-
PDF
- Split View
-
Views
-
Cite
Cite
Mark F. Belmonte, Muhammad Tahir, Dana Schroeder, Claudio Stasolla, Overexpression of HBK3, a class I KNOX homeobox gene, improves the development of Norway spruce (Picea abies) somatic embryos, Journal of Experimental Botany, Volume 58, Issue 11, August 2007, Pages 2851–2861, https://doi.org/10.1093/jxb/erm099
Close - Share Icon Share
Abstract
In order to investigate the effects of HBK3, a spruce gene member of the class I KNOX family, during somatic embryogenesis, sense (HBK3-S) and antisense (HBK3-A) Norway spruce (Picea abies) lines were generated. Somatic embryos produced from these lines were then analysed at morphological and structural levels. Compared with control, differentiation of immature somatic embryos from pro-embryogenic masses (PEMs) was accelerated in lines overexpressing HBK3 (HBK3-S). Such immature embryos showed enlarged embryogenic heads and were able to produce fully developed cotyledonary embryos at higher frequency. Furthermore, HBK3-S embryos had enlarged shoot apical meristems (SAMs) and enlarged expression pattern of PgAGO, a molecular marker gene specific to meristematic cells. Lines in which HBK3 (HBK3-A) was down-regulated had reduced ability to produce immature somatic embryos from PEMs and were not able to complete the maturation processes. To assess the function of HBK3 in comparison with that of angiosperm KNOX genes, this gene was ectopically expressed in Arabidopsis plants. As observed for spruce, Arabidopsis embryos overexpressing HBK3 had enlarged meristems and enlarged expression pattern of SHOOTMERISTEMLESS, a SAM molecular marker gene. In addition, transformed embryos were able to germinate at a higher rate and the resulting plants showed a variety of phenotypic aberrations, including abnormal leaves and reduced apical dominance. Overall, these data confirm the importance of KNOTTED genes during development and reveal the participation of HBK3 in conifer embryogeny. Furthermore, the results show redundant functions of this gene during embryonic growth of spruce and Arabidopsis, but not during post-embryonic growth.
Introduction
Over the past number of years, somatic embryogenesis, the formation of embryos in culture from asexual cells, has been extensively employed to investigate the physiological and molecular mechanisms governing embryogenesis (Thorpe and Stasolla, 2001). Using this process, a large number of immature and mature embryos can be easily obtained for experimental studies which would otherwise be impossible to perform with seed embryos. The developmental pathway of in vitro embryogenesis has been well documented in the conifer Norway spruce (Picea abies) (Filonova et al., 2000a; von Arnold et al., 2002). In this system, the formation of cotyledonary embryos passes through a sequence of developmental stages corresponding to specific culture treatments (Filonova et al., 2000a). The embryogenic potential is maintained through proliferation of the pro-embryogenic masses (PEMs) which appear as defined structural aggregates of different complexity. Over the 7 d proliferation period in the presence of the plant growth regulators (PGRs) auxin and cytokinin, PEMsI develop into PEMsIII via the intervening PEMsII. Formation of somatic embryos from PEMsIII is initiated upon withdrawal of the PGRs auxin and cytokinin, and completed in the presence of abscisic acid (ABA) (Filonova et al., 2000a). Fully developed cotyledonary embryos are similar in morphology to their zygotic counterparts and are characterized by the presence of well-developed shoot and root apical meristems (SAM and RAM, respectively). Proper execution of this embryogenic developmental pathway is often precluded if some physiological parameters are not met (Filonova et al., 2000b, Bozhkov et al., 2002, Smertenko et al., 2003) or in the absence of a defined gene expression pattern (Stasolla et al., 2004).
KNOTTED-like homeobox (KNOX) genes are a large group of transcription factors that play fundamental roles during different phases of plant growth and development (Chang et al., 1998). Unique features of these proteins are six conserved regions which include a long homeobox domain involved in DNA binding as well as the KNOX and ELK domains, both N-terminal of the homeodomain (Ito et al., 2002). Based on their localization pattern and sequence similarity within the homeodomain region, KNOX genes are grouped into two classes. While class I genes have been shown to play a key role during plant development where they regulate cell specification and pattern formation, no specific function has been assigned to members of class II. A well studied example of a class I KNOX gene in Arabidopsis is SHOOTMERISTEMLESS (STM). This gene, which is expressed in the apical pole during embryonic and post-embryonic development, regulates the architecture of the SAM by maintaining a balance between cell division and differentiation (Long et al., 1996). Loss-of-function mutations of STM result in the degeneration of the embryonic SAM and culminate in reduced post-embryonic growth and, in extreme cases, embryo abortion (Long and Barton, 1998).
Unlike their angiosperm counterparts where a large number of KNOX genes have been identified and characterized, only a few KNOX genes have been reported in gymnosperms (Sundas-Larsson et al., 1998; Hjortswang et al., 2002; Guillet-Claude et al., 2004), and their functions remain almost completely unknown. In Norway spruce, three class I KNOX genes, HBK1, HBK2, and HBK3, have been described (Hjortswang et al., 2002). While it has been suggested that HBK1 is involved in SAM regulation, based on its localization pattern (Sundas-Larsson et al., 1998; Belmonte et al., 2005), no information is currently available on the role played by the other two genes.
To understand further the role of KNOX genes in conifers, the role of HBK3 during Norway spruce somatic embryogenesis has beeen investigated using transformation approaches. Analyses of embryogenic lines transformed with HBK3 in sense or antisense orientation suggest that HBK3 may be involved in transdifferentiation of PEMs into somatic embryos and in the formation of the embryonic SAM.
