-
PDF
- Split View
-
Views
-
Cite
Cite
Y. Genc, C. Y. Huang, P. Langridge, A study of the role of root morphological traits in growth of barley in zinc-deficient soil, Journal of Experimental Botany, Volume 58, Issue 11, August 2007, Pages 2775–2784, https://doi.org/10.1093/jxb/erm142
Close - Share Icon Share
Abstract
Zinc (Zn) deficiency reduces crop yields globally. This study investigated the importance of root morphological traits, especially root hairs, in plant growth and Zn uptake. Wild-type barley (Hordeum vulgare) Pallas and its root-hairless mutant brb were grown in soil and solution culture at different levels of Zn supply for 16 d. Root morphological traits (root length, diameter, and surface area) were measured using the WinRHIZOPro Image Analysis system. In soil culture, Pallas had greater shoot dry matter, shoot Zn concentration, shoot Zn content, and Zn uptake per cm2 root surface area than brb, primarily under zinc deficiency. Both Pallas and brb developed longer roots under Zn deficiency. Development of root hairs was not affected by plant Zn status. In solution culture, there were no significant genotypic differences in any of the parameters measured, indicating that mutation in brb does not affect growth and Zn uptake. However, both Pallas and brb developed longer and thinner roots, and root hair growth was less than in soil culture, and was not affected by plant Zn status. The better growth and greater Zn uptake of Pallas compared with brb in Zn-deficient soil can be attributed primarily to greater root surface area due to root hairs in Pallas rather than other root morphological differences.
Introduction
Worldwide, zinc (Zn) deficiency and its negative impact on grain yield of cereals have been recognized for many years (Graham et al., 1992; Takkar and Walker, 1993; Cakmak et al., 1996). Zn deficiency in most cases can be corrected by the application of Zn fertilizers, but the use of minimum Zn fertilizer together with Zn-efficient genotypes is regarded as the most practical approach (Graham et al., 1992; Cakmak et al., 1999). A Zn-efficient genotype is able to grow and yield well in soils too deficient for a standard genotype (Graham, 1984). Research efforts over the last decade have resulted in considerable genotypic variation and a better understanding of underlying mechanisms of Zn efficiency in cereals. Some studies attributed Zn efficiency to better internal Zn utilization (Hacisalihoglu et al., 2003, 2004), while others attributed it to greater Zn uptake by the root (Graham and Rengel, 1993; Cakmak et al., 1998; Erenoglu et al., 1999; Wissuwa et al., 2006). Most recent studies in wheat (Genc et al., 2006) and rice (Gao et al., 2005) suggest that amongst numerous other mechanisms, Zn uptake is most important. However, there has been little critical appraisal of root morphological traits such as length, diameter, and surface area, and their relationships to plant growth and Zn uptake in crop species. Dong et al.(1995) showed that a Zn-efficient bread wheat developed longer and thinner roots than a less Zn-efficient bread wheat and a Zn-inefficient durum. This root trait presumably would enable plants to extract more of the slowly diffusible Zn ions from a given soil volume (Rengel and Graham, 1995a, b). A recent study in rice reported that root surface area explained 32% of the variation observed in Zn uptake (Gao et al., 2005). However, in these studies, only one or two root traits were examined and clearly there is a need to examine major root traits such as length, diameter, and surface area simultaneously.
The presence of long and dense root hairs (e.g. 1–2 mm long subcellular extensions from the root epidermis) may further contribute to plant growth and/or Zn uptake by increasing the effective surface area of the root, thus allowing exploitation of a greater soil volume. Support for this assumption of improved growth and/or Zn uptake through root hairs comes from phosphorus (P) studies in Arabidopsis thaliana (Bates and Lynch, 2000) and barley (Gahoonia and Nielsen, 1997). In these studies, greater P uptake was correlated to length and density of root hairs. This finding was reinforced by another study demonstrating considerable genotypic variation in root hair length amongst wheat and barley cultivars and its importance in P acquisition especially under low P conditions (Gahoonia et al., 1997). Like P, Zn is transported to the root surface mainly by diffusion (Barber, 1984). It has been estimated that for a given amount of diethylene triamine penta-acetic acid (DTPA)-extractable Zn, the spatial accessibility of this fraction increases about 2-fold when the root surface area is doubled (Marschner, 1993). However, there have been no studies to determine the contribution of root hairs to Zn uptake under either deficient or adequate conditions in any crop species.
