), and internal, specifically auxins (Ross et al., 2000, 2001; Wolbang and Ross, 2001; Goda et al., 2004; Frigerio et al., 2006), which were shown to induce the gibberellin biosynthetic genes GA20ox and GA3ox (O'Neill and Ross, 2002; Frigerio et al., 2006; Nemhauser et al., 2006). This effect of auxins on gibberellin biosynthesis is mediated by the auxin receptor TRANSPORT INHIBITOR RESPONSE1 (TIR1) (Frigerio et al., 2006), and occurs via the degradation of Aux/IAA proteins and the resulting activation of AUXIN RESPONSE FACTOR7 (ARF7) (reviewed in Teale et al., 2006; Fig. 1). Furthermore, TIR3, identified as a protein involved in auxin transport, appears to influence the C20 oxidation of gibberellins, since tir3 mutants display an abnormal stem elongation response to C20-gibberellin (Sponsel et al., 1997).
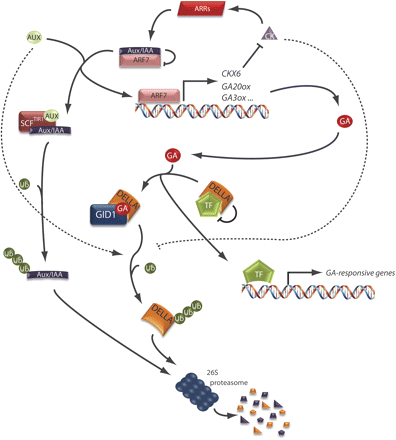
Interplay of auxins (AUX) and gibberellins (GA) in gene regulation. The regulation of auxin- and gibberellin-induced genes occurs in a similar manner; in the presence of the phytohormone, a phytohormone–receptor–repressor complex (auxin–SCFTIR1–Aux/IAA and gibberellin–GID1–DELLA, respectively) is formed. This leads to the degradation of the repressors via the ubiquitin–proteasome pathway, thus releasing transcription factors (ARF7 and ‘TF’, respectively) from inactive complexes with the repressors. Auxin appears to work upstream of the gibberellin signalling pathway, since gibberellin accumulation occurs as a result of auxin-induced transcription of the gibberellin biosynthetic genes GA20ox and GA3ox. Furthermore, auxin induces the expression of CKX6, a gene encoding a cytokinin-degrading enzyme. This results in a reduced cytokinin level, leading to the repression of cytokinin-induced, ARR-mediated accumulation of Aux/IAA proteins, amongst others, which provide a positive feedback regulation of auxin-induced transcription. Both auxin and cytokinin affect the degradation of DELLA proteins (dotted lines), either by stabilizing DELLAs (cytokinin) or by being required for their degradation (auxin) in an as yet unknown fashion. Arrows indicate activation, and blocked arrows indicate repression of either protein function or accumulation.
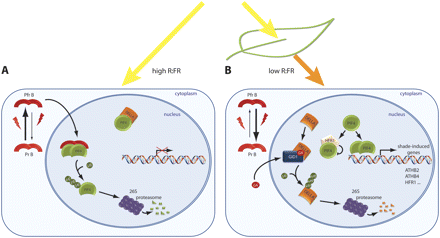
PIF4-mediated shade-induced gene regulation. In white light (high R:FR), PIF4-mediated transcriptional activation is inhibited. Part of the PIF4 protein pool is sequestered in inactive complexes with DELLA proteins, whereas another part is bound by the FR-absorbing active phyB (PfrB), which leads to the degradation of PIF4 via the ubiquitin–proteasome pathway. In FR light-enriched environments, e.g. due to canopy shade, PfrB is converted to its R-absorbing inactive form (PrB), which abolishes its ability to bind PIF4. Thus, PIF4 can accumulate, allowing transcription of shade-induced genes. In addition, gibberellin levels increase in response to shade, which leads to the degradation of DELLA proteins, releasing an additional pool of PIF4 protein. In prolonged shade, HFR1 accumulates and forms heterodimers with PIF4 that do not bind to DNA any more, thus preventing an exaggerated response.
For the case of one of the gibberellin biosynthetic genes, GA20ox, Desgagné-Penix and Sponsel (2008) propose four different levels of regulation following a strict hierarchy of spatial, developmental, metabolic, and auxin-mediated regulation. The authors suggest that the spatial regulation of GA20ox expression (i.e. high expression in cotyledons and leaves, and low expression in roots) over-rides all other levels of regulation.