Materials and methods
Production of spruce somatic embryos
The Norway spruce embryogenic line 95:88:22 (kindly provided by Professor von Arnold) (Elfstrand et al., 2001) was used for all experiments. Maintenance of PEMs and initiation of embryo development were performed exactly as previously reported (Bozhkov et al., 2002). Briefly, proliferation of PEMs was stimulated using half-strength LP medium (modified after Filonova et al., 2000a) supplemented with PGR, auxin (9.0 μM 2,4-dichlorophenoxyacetic acid; 2,4-D), and cytokinin (4.4 μM N6-benzyladenine; BA). For maintenance of embryogenic potential, PEMs were subcultured weekly into fresh PGR-containing medium. Transdifferentiation of PEMs into somatic embryos was accomplished by transferring cells into half-strength LP medium devoid of PGR for a week. Continuation of embryo development, i.e. generation of cotyledonary somatic embryos, was carried out by plating small cell aggregates on solidified half-strength LP medium supplemented with 30 μM ABA. Cotyledonary embryos were harvested after 35 d in culture.
PCR
Total RNA was extracted with an RNeasy Plant mini kit (Qiagen) from Norway spruce embryogenic tissue. cDNA was synthesized using 2 μg of total RNA, 500 ng of oligo(dT17)-adapter primer (5′-GACTCGAGTCGACATCGA(T)17V-3′), and 15 U of M-MuLV reverse transcriptase in a 20 μl reaction. Full-length HBK3 (accession no. AF483278) was amplified using specific primers (5′-CAGTAAATACAAGGTCTTGAAGAT-3′ and 5′-CCGCCAGTGTGCTGGAATTCGCCC-3′). Amplification by PCR was as follows: 94 °C for 30 s, 55 °C for 30 s, 72 °C for 2 min, 30 cycles.
Spruce transformation
To generate the plasmids for the transformation experiments, the GUS (β-glucuronidase) reporter gene downstream from the maize ubiquitin promoter was cleaved from pUTV45 (Clapham et al., 2000) with SacI and BamHI, and replaced with HBK3 in sense or antisense orientation. Full-length HBK3 was re-amplified by PCR using primers designed to add SacI and BamHI restriction sites to the 5′ and 3′ ends, respectively, and the amplification products were ligated to the cut pUTV45. The resulting plasmids were named pUTV45-HBK3 sense and pUTV45-HBK3 antisense.
Production of transgenic material was carried out exactly as reported by Clapham et al. (2000). Briefly, Norway spruce embryogenic cells were transformed with a particle gene gun in which the gold particles were coated with an equal amount of pUTV45-HBK3 sense or pUTV45-HBK3 antisense and pUbi-BAR, in which the BASTA resistance gene is driven by the ubiquitin promoter (Clapham et al., 2000). Control cells were transformed with an equimolar mixture of pUTV45 and pUbi-BAR. Selection of transformed cells was carried out on proliferation medium containing 1 mg l−1 BASTA, exactly as reported by Clapham et al. (2000).
Transformed cells were screened by PCR using genomic DNA as a template and a forward primer annealing to the maize ubiquitin promoter (5′-GCTTTTTGTTCGCTTGGTTGTG-3′) and reverse primers specific to HBK3 (5′-CCGCCAGTGTGCTGGAATTCGCCC-3′ for sense transformants and 5′-CAGTAAATACAAGGTCTTGAAGAT-3′ for antisense transformants).
Embryos from the transformed cells were matured and germinated according to Bozhkov and von Arnold (1998).
RNA blot analysis
Total RNA from transformed cells was isolated using an RNeasy Plant mini kit (Qiagen). A 15 μg aliquot was fractionated on a 1.0% (v/v) formaldehyde agarose gel and transferred to a nylon membrane (Sambrook and Russell, 2001). Duplicate blots were hybridized with the digoxigenin (DIG)-labelled sense or antisense HBK3 probes which were prepared using DIG-11-UTP, as described in the DIG RNA labelling kit (Roche Molecular Biochemicals). Probe hybridization and colour development was carried out exactly as described in the DIG Application Manual (Roche). Spruce actin (GeneBank accession no. AF172094) probes were also hybridized to verify that equal amounts of RNA were loaded.
Cell culture composition
Norway spruce suspension cultures were sampled at 7 d in the presence of PGRs and at days 3 and 7 in the hormone-free treatment (–PGR medium). The percentage composition of PEMI, PEMII, PEMIII, and somatic embryos from a 1.5 ml aliquot was determined as reported in the methods of Bozhkov et al. (2002).
RNA in situ hybridization of PgAGO, a gene expressed in SAM and RAM of spruce embryos
Chemical fixation and tissue processing were performed exactly as described by Tahir et al. (2006). The full-length PgAGO gene was amplified with primers containing T7 and T3 sites (5′- TAATACGACTCACTATAGGGGCGTTTCTCTGGCTTTGAGG and 5′- AATTAACCCTCACTAAAGGTTTGGCAATTTCTCGACGAT). The PCR product was then used for in vitro transcription using DIG-11-UTP, as described in the DIG RNA labelling kit (Roche Molecular Biochemicals). Sense and antisense probes were hydrolysed for 40 min at 60 °C in the presence of 60 mM Na2CO3 and 40 mM NaHCO3, and stored at –80 °C prior to hybridization.
Tissue treatments and pre-hybridization washes were conducted exactly as described in Canton et al. (1999). Post-hybridization washes and antibody treatment were carried out according to Regan et al. (1999).
Light microscopy
Samples were fixed in 2.5% glutaraldehyde and 1.6% paraformaldehyde buffered with 0.05 M phosphate buffer, pH 6.9, dehydrated with methyl cellosolve followed by two changes of absolute ethanol, and then infiltrated and embedded in Historesin (Leica Canada, Toronto) (Yeung, 1999). Serial sectioning (3 μm) was carried out on a Reichert-Jung 2040 Autocut rotary microtome. These sections were stained with periodic acid–Schiff (PAS) for total carbohydrates, and counterstained with amido black 10B for protein or toluidine blue O (TBO) for general histological organization (Yeung, 1984).
Arabidopsis transformation
Seeds of the Arabidopsis ecotype Landsberg erecta were obtained from the Arabidopsis Biological Resource Stock Center (Ohio State University, Columbus).
Full-length HBK3 cDNA was inserted downstream of the cauliflower mosaic virus 35S promoter of the pCHF3 binary vector, a pPZP211-based plant expression vector carrying the 35S promoter and a pea ribulose 1,5-bisphosphate carboxylase/oxygenase terminator (C Fankhauser, K Hanson, and J Chory, unpublished data). The construct was then introduced into the Agrobacterium tumefaciens strain GV3101 with Ti plasmid pMP90 by freeze–thawing. Agrobacterium cultures were grown for 3 d in the presence of spectinomycin (selectable antibiotic marker). Positive colonies were screened by PCR using a forward primer annealing to the 35S promoter (5′-GATGTGATATCTCCACTGACGTAAGGG-3′) and the reverse gene primer (5′-CCGCCAGTGTGCTGGAATTCGCCC-3′).
Selected colonies were grown overnight in YEP medium supplemented with gentamycin (25 μg ml−1) and spectomycin (25 μg ml−1). The resulting culture was then spun down and resuspended to OD600=0.8 in 1% sucrose, 0.05% Silwet L-77, and used to spray-transform wild-type Arabidopsis plants via standard Agrobacterium-mediated techniques (Weigel and Glazebrook, 2002). All plants were grown at 22 °C under long day conditions (16 h light and 8 h dark).
Seeds of transformed plants were plated on 1× MS medium supplemented with 1% sucrose, kanamycin (100 μg ml−1), and carbenicillum (100 μg ml−1). The resulting 35S:HBK3 T2 plants were screened by PCR and RNA blots as indicated above, and examined for morphological analysis.
GUS assay
The Arabidopsis STM:GUS reporter lines (kindly provided by Professor Barton) were transformed with HBK3, as described above. Whole Arabidopsis seeds and dissected embryos were stained and vacuum infiltrated in a solution containing 25 mM phosphate buffer (pH 7), 0.25% Triton X-100, 1.25 mM potassium ferricyanide, 1.25 mM potassium ferrocyanide, 0.25 mM EDTA, 1 mg ml−1 5 bromo-4 chloro-3-indolyl-β-D-glucuronide (X-Gluc) for 12 h at 37 °C (Weigel and Glazebrook, 2002). Tissues were then cleared in a modified Hoyer's solution according to the methods of Strangeland and Salehian (2002), and utilized for microscopic observations.
Statistical analysis
Unless specified, all experiments were performed using at least three biological replicates, and Tukey's post hoc test for multiple variance (Zar, 1999) was used to compare differences between treatments and control.
Results
Generation of transformed spruce lines and cell tracking experiments
After transformation experiments, four antisense (HBK3-A1 to A4) and four sense (HBK3-S1 to S4) spruce sublines were identified. The former sublines were screened for the presence of antisense HBK3 by PCR. As expected, no amplification product was obtained from genomic DNA isolated from control cells (Fig. 1A). Northern blot hybridization analysis using the RNA sense probe revealed the presence of antisense HBK3 transcripts in all the transformed sublines, but not in control cells (Fig. 1A). In all HBK3-A sublines a reduction of sense HBK3 transcripts was also detected (Fig. 1A). Insertion of sense HBK3 in all the HBK3-S sublines was confirmed by PCR (Fig. 1B). Compared with their control counterparts, all HBK3-S cells showed increased levels of HBK3 transcripts as revealed by hybridization with antisense probes (Fig. 1B).
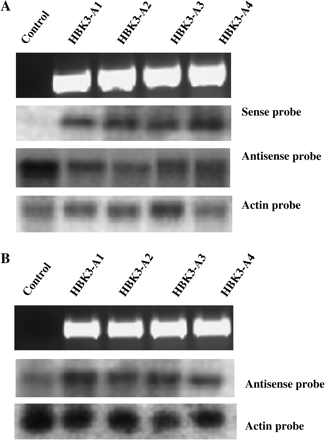
Screening of positive spruce cells transformed with HBK3 in antisense (HBK3-A1–A4) and sense (HBK3-S1–S4) orientation. (A) The presence of the pUTV45-HBK3-antisense construct in the cultures was verified using ubiquitin/HBK3 primers from genomic DNA (see Materials and methods for details). The expected amplified products were observed in the four antisense lines, but not in the control line (top panel). RNA gel blot analysis showing the presence of antisense HBK3 transcripts (second panel) and a reduction of sense HBK3 transcripts (third panel) in the HBK3-A lines. Equal loading was ensured by staining with ethidium bromide before blotting and by re-probing the same RNAs with spruce actin (fourth panel). (B) The presence of pUTV45-HBK3-sense construct was verified in the four sense (HBK3-S) lines (top panel). Compared with control, these lines had increased levels of HBK3 transcripts which were probed with the HBK3 antisense probe (second panel). Equal loading was ensured by staining with ethidium bromide before blotting and by re-probing the same RNAs with spruce actin (third panel).