DELLA proteins are the key negative regulators of gibberellin signalling; they repress gibberellin-induced growth responses, which is relieved mostly, but not exclusively, by their degradation via the ubiquitin–proteasome pathway (reviewed in Schwechheimer and Willige, 2009). Arabidopsis contains five DELLA proteins (GAI, RGA, RGL1, RGL2, and RGL3) with both overlapping and unique functions (Lee et al., 2002; Cheng et al., 2004; Cao et al., 2005). The current model of gibberellin action suggests that DELLAs sequester transcription factors in inactive complexes. Through the recognition of gibberellins, the receptor GID1 changes its conformation and binds to DELLA proteins (Murase et al., 2008; Shimada et al., 2008). This complex also recruits the SCFSLY E3 ubiquitin ligase, leading to the degradation of DELLA proteins, and the relief of the transcription factors. This transcriptional regulation by binding to transcription factors might well represent the main mode of DELLA function, since to date there are no reports of DELLAs directly associating with DNA, and chromatin immunoprecipitation studies with DELLA proteins only lead to subtle promoter enrichment (Zentella et al., 2007). Interestingly, all DELLA-regulated genes identified in this study were shown to be gibberellin repressed and DELLA induced. This indicates that DELLAs might not only repress transcription by sequestering transcription factors, but also activate transcription by forming either active complexes with transcription factors or inactive complexes with transcriptional repressors.
DELLAs have been proposed to integrate several other phytohormone pathways on several occasions. For example, in the case of gibberellin-induced root elongation, auxin was shown to be necessary for DELLA degradation to occur (Fu and Harberd, 2003). The endodermis seems to represent the main tissue governing this elongation response, since gibberellin signalling in other root tissues was shown not to affect the overall growth rate (Ubeda-Tomás et al., 2008). On the other hand, both ethylene and abscisic acid seem to stabilize DELLA proteins during root growth (Achard et al., 2003, 2006).
In the SAM, stem cell maintenance requires high cytokinin and low gibberellin levels (Sakamoto et al., 2001; Jasinski et al., 2005; Yanai et al., 2005). This is achieved by cytokinins, inducing DELLA protein expression (Brenner et al., 2005). Cytokinins were also shown to control gibberellin levels by inhibiting gibberellin biosynthesis and inducing gibberellin catabolism (Brenner et al., 2005; Jasinski et al., 2005). This negative regulation is reflected in the generally antagonistic action of cytokinins and gibberellins, for example in the regulation of shoot and root elongation, cell differentiation, shoot regeneration in culture, or meristem activity (Greenboim-Wainberg et al., 2005; Jasinski et al., 2005). However, Greenboim-Wainberg et al. (2005) showed that cytokinins do not affect gibberellin biosynthesis or signalling. These seemingly contradictory results might simply be due to the different approaches used. Whereas Brenner et al. (2005) performed a genome-wide expression analysis of Arabidopsis seedlings at several time points after cytokinin treatment, Greenboim-Wainberg et al. (2005) based their conclusion on phenotypic analyses (germination in the presence of the gibberellin biosynthesis inhibitor paclobutrazol as well as the gibberellin effect on flowering time) and the expression level of one gibberellin-regulated gene. In line with the hierarchy of regulation proposed by Desgagné-Penix and Sponsel (2008), feedback mechanisms might regulate the misexpression of genes on a developmental level following a single exogenous phytohormone application; the actual change in expression levels could therefore remain without effect on the overall growth and development.
Despite the emerging evidence of DELLAs acting as key integrators for several hormonal as well as environmental pathways, growth control is not entirely DELLA dependent. Another negative regulator of gibberellin signalling, SPINDLY (SPY), was shown to suppress cytokinin responses by inhibiting the induction of a subset of the cytokinin signalling intermediates ARABIDOPSIS RESPONSE REGULATORs (ARRs) (Greenboim-Wainberg et al., 2005). Swain et al. (2001) showed that SPY can act in those two different pathways through the interaction with different proteins. Although evidence has yet to be reported, it is proposed that SPY might distinguish between different branches of cytokinin signalling by specifically targeting only a subset of ARRs (Greenboim-Wainberg et al., 2005).
The requirement for gibberellins in the elongation response upon a monochromatic FR stimulus was shown by Martínez-García et al. (2000), and the rapid induction of one of the gibberellin biosynthetic genes, GA20ox, in FR-enriched light (Devlin et al., 2003) reinforces this view. The involvement of DELLA proteins in the shading-induced elongation response has also been determined on many occasions. Their exact role, however, was only recently shown by Djakovic-Petrovic et al. (2007) and Pierik et al. (2007). According to these studies, the elongation response to canopy shade in dense stands is mediated by the breakdown of DELLA proteins due to an increase in gibberellin action. However, they also provided evidence that this degradation of DELLA proteins is necessary, but not sufficient, to induce the hypocotyl elongation response upon shading; quadruple DELLA knockout mutants (gai rga rgl1 rgl2) do not exhibit a constitutive shade-avoiding phenotype, and react to shading with an induced elongation similar to that of wild-type plants (Djakovic-Petrovic et al., 2007; Pierik et al., 2007). On the other hand, Feng et al. (2008) observed that a mutant lacking all five DELLAs (gai rga rgl1 rgl2 rgl3) shows a slightly altered response to red light. This leaves room to speculate that RGL3 could be the DELLA protein with the main role in the shade-induced elongation growth, or that all five DELLA proteins are required to regulate this response in a cumulative way.
Nonetheless, DELLA proteins are accorded an important role in the shade avoidance response. They were shown to bind to the light-responsive transcription factors PIF3 and PIF4, sequestering them in inactive complexes, preventing transcriptional activation of downstream genes (de Lucas et al., 2008; Feng et al., 2008). PIF4 is thought to be involved in shade avoidance, regulating genes associated with cell elongation. Therefore, the degradation of DELLA proteins upon shading-induced gibberellin biosynthesis would lead to the release of PIF4, allowing transcription of target genes. DELLA stability, as discussed earlier, is regulated not only by gibberellins, but also by auxins and ethylene (Achard et al., 2003; Fu and Harberd, 2003). This further reinforces the view that DELLA proteins might be acting as key integrators of different environmental as well as endogenous (hormonal) cues. Furthermore, based on the hypothesis of Franklin and Quail (2010) that phytochrome-mediated regulation of gene expression could be the main mechanism of growth regulation in response to light signals, it is tempting to speculate that DELLA proteins could be key players in this gene expression control, integrating hormonal control of growth with light signals.
Cytokinins antagonistically regulate shade-induced morphological changes
Cytokinins were discovered based on their ability to induce cell division (Miller et al., 1955), but are now known also to have roles in the regulation of germination, shoot and root development, leaf senescence, interaction with pathogens, as well as circadian rhythms (To and Kieber, 2008).
In the induction of cell proliferation, cytokinins were shown to up-regulate CycD3 transcripts in shoots (Nogue et al., 2000). However, the G1 transition in the cell cycle can only occur if both cyclin-dependent kinase and the corresponding cyclin are present. Intriguingly, auxins can induce cell proliferation by up-regulating the Cdc2 class of cyclin-dependent kinases (John et al., 1993), resulting in a situation where both phytohormones need to be present in order to induce cell proliferation.
In roots, on the other hand, auxins and cytokinins appear to regulate Cyc2 expression and thus cell division antagonistically (John et al., 1993). An opposing effect of cytokinins and auxins seems to be more frequent, and is reflected in the regulation of each other's abundance. Auxin, if overproduced in tobacco plants, leads to reduced levels of cytokinins, and vice versa (Eklöf et al., 1997, 2000). It was suggested that auxins can reduce the pool of active cytokinins by directly regulating the activity of cytokinin oxidase (Zhang et al., 1995).