Time lapse tracking experiments were used to follow the dynamics of embryo production in control and transformed sublines. At the end (day 7) of the proliferation treatment in the presence of PGRs, a similar embryo composition was observed among all lines (Fig. 2A). Overall, the percentage of PEMsIII was predominant compared with other cellular aggregates (Fig. 2A) Upon removal of PGRs, transdifferentiation of PEMsIII into somatic embryos increased in the control line and lines overexpressing HBK3 (HBK3-S) after only 3 d. This transition was precluded in the antisense HBK3-A lines, where the percentage embryo composition remained almost unchanged (Fig. 2B). Production of somatic embryos from PEMsIII continued to increase in both control and HBK3-S lines in the absence of PGRs at day 7 (Fig. 2C). This increase was more pronounced in all the four HBK3-S lines. On this day in culture, the percentage of somatic embryos remained low in all the HBK3-A lines (Fig. 2C).
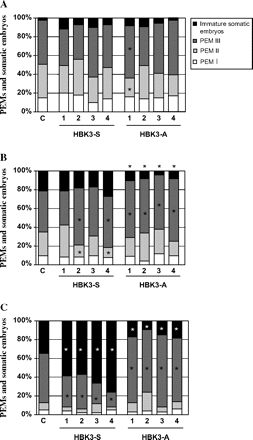
Percentage composition of cell aggregates observed in the control (c) line, sense lines (HBK3-S), and antisense lines (HBK3-A) during the initial phases of embryo development. (A) At day 7 in maintenance (+PGR) medium a similar embryo composition was observed among all lines, and the percentage of PEMsIII was predominant compared with that of other cellular aggregates. (B) After 3 d in hormone-free medium (–PGR) the formation of immature somatic embryos from PEMsIII increased in the control line and lines overexpressing HBK3 (HBK3-S). No increase was observed in the lines down-regulating HBK3 (HBK3-A). (C) Production of somatic embryos from PEMsIII continued to increase in both control and HBK3-S lines in the absence of PGRs at day 7 (Fig. 3C). This increase was more pronounced in all the four HBK3-S lines. The percentage of somatic embryos remained low in all HBK3-A lines. PEM, pro-embryogenic masses. Each value is the average of three independent replicates. More than 1000 cell structures were counted for each replicate. Asterisks indicate values that are statistically different (P ≤0.01) from control values.
Further embryonic growth was examined by counting the number of fully developed cotyledonary somatic embryos produced by the different lines. Compared with control cells, the percentage of embryo yield in the four HBK3-S lines increased >20% (Fig. 3). Formation of cotyledonary embryos was not observed in the HBK3-A lines. Both embryos produced by control and HBK3-S lines were able to germinate and convert into viable plants. No differences in morphology and post-embryonic performance were observed between these embryos.

Comparison of the percentage of fully developed cotyledonary embryos produced by the control (C) line (100%) and the four lines overexpressing HBK3 (HBK3-S). Each value is the average of four independent replicates. More than 80 embryos were plated for replicates. An asterisk indicates values that are significantly different from control (P <0.01).
Morphology and anatomy of developing spruce embryos
Embryos produced by control cells followed a precise developmental pathway (described in detail by Filonova et al., 2000a). PEMsI were characterized by the presence of a small embryogenic head subtended by a long vacuolated cell (Fig. 4A). Formation of additional vacuolated cells delineated the transition from PEMsI to PEMsII (Fig. 4B). A further increase in size of the embryogenic head and a change in distribution of the vacuolated cells characterized the formation of PEMsIII (Fig. 4C). Upon removal of PGRs, PEMsIII differentiate into immature somatic embryos. Both the external (Fig. 4D) and internal (Fig. 4E) morphology of these embryos revealed the presence of an elongated suspensor tail subtending the embryo proper which developed a protoderm. Inclusion of ABA triggered further development. After 20 d in ABA-containing medium, the embryo proper increased in size and the apical meristems were formed. At this stage, the SAM was fully differentiated and starch accumulated heavily within the subapical cells (Fig. 4F). In fully developed cotyledonary embryos the SAM was generally dome shaped, although variations were observed. The apical cells became distinguishable from the subapical cells, as they were devoid of starch granules (Fig. 4G). Starch and, to a lesser extent, protein bodies were the predominant storage products in the cortical cells of cotyledonary embryos (Fig. 4H).
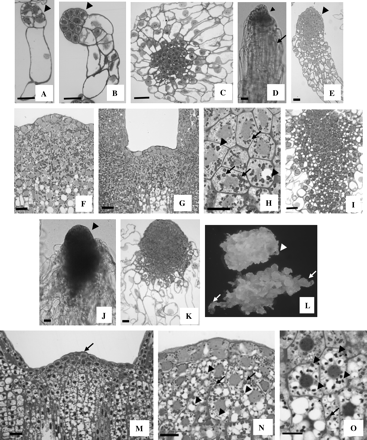
Embryonic development in the control line (A–H) and in lines overexpressing HBK3 (I–O). (A) A control pro-embryogenic mass I (PEMI) characterized by an elongated cell subtending a small cluster of cytoplasmic cells (arrowhead). (B) PEMII with a more elaborated suspensor region composed of vacuolated cells and an embryo proper (arrowhead) formed by a cluster of cytoplasmic cells. (C) PEMIII in which the vacuolated cells radiate from a central group of cytoplasmic cells. (D) External morphology of an immature control somatic embryo collected in the hormone-free medium (–PGR). The embryo is composed of a small embryo proper (arrowhead) and an elongated suspensor region (arrow). (E) Internal morphology of an immature control somatic embryo at a similar stage to that shown in (D). A differentiating protoderm (arrowhead) is visible within the embryo proper. (F) After 20 d in the ABA-containing medium the SAM of control embryos is fully formed and a heavy accumulation of starch is visible in both apical and subapical cells. (G) In fully developed cotyledonary embryos the SAM is generally dome shaped, although variations in shape are often observed. Accumulation of starch is prevalent in the subapical cells. (H) Starch (arrows) and, to a lesser extent, protein bodies (arrowheads) are visible in the cortical cells of fully developed control embryos. (I) In HBK3-S lines, the PEMsIII are composed of a large central group of cytoplasmic cells and a peripheral cluster of vacuolated cells. (J) External morphology of an immature HBK3-S embryo collected in the hormone-free medium (–PGR). The embryo is characterized by an enlarged embryo proper (arrowhead) and an extensive suspensor tail. (K) Internal structure of an embryo similar to that depicted in (B). (L) Morphology of control cells (top) and HBK3-S cells (bottom) cultured on solid hormone-free medium (–PGR) for 3 d. Large immature embryos (arrows) emerge from the HBK3-S embryogenic tissue. The size of the embryos is much smaller in control cells (arrowhead). (M) The shoot apical meristems of fully developed cotyledonary HBK3-S embryos are large and composed of defined cytoplasmic apical cells (arrows) and vacuolated subapical cells. (N) Both starch (arrows) and protein bodies (arrowheads) are visible within the cells of the shoot apical meristem. (O) Protein bodies (arrowheads) accumulate preferentially in the cortical cells. Reduced deposition of granules of starch (arrows) also occurs. Scale bar=20 μm.
The developmental pathway of embryos produced by the HBK3-S lines was generally similar to that described for control cells, although some differences were observed. The PEMsIII varied in shape but were composed of large clusters of cytoplasmic cells (Fig. 4I), rarely observed in the control line. Structural deviations from control embryos became more evident upon transfer onto hormone-free (–PGR) medium. The somatic embryos produced by cells overexpressing HBK3 had a bigger embryogenic head than their control counterparts and their suspensor-like tails were enlarged and partially covered the flanks of the embryo proper (Fig. 4J, K). The appearance of these enlarged embryos emerging from the subtending embryogenic tissue was more noticeable when cells were cultured on solid medium (arrows in Fig. 4L). As for the control, differentiation of the SAM in HBK3-S embryos also occurred around day 20. The SAMs of HBK3-S embryos were always larger than their control counterparts (Fig. 4M) and accumulated both protein bodies and starch in the subapical cells (Fig. 4N). Protein, as opposed to starch, was the major storage product in the cortical cells of HBK3-S embryos (Fig. 4O).
Embryo development was arrested in the lines in which HBK3 (HBK3-A lines) was down-regulated. The structure of the different PEM aggregates was very similar to that described for the control line (Fig. 4A–C). Although a few immature somatic embryos with similar morphology to those observed in control lines (Fig. 4D, E) differentiated from PEMsIII, they did not increase in size in the presence of ABA and failed to produce fully developed cotyledonary embryos.
Localization of PgAGO, a root and shoot apical meristem molecular marker gene
SAM identity in the transformed lines was further investigated by following the localization pattern of PgAGO, a gene with preferential expression in both RAMs and SAMs of spruce (Tahir et al., 2006). Localization of PgAGO transcripts in control cotyledonary embryos was restricted to the apical layer of the shoot apex (Fig. 5A), whereas it was extended into the subapical cells of embryos overexpressing HBK3 (Fig. 5B). Expression of PgAGO in the root meristem was similar in both control (Fig. 5C) and HBK3-S embryos (Fig. 5D). Control experiments with sense probes confirmed the specificity of the antisense probe (data not shown).
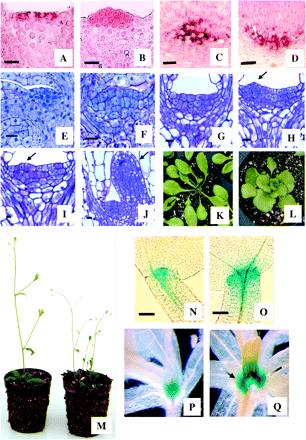
PgAGO expression in spruce embryos (A–D) and development of Arabidopsis with ectopic HBK3 expression (E–Q). (A) In shoot apical meristems (SAMs) of control spruce embryos the expression of PgAGO (red colour) is restricted to the apical cells. (B) PgAGO expression extends in the subapical cells of embryos which overexpress HBK3. (C) In control embryos expression of PgAGO is localized in the cells of the root apical meristem. (D) A similar expression pattern was also observed in embryos overexpressing HBK3. (E) SAM of wild-type Arabidopsis embryos at the cotyledonary stage of development. (F) The SAMs of Arabidopsis embryos expressing HBK3 are enlarged and characterized by a large cluster of cytoplasmic cells. (G) After 2 d of germination, the SAM of control embryos is still quiescent. (H) On the same day primordia inception (arrow) was visible in the 35S:HBK3 embryos. (I) Primodia inception (arrow) was observed around day 4 in control embryos. (J) On this day immature leaves (arrow) had already emerged from the SAM of embryos expressing HBK3. (K) Rosette leaves of wild-type Arabidopsis. (L) The leaves of plants expressing HBK3 were lobed and had short petioles. (M) Compared with the wild type (left), plants expressing HBK3 (right) were shorter and exhibited reduced apical dominance. (N) In wild-type cotyledonary embryos of the 35:GUS reporter line, GUS staining was restricted to a small cluster of apical cells. (O) The expression of GUS in the embryos of the STM:GUS reporter line transformed with HBK3 was extended to a larger group of apical and subapical cells. (P) GUS expression in wild-type STM:GUS plants 10 d after germination. (Q) In STM:GUS plants transformed with HBK3 the expression of GUS was enlarged as lateral meristems (arrows) were produced. Scale bars=10 μm (A–M, P, Q) and 50 μm (N, O).