A similar contrast in the cytokinin–auxin interaction between shoot and root can be observed in the determination and maintenance of meristematic tissue. Auxin accumulation and response is required for the specification of the embryonic root meristem founder cell (hypophysis), which will give rise to the quiescent centre and the neighbouring stem cells of the root meristem in the adult plant (Friml et al., 2003; Aida et al., 2004; Weijers et al., 2006). Cytokinins have also been shown to be required for the formation of the root stem cell niche during embryogenesis (Müller and Sheen, 2008). The authors show, however, that high endogenous auxin content directly induces the cytokinin signalling repressors ARR7 and ARR15. Furthermore, phytohormone levels visualized by green fluorescent protein (GFP) expression under phytohormone-inducible promoter control indicate adjacent, rather than overlapping, functions of cytokinins and auxins in the hypophysis-derived cells. In the adult root, cytokinins are implicated to act at the border between the proliferation and differentiation zone (transition zone) and inhibit auxin responses via ARR1-mediated induction of SHY2 (IAA3), a negative regulator of auxin signalling (Dello Ioio et al., 2007). Auxin, on the other hand, retains its maximum concentration in the quiescent centre of the root during post-embryonic development, where it functions in stem cell maintenance, and inhibits cytokinin biosynthesis and signalling. Interestingly, Miyawaki et al. (2004) have shown that auxin treatment can induce some of the cytokinin biosynthetic ISOPENTENYLTRANSFERASE (IPT) genes in Arabidopsis. Although it has to be taken into consideration that exogenously applied auxin does not mirror physiological conditions with respect to the actual amount of phytohormone or the normal site of its action, this induction of cytokinin biosynthesis might represent a dose-dependent effect of auxins. A dose-dependent activation of inward and outward K+ channels in stomata by auxins has been shown earlier (Blatt and Thiel, 1994), and could also be the case in roots, where an obvious auxin gradient is present.
In shoots, cytokinins accumulate in the meristematic tissue, whereas auxin levels are generally low due to the polar (basipetal) transport. The homeobox transcription factors SHOOT MERISTEMLESS (STM) and WUSCHEL (WUS) play a key role in the establishment and maintenance of the SAM, and their functions appear to be tightly linked with cytokinins (Wolters and Jürgens, 2009). STM belongs to the KNOTTED-like homeobox proteins, and was shown to induce the cytokinin biosynthetic gene IPT7 (Jasinski et al., 2005; Yanai et al., 2005), while WUS down-regulates the cytokinin signalling repressors ARR5 and ARR7 (Leibfried et al., 2005). Auxins, on the other hand, only locally accumulate in the periphery of the shoot meristem, where they trigger the initiation of organ primordia (Reinhardt et al., 2003). At those sites auxins also inhibit cytokinin biosynthesis (Nordström et al., 2004), and down-regulate STM expression (Furutani et al., 2004; Heisler et al., 2005). It thus appears that auxins play a role in limiting both CK and STM action in maintaining the stem cell identity in the SAM.
The role of cytokinin breakdown in the inhibition of leaf development during shade avoidance was substantiated when Carabelli et al. (2007) showed that CKX6, a cytokinin oxidase, is induced in simulated shade and promotes cytokinin breakdown specifically in pre-procambial cells of developing leaf primordia. This, in turn, appears to be sufficient to arrest leaf primordial growth, which was shown to be due to a reduction in cell proliferation rather than elongation. They showed furthermore that this CKX6 induction is an auxin response, and is mediated by the auxin receptor TIR1, which does not seem to be involved in the hypocotyl elongation response to shading. It is therefore tempting to conclude that multiple receptors of one phytohormone might be involved in non-redundant responses, either in different tissues, in different developmental stages, or upon different environmental cues. In fact, this hypothesis was corroborated recently, when Vidal et al. (2010) showed that microRNA 393 (miR393) targets transcripts of a bHLH transcription factor as well as the auxin receptors TIR1, AFB1, AFB2, and AFB3. However, in response to nitrate, miR393 specifically down-regulates only AFB3 in Arabidopsis roots to induce changes in root architecture. Similar regulation by other microRNAs in shoot development may occur in response to phytohormones and/or environmental signals, adding an additional level of complexity to the growth-regulating network.
Another role for cytokinins in perception and response to shading was elucidated when Pons et al. (2001) showed that the transpiration rate of leaves correlates with the vertical light gradient in tobacco plants, and that this correlation is potentiated in dense, more shaded stands. This reduced transpiration rate in shaded leaves leads to a reduced import of compounds from the transpiration stream, of which cytokinin was shown to be responsible for the induced re-allocation of resources as well as the adaptation of photosynthesis, which is reflected by a reduced chlorophyll a/b ratio (Boonman and Pons, 2007; Boonman et al., 2007, 2009). Furthermore, this photosynthetic adaptation to canopy density appears to be redundantly regulated by cytokinin and phyD, since it is reduced in both phyD and cytokinin signalling mutants (Boonman et al., 2009). Hence it is clear that cytokinins are involved in regulating the response to shading along with the other major phytohormones, such as gibberellins and auxins. The complex signalling cross-talk between cytokinins and auxins as well as cytokinins and gibberellins described earlier demonstrates the intricate regulatory network in the process.
Ethylene appears to be the initiator of stress-induced growth
The phytohormone ethylene is involved in several growth responses, including seed germination, seedling growth, development of leaves, root, stem, and flowers, fruit ripening, senescence, and abscission. Its biosynthesis is greatly enhanced by various stresses as well as a range of other phytohormones. It is therefore thought to have a major role in the integration and coordination of environmental and endogenous cues.
Interestingly, Finlayson et al. (1999) observed a circadian rhythm for ethylene production in Sorghum bicolor. They also showed that the amplitude of the circadian rhythm is increased dramatically in the phyB-1 mutant which exhibits a constitutive shade-avoiding phenotype, providing an interesting link between circadian rhythm, phytochromes, and the phytohormone ethylene.
Ethylene has been shown to be involved in the shade-induced stem and petiole elongation in tobacco (Pierik et al., 2004), and recently this growth stimulation of ethylene was shown to occur through the auxin pathway, since auxin signalling mutants lose the ability to respond to exogenous ethylene, but ethylene signalling mutants retain their responsiveness to auxins (Pierik et al., 2009). The authors therefore infer that auxins might be a downstream regulator of ethylene-induced hypocotyl elongation. This in fact has been shown to be the case in root elongation, where ethylene stimulates both auxin biosynthesis and transport (Ruzicka et al., 2007; Stepanova et al., 2007; Swarup et al., 2007). On the other hand, Pierik et al. (2009) determined that the shade-induced hypocotyl elongation mediated by ethylene can occur independently of gibberellins. Despite the observation that DELLA proteins accumulate in response to ethylene, the full gibberellin-deficient ga1-3 mutant shows only a slight reduction in light quality-triggered hypocotyl elongation. On the other hand, evidence is emerging linking ethylene to gibberellin signalling in the morphologically similar response to flooding.
Temporary submergence triggers a similar response, and involves similar key players
Temporary flooding leads to a highly increased internode elongation in some rice cultivars as well as other semi-aquatic species in the so-called deepwater response. It results in morphological changes similar to the shade avoidance response, most importantly a rapid elongation of the stem at the expense of leaf development and size. Under water, the diffusion of oxygen and carbon dioxide (CO2) is reduced 10 000-fold compared with air. Thus, if a plant is submerged, hypoxic and anoxic conditions affect the respiratory metabolism, and the limited CO2 availability slows down the photosynthetic rate. To date, both the actual signal(s) and the corresponding receptor(s) that translate flooding into an elongation response remain elusive. It has been proposed, however, that changes in reactive oxygen species (ROS), pH changes, metabolic changes, and/or changes in the availability of nutrients could serve as signals (Dat et al., 2004). As in canopy shade, the light intensity is reduced under water; however, the spectral composition differs. Light that reaches submerged plants is enriched in R, resulting in a higher R:FR ratio, whereas the shade avoidance response is triggered by a lower R:FR ratio. Thus, although the triggers for inducing the rapid stem elongation differ in shading and flooding, the molecular mechanisms involved in these responses show close parallels. In fact, the downstream mechanisms regulating the submergence-induced elongation response are believed to be so similar that it was proposed to use the shade avoidance response as a tool for flooding research (Pierik et al., 2005).
It was shown very early that ethylene is involved in this response (Métraux and Kende, 1983), and that gibberellins are required for the ethylene-mediated elongation response of rice (Raskin and Kende, 1984). It was furthermore shown that both submergence and ethylene treatment result in the degradation of abscisic acid and an increased sensitivity to gibberellins in rice (Hoffmann-Benning and Kende, 1992), leading the authors to conclude that the ratio of growth-promoting (gibberellins) to growth-inhibiting (abscisic acid) phytohormones determines the elongation response. It thus appears that mainly ethylene, gibberellins, and abscisic acid control this elongation response. The exact mechanism of their interaction, however, has yet to be determined. Recently, however, two genes involved in this elongation response have been identified and named SNORKEL1 (SK1) and SK2 (Hattori et al., 2009). These genes were identified to be novel ETHYLENE RESPONSE FACTORS (ERFs), and were shown to be connected to gibberellin signalling, since gibberellins appear to be required for this elongation response. The authors speculated that SK1 and SK2 may also stimulate the biosynthesis of gibberellins (Hattori et al., 2009). Interestingly, the authors showed a strong induction of SK1 and SK2 transcripts upon ethylene treatment after 1 h. An induction of SK1 and SK2 transcripts, even though it is lower and occurs after 3 h only, can be observed upon treatment with gibberellin, the auxin IAA, and also cytokinins. Despite the authors’ conclusion that SK1 and SK2 are responsive to ethylene only, this slight induction could be interpreted as a contribution of these other phytohormones to the expression of SK1 and SK2, either through independent pathways following submergence, or as a result of feedback regulation.