Ectopic expression of HBK3 during Arabidopsis development
Ectopic expression of HBK3 in Arabidopsis led to the generation of many independent transformants, eight of which were selected for further studies. Northern blot hybridization using DIG-labelled antisense probes revealed intermediate levels of HBK3 transcripts in four lines (1–4) and high levels of HBK3 transcripts (5–8) in the remaining lines (Fig. 6). In this report only data obtained for line 8 are presented since, unless specified, no morphological differences were observed among the transformed lines regardless of the HBK3 expression level.
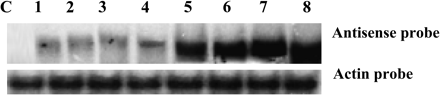
Screening of positive Arabidopsis lines transformed with HBK3. The presence of the transgene was detected using the antisense HBK3 probe and confirmed in all the transformed lines (1–8) but not in the control wild-type line (top panel). Equal loading was ensured by staining with ethidium bromide before blotting and by re-probing the same RNAs with spruce actin (second panel).
Arabidopsis early embryogenesis followed a similar developmental pattern in both control lines and the 35S:HBK3 lines (data not shown). However, the SAM of fully developed control embryos (Fig. 5E) was always smaller than that of the 35S:HBK3 embryos (Fig. 5F). In these latter embryos, the SAM was composed of a larger number of cytoplasmic cells (compare Fig. 5E and F) which contributed to the increased size of the tunica region (L1 and L2 layers; Table 1). Upon germination, reactivation of the SAM was more pronounced in embryos overexpressing HBK3 (Table 2). Anatomical studies revealed that after 2 d of germination the SAM of control embryos was still quiescent (Fig. 5G), whereas leaf primordia appeared in the 35S:HBK3 embryos (arrow in Fig. 5H). On average, primordia inception was observed around day 4 in control embryos (Fig. 5I). On this day immature leaves had already emerged from the SAM of embryos overexpressing HBK3 (Fig. 5J).
The effect of HBK3 on both cell number and cell size in the tunica region of shoot apical meristem of Arabidopsis embryos
| Genotype | Average L1 cell number | Area per L1 cell, μm2 | Average L2 cell number | Area per L2 cell, μm2 |
| Wild type | 5±0.3 | 30±1.6 | 5±0.2 | 31±1.1 |
| 35S:HBK3 | 7±0.5 | 38±2.58 | 7±0.1 | 39±2.3 |
| Ratio | 1.4 | 1.3 | 1.4 | 1.3 |
| Genotype | Average L1 cell number | Area per L1 cell, μm2 | Average L2 cell number | Area per L2 cell, μm2 |
| Wild type | 5±0.3 | 30±1.6 | 5±0.2 | 31±1.1 |
| 35S:HBK3 | 7±0.5 | 38±2.58 | 7±0.1 | 39±2.3 |
| Ratio | 1.4 | 1.3 | 1.4 | 1.3 |
Cells were counted in both the L1 and L2 layers. Shoot apical meristems of mature embryos were sectioned (see Materials and methods) and photographed. Cell number and cell area were measured using ACCESS imaging software. Perfect median longitudinal sections of at least 20 embryos were used for analysis in each genotype. Means ±SE are shown. All values for areas and cell numbers obtained in the 35:HBK3 embryos are statistically different from those recorded in control embryos (P <0.01).
The effect of HBK3 on both cell number and cell size in the tunica region of shoot apical meristem of Arabidopsis embryos
| Genotype | Average L1 cell number | Area per L1 cell, μm2 | Average L2 cell number | Area per L2 cell, μm2 |
| Wild type | 5±0.3 | 30±1.6 | 5±0.2 | 31±1.1 |
| 35S:HBK3 | 7±0.5 | 38±2.58 | 7±0.1 | 39±2.3 |
| Ratio | 1.4 | 1.3 | 1.4 | 1.3 |
| Genotype | Average L1 cell number | Area per L1 cell, μm2 | Average L2 cell number | Area per L2 cell, μm2 |
| Wild type | 5±0.3 | 30±1.6 | 5±0.2 | 31±1.1 |
| 35S:HBK3 | 7±0.5 | 38±2.58 | 7±0.1 | 39±2.3 |
| Ratio | 1.4 | 1.3 | 1.4 | 1.3 |
Cells were counted in both the L1 and L2 layers. Shoot apical meristems of mature embryos were sectioned (see Materials and methods) and photographed. Cell number and cell area were measured using ACCESS imaging software. Perfect median longitudinal sections of at least 20 embryos were used for analysis in each genotype. Means ±SE are shown. All values for areas and cell numbers obtained in the 35:HBK3 embryos are statistically different from those recorded in control embryos (P <0.01).
Production of shoots in germinating wild-type Arabidopsis embryos and embryos expressing HBK3
| Day | Shoot developmenta (%) | |
| Wild type | 35S:HBK3 | |
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 1 | 5 |
| 4 | 23 | 40 |
| 5 | 38 | 62 |
| 6 | 55 | 79 |
| 7 | 75 | 81 |
| Day | Shoot developmenta (%) | |
| Wild type | 35S:HBK3 | |
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 1 | 5 |
| 4 | 23 | 40 |
| 5 | 38 | 62 |
| 6 | 55 | 79 |
| 7 | 75 | 81 |
Shoots were counted over a 7 d culture period.