The aim of this phytohormone interaction network in response to either shade or flooding is cell elongation in order to emerge from the blockage. Interestingly, Vreeburg et al. (2005) showed that ethylene can quickly induce both acidification of the apoplast and the expression of the cell wall-loosening expansin A1 (RpEXPA1) in Rumex palustris. This process of acidification is similar to the effect of auxins on cell expansion (Rayle and Cleland, 1970, 1992; Cleland, 1973; Rayle et al., 1977), suggesting that the two types of phytohormones might be mediating this response in concert. Alternatively, the ethylene-induced acidification of the apoplast could be mediated by the auxin pathway, as was shown to be the case for shade-induced stem and petiole elongation (Pierik et al., 2009).
Conclusions and future perspectives
Auxins can be attributed a major role in the enhanced elongation response upon shading or flooding, since the inhibition of both biosynthesis and transport abolishes submergence- as well as shade-induced growth. On the other hand, gibberellins were shown to be required (even though not sufficient) for the shade avoidance response. Several lines of evidence show that these two phytohormones not only act synergistically, but also positively regulate each others’ abundance in a normal growth context (Fig. 1). In the shade avoidance response, however, pathways of auxin and gibberellin appear to be parallel rather than connected, which could be achieved by the activation of different sets of genes in response to shading as compared with a normal growth context. Future studies should thus be aimed at understanding to what extent the auxin and gibberellin pathways intersect or depend on each other.
The sequestering by DELLA proteins of PIF4, a major transcription factor positively regulating the transcription of genes associated with cell elongation (de Lucas et al., 2008; Feng et al., 2008), reinforces the importance of gibberellin signalling in shade-induced growth. DELLA proteins are, furthermore, thought to integrate several hormonal signals (Achard et al., 2003; Fu and Harberd, 2003), making them good candidates for key regulators of growth in response to environmental as well as hormonal cues. However, this hypothesis is difficult to reconcile with the observation that quadruple DELLA knockout mutants elongate in response to shading similarly to wild-type plants (Djakovic-Petrovic et al., 2007; Pierik et al., 2007) and pentuple DELLA knockout mutants showing only slightly altered elongation in red light (Feng et al., 2008). Thus, elongation growth mediated by the release of PIF4 through shade-induced DELLA degradation very probably represents a redundant mechanism. It is imaginable that, under white light, DELLAs sequester only a part of the available PIF4 protein, whereas another part is bound by the active phyB (PfrB) and subsequently degraded via the ubiquitin–proteasome pathway (Fig. 2A). Upon shading, both the conversion of PfrB into its inactive isoform (PrB), and the gibberellic acid-mediated DELLA degradation release PIF4, allowing the transcription of shade-induced genes to occur (Fig. 2B).
Relatively little is known about the involvement of cytokinins in the shade avoidance response. It seems, however, that cytokinin breakdown mediated by the CKX family of proteins (Sessa et al., 2005; Carabelli et al., 2007) upon shading plays an important role in morphological changes other than stem elongation (e.g. arrest of leaf primordial growth). A reduction of cytokinin content also appears to occur passively, due to a decreased import through the transpiration stream into shaded leaves (Boonman and Pons, 2007; Boonman et al., 2007, 2009; Pons et al., 2001), and seems to play an important role in photosynthetic adaptations to shading. Nevertheless, it is clear that additional work needs to be done on the complex interactions between cytokinin signalling and gibberellin and auxin signalling.