Shoot development was scored as positive based on the emergence of the first leaf pair. Percentage shoot development was calculated by dividing the number of plants with the first leaf pair by the number of seeds plated. For each replicate, at least 200 seeds were plated.
Production of shoots in germinating wild-type Arabidopsis embryos and embryos expressing HBK3
| Day | Shoot developmenta (%) | |
| Wild type | 35S:HBK3 | |
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 1 | 5 |
| 4 | 23 | 40 |
| 5 | 38 | 62 |
| 6 | 55 | 79 |
| 7 | 75 | 81 |
| Day | Shoot developmenta (%) | |
| Wild type | 35S:HBK3 | |
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 1 | 5 |
| 4 | 23 | 40 |
| 5 | 38 | 62 |
| 6 | 55 | 79 |
| 7 | 75 | 81 |
Shoots were counted over a 7 d culture period.
Shoot development was scored as positive based on the emergence of the first leaf pair. Percentage shoot development was calculated by dividing the number of plants with the first leaf pair by the number of seeds plated. For each replicate, at least 200 seeds were plated.
A variety of phenotypic aberrations were observed during post-embryonic growth of Arabidopsis plants with ectopic HBK3 expression. Compared with wild-type plants (Fig. 5K), the rosette leaves of 35S:HBK3 plants were mis-shapen (spatulate with slight serrations) and lacked elongated petioles (Fig. 5L). The first leaves to appear after germination had fewer lobes or rumples, while those leaves produced later were severely rumpled and mis-shapen. Overall, 35S:HBK3 plants were dwarfed and showed reduced apical dominance (Fig. 5M). This phenotype was shared among all independent transformant lines, albeit that a less severe phenotype was observed for the transformed lines 1–4, which showed a lower expression of HBK3 (Fig. 6).
To examine further the effect of HBK3 on Arabidopsis SAM development, this gene was introduced into a STM:GUS reporter line. GUS expression, which in control embryos was restricted to a small group of meristematic cells in the apical pole (Fig. 5N), was extended to a larger area in embryos expressing HBK3 (Fig. 5O). This expression pattern was retained during post-embryonic growth. Compared with control plants after 10 d of germination (Fig. 5P), GUS expression was enlarged in the apical poles of HBK3-expressing plants (Fig 5Q).
Discussion
Class I KNOX genes are transcription factors which play crucial roles in plant growth and development. Genetics and molecular analyses have revealed that this class of genes regulates SAM formation and maintenance by promoting indeterminate growth of meristematic cells. In maize, ectopic expression of knotted1 results in the formation of meristemoids originating from leaf cells, which lose their cell fate and embark on a new developmental pathway (Vollbrecht et al., 1991). Similarly, STM, another member of the KNOX family in Arabidopsis, promotes meristematic cell identity in the embryonic and post-embryonic SAM (Long et al., 1996). In conifers, several class I KNOX genes have been identified, including HBK3 (Hjortswang et al., 2002; Guillet-Claude et al., 2004). This gene is phylogenetically related to other KNOX members from other species and encodes functional domains unique to KNOX proteins (Hjortswang et al., 2002). Transformation experiments have revealed that overexpression of HBK3 encourages the completion of the embryogenic pathway in spruce as it promotes the formation of immature somatic embryos from PEMsIII and facilitates their subsequent development. Differentiation of PEMsIII into early somatic embryos is accelerated in cells overexpressing HBK3 and is inhibited in those with reduced HBK3 levels (Fig. 2). Furthermore, completion of embryo growth is enhanced in the HBK3-S lines and precluded in the HBK3-A lines (Fig. 3).
One possible way in which overexpression of HBK3 may favour the PEMIII–somatic embryo transdifferentiation is by increasing the number of cytoplasmic cells of PEMsIII from which somatic embryos originate. Although difficult to quantify, as they are disorganized structures, PEMsIII tend to be bigger in HBK3-S lines compared with the control (compare Fig. 4C and I). Larger PEMsIII can in turn give rise to an increased number of somatic embryos which are also larger in size (Fig. 4J, K). As suggested by Gupta and Pullman (1990), immature somatic embryos with larger embryogenic heads, such as those observed in lines overexpressing HBK3, are more responsive to the maturation conditions imposed by ABA and produce cotyledonary embryos at higher frequency. This notion, which is confirmed by the present work (Fig. 3), is also validated by previous studies which showed a positive relationship between increased embryogenic head size, effected by glutathione treatments, and improved embryo yield (Belmonte et al., 2005). A positive relationship between SAM size and embryogenic competence was also established in Arabidopsis. Production of somatic embryos is facilitated in primordia timing zygotic embryos, characterized by an enlarged SAM containing a higher number of uncommitted meristematic cells (Mordhorst et al., 1998).
Compared with their control counterparts, fully developed embryos overexpressing HBK3 have a larger SAM and a different pattern of storage product accumulation, with protein bodies prevailing over starch granules (compare Fig. 4F with N). Control of meristem size by members of KNOX genes was also demonstrated in other systems. Endrizzi et al. (1996) have shown that in stm mutants the embryonic SAM is either reduced in size or completely absent. Conversely, meristematic identity is promoted by increasing STM levels (Williams, 1998). The larger SAM observed in the HBK3-S lines may be due to alterations in endogenous hormone levels, especially cytokinins which are implicated in shoot formation (Steeves and Sussex, 1989). Cytokinin-autotrophic growth was observed in maize cells with ectopic KNOTTED 1 expression (Hewelt et al., 2000). Similarly, Frugis et al. (2001) reported an accumulation of isopentenyl-type cytokinins in lettuce leaves overexpressing KNAT1. SAM integrity was also examined by following the expression pattern of PgAGO, a marker gene which is preferentially expressed in the apical poles of spruce somatic embryos (Tahir et al., 2006). The expression of this gene, which has been used previously to estimate meristem integrity, is enlarged in the shoot apexes of mature HBK3-S embryos (compare Fig. 5A with B). This observation is indicative of a higher number of embryogenic cells present in the SAM of embryos overexpressing HBK3. Furthermore, the fact that the expression pattern of PgAGO in the root meristem is unaltered between control and HBK3-S embryos (Fig. 5C, D) indicates that the effect of HBK3 is restricted to the shoot apex and that different regulatory mechanisms operate in the development of root and shoot meristems in spruce embryos.
The preferential accumulation of protein bodies observed in HBK3-S embryos is an indication that an up-regulation of this gene promotes a ‘zygotic-like’ storage deposition pattern, which is characterized by protein bodies prevailing over starch granules (Yeung et al., 1998). The observation that accumulation of protein bodies is induced by ABA (Stasolla and Yeung, 2003) leads to the speculation that the level of this hormone is increased in HBK3-S embryos. Kusaba et al. (1998) documented an accumulation of ABA in tobacco plants overexpressing the KNOX gene OSH1 (Kusaba et al., 1998). An increasing level of ABA in HBK3-S lines would also explain the improvement of embryo yield reported in this study (Fig. 3), as ABA is required for the completion of the embryogenic process. Based on the above evidence, it appears that overexpression of HBK3 is beneficial not only during the early phases of embryogenesis, but also during the successive stages, possibly by altering the endogenous hormone levels. Overexpression of HBK3 does not seem to affect post-embryonic growth. No differences in morphology and performance were in fact observed between control and HBK3-S embryos during germination. Embryos overexpressing HBK3 were able to generate viable plants with no phenotypic deviations from their control counterparts.
Transition of PEMsIII into somatic embryos was reduced in lines in which HBK3 was down-regulated (Fig. 2), and the few immature embryos produced failed to complete the maturation programme (data not shown). This developmental arrest, however, is not due to the changes in morphology as the PEMs III and immature somatic embryos produced by the HBK3-A lines are similar to those observed in control embryos (data not shown). The cause of the developmental arrest observed in the HBK3-A lines remains unclear. One possible explanation is due to the fact that HBK3 shares a high degree of similarity with other spruce KNOX genes, including HBK1 (86% amino acid identity; Hjortswang et al. 2002). Therefore, it cannot be excluded that the introduction of the antisense HBK3 might have caused the down-regulation of more than one KNOX gene.
To assess the function of HBK3 in comparison with that of angiosperm KNOX genes, this gene has been ectopically expressed in Arabidopsis plants and the phenotypic properties of the resulting transformant lines in the T2 generation have been analysed. As observed for spruce, expression of HBK3 increases the size of the SAM in cotyledonary Arabidopsis embryos (Fig. 5E, F) by increasing the number and size of the tunica cells (L1 and L2 layers) (Table 1). However, unlike spruce, HBK3 expression affects post-embryonic growth. Seeds overexpressing HBK3 reactivate early at germination (Fig. 5G–J, Table 2), suggesting that the larger SAMs are more responsive to the germination conditions and are able to form viable shoots at a faster rate. The formation of ‘better quality’ SAMs in the transformed embryos may be the result of a large number of meristematic cells present in the apical pole, as estimated by the enlarged STM expression pattern (Fig. 5N, O). Phenotypic deviations from control were observed in transformed plants during post-embryonic growth. Rosettes of 35S:HBK3 plants had highly lobed leaves and drastically reduced petioles (Fig. 5K, L), common characteristics of plants overexpressing members of the KNOX gene family, including the Arabidopsis STM and its Populus orthologue ARBORKNOX1 (Groover et al., 2006). Furthermore, plants overexpressing HBK3 were generally dwarf and had several axes emerging from the rosette (Fig. 5M). These characteristics were ascribed to new meristems forming within the rosette, as also revealed by the enlarged GUS expression domain (Fig. 5P, Q).
From the present study it is not clear why the severe post-embryonic changes observed in Arabidopsis plants ectopically expressing KBK3 are not reproducible in spruce. One explanation may be in the different promoters driving the transgene in the two systems (ubiquitin in spruce and the 35S promoter in Arabidopsis).
Overall, these data show the importance of HBK3 during embryo development in spruce. Overexpression of this gene promotes the transdifferentiation of immature somatic embryos from PEMIIIs and the formation of the embryonic SAM. The function of this gene seems to be retained in Arabidopsis, albeit that its effect was also extended during post-embryonic growth. Ongoing studies investigating the role of other KNOX genes during embryogenesis in conifers are being carried out.
Abbreviations
- ABA
abscisic acid
- DIG
digoxigenin
- GUS
β-glucuronidase
- PEM
pro-embryogenic mass
- PgAGO
Picea glauca ARGONAUTE gene
- PGR
plant growth regulator (auxin plus cytokinin)
- RAM
root apical meristem
- SAM
shoot apical meristem
- STM
SHOOTMERISTEMLESS
This research was supported by the Natural Sciences and Engineering Research Council of Canada in the form of grants to CS and DS and a PGS D to MB. The generous financial contribution of CELLFOR and the technical help of Mr Luit are also appreciated. The authors wish to thank Professor von Arnold and Dr Clapham for providing the Norway spruce cells used for the experiment and for their kind assistance and suggestions on the transformation studies.




Comments