Accumulation of ethylene has been identified as one of the early responses to various stresses in plants. The findings that ethylene appears to act upstream of auxin signalling in shade-induced growth (Pierik et al., 2009), as well as upstream of the gibberellin signalling pathway in response to flooding (Raskin and Kende, 1984) substantiate this view. Other studies have shown that ethylene can induce the degradation of abscisic acid upon submergence (Hoffmann-Benning and Kende, 1992). It is therefore tempting to conclude that ethylene could act as the initiator of stress-related morphological changes, regulating both abundance and signalling of other phytohormones. However, the complete regulatory network of shade avoidance and/or flooding responses is likely to contain both intersecting and functionally parallel pathways.
Furthermore, the strict hierarchy of regulation of phytohormone signalling described for GA20ox (Desgagné-Penix and Sponsel, 2008) is likely to be a mechanism common to most, if not all phytohormone signalling intermediates. If the spatial regulation thus over-rides all other regulatory mechanisms, specifically altering endogenous phytohormone levels in the target tissue instead of applying phytohormones exogenously will provide a much more powerful tool to determine the actual phytohormone responses. This is more likely to represent the actual phytohormone delivery and concentration in planta.
As more interconnections of phytohormone pathways are unravelled, and more interactions of phytohormone biosynthesis and signalling pathways are being discovered, it is becoming more and more clear that no single phytohormone affects plant growth and development on its own. Rather it is a complicated network connecting external cues with phytohormone signalling, which are only beginning to be understood (Fig. 3). In many cases of phytohormone interactions described here it remains to be determined exactly how and when these specific interactions occur. Thus, in order to gain a holistic view of how phytohormones regulate plant growth it is crucial in future studies to examine not only the overall phytohormone context in the plant, but also possible spatial and/or temporal (developmental) differences in interactions. In this regard, it is worth noting that cDNA microarray studies can now be attempted with only a few cells as starting material, which will permit extremely fine resolution for analysing tissue-specific differences in gene expression.
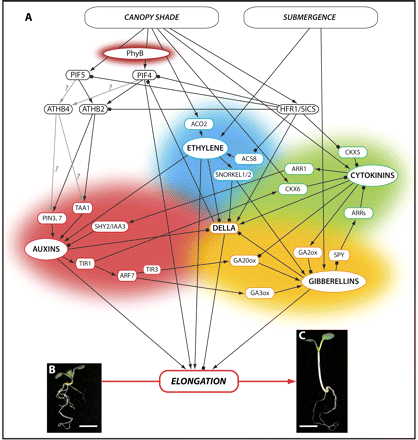
Phytohormone signalling network regulating shade-induced elongation growth. Canopy shade leads to a series of reactions in a plant, some of the earliest including the phyB-mediated transcript accumulation of the transcription factors ATHB2, ATHB4, and HFR1/SICS, as well as the accumulation of ethylene. These fast responses are followed by a network of interactions of other phytohormones such as auxin, gibberellin, and cytokinin, whose signalling intermediates interact with and influence each other at multiple levels (A). The result is a complex change in plant architecture, the most obvious effect being a dramatic elongation compared with plants growing in white light. This is illustrated by Arabidopsis wild-type (Col-0) seedlings that were induced to germinate in white light for 4 d and either kept in white light (B) or incubated in simulated shade in a red-tinted phytatray II (Sigma-Aldrich®) (C) for another 4 d. Scale bar=3 mm. A plant's response to submergence includes similar phenotypic changes, the most obvious being a rapid stem elongation, and is likely to involve a similar, if not the same, network of phytohormone interactions. This is represented by submergence forming a second input into the same network, as illustrated by the increased levels of ethylene and gibberellin observed in submerged plants, and the induction of SNORKEL1 and SNORKEL2, which in turn increase gibberellin levels and enhance DELLA degradation. Each phytohormone and its respective signalling intermediates involved in this network is shaded in the same colour as follows: auxin, red; ethylene, blue; cytokinin, green; gibberellin, yellow. Arrows indicate positive effects (accumulation of transcript and/or protein and hormone level, respectively; activation through interaction, etc.), and blocked arrows indicate negative effects.
In the long term, detailed knowledge of the molecular mechanism of the shade avoidance response will not only contribute to our understanding of how plants modulate a network of internal and external factors to regulate growth, future crop improvement efforts will also benefit from such knowledge, since this will allow us to target and modulate specific plant responses in greater detail.




Comments