Root and root hair growth are influenced by both genetic and environmental factors. It has long been known that plant species (Dittmer, 1949) and genotypes within the same species (Gahoonia et al., 1997) differ in root hair length and density. Root hair growth has been shown to increase under limited soil moisture conditions (Mackay and Barber, 1985) and nutrient deficiencies such as P, Fe, and nitrate (Foehse and Junk, 1983; Muller and Schmidt, 2004), while Ca deficiency can inhibit root hair growth (Tanaka and Woods, 1973). However, very little is known about whether root hair growth is affected by plant Zn status or Zn concentrations at the root surface. To our knowledge, there has been only one study, showing a 0.7-fold increase in root hair density under Zn-deficient conditions compared with Zn-adequate conditions (Ma et al., 2001). This study was conducted in A. thaliana, and in agar growth medium which is different from soil or solution culture. It remains to be seen whether Zn deficiency would affect root hair growth in barley grown in soil or solution culture.
Soil culture is commonly used for analysing roots, but difficulties are encountered in separating roots from soil particles and accurately determining nutrient concentrations of roots (Mackay and Barber, 1984). To avoid these problems, solution culture is frequently employed. However, solution culture creates a different environment for root growth, and may produce different results when compared with soil culture. For example, Mackay and Barber (1984) reported that under low P conditions, root hair length and density in corn were greater in soil-grown than solution-grown plants, which was attributed to differences in moisture contents between the two culture systems. In addition, root hair growth did not increase in solution culture as P concentration declined. The authors concluded that the usefulness of solution culture for determining factors that control root growth and root hair growth in soil was limited. In contrast, a P study with two barley genotypes differing in root hair length demonstrated that although root hairs were shorter in solution than in soil, the difference between growth media was not significant (Gahoonia and Nielsen, 1997). Perhaps it can be inferred from this study that solution culture could be useful at least for analysing root traits in barley, if not for nutrient uptake. In A. thaliana, it was shown that root hairs grew longer and denser under low P availability, and this response was the same in nutrient solution and sand–alumina media (Bates and Lynch, 2000). In the light of these discrepancies, which may be due to different growth conditions and plant species used in these studies, it would be useful to assess the suitability of solution culture for root analysis and Zn responses at least in barley.
Recent technical advances in image analysis of root morphological traits and availability of a root-hairless barley mutant have provided tools and plant material for examining the role of root morphological traits in plant growth and Zn accumulation under variable Zn supply and growth conditions, which was the focus of the present study.
Materials and methods
Plant material
Barley (Hordeum vulgare) genotype Pallas and its root-hairless mutant, brb (Gahoonia et al., 2001) were used in this study. The seeds of both genotypes were multiplied in a potting mix to minimize genotypic differences in seed nutrient content as a previous study showed that seed Zn content had a significant effect on growth responses in barley especially under Zn deficiency (Genc et al., 2000). In the present study, the difference in seed Zn content between Pallas (3.4 μg Zn per seed) and the brb mutant (2.7 μg Zn per seed) did not affect growth and Zn accumulation significantly, as indicated by the similar growth responses in both genotypes grown in solution culture with negligible Zn supply (Figs 3, 4).
Growth conditions
Soil culture:
A Zn-deficient siliceous sand, collected from Mt Compass, South Australia, was used in the present study. The sand was passed through a 2 mm sieve, washed three times with deionized water to remove soluble salt and organic matter, and air-dried. The properties of washed and air-dried sand are given in Table 1. The air-dried sand was packed into clear plastic bags in 1 kg portions. As Zn deficiency is often associated with calcareous soils and high pH, calcium carbonate powder (0.5% w/w) was added to the sand and mixed in thoroughly to reduce the availability of Zn and to maintain a high pH (8.0). The following basal nutrients (in mg kg−1 dry sand) were added to the sand in each bag; NH4NO3 (350), KH2PO4 (75), K2SO4 (120), MgSO4.7H2O (90), MnSO4.H2O (3), CuSO4.5H2O (5), H3BO3 (0.3), CoSO4.7H2O (1); FeSO4.7H2O (16.8), H2MoO4.H2O (0.005), and NiSO4.6H2O (0.15). There were three Zn treatments: 0, 0.1, and 1 mg kg−1 sand, which produced plants with deficient, marginal, and adequate levels of Zn, respectively. Once all nutrients were added, they were allowed to dry and mixed throughout the soil. The soil was placed into polyethylene-lined cylindrical PVC pots with approximate dimensions of 6.5×30 cm (diameter×depth).
| Properties | Sand |
| pH (water) | 6.8 |
| Conductivity (dS m−1) | 0.012 |
| Organic carbon (g kg−1) | 0.1 |
| Nitrate-nitrogen (mg kg−1) | 1 |
| Ammonium (mg kg−1) | 1 |
| Phosphorus (mg kg−1) | 2 |
| Potassium (mg kg−1) | 10 |
| DTPA-extractable (mg kg−1)a | |
| Zn | 0.12 |
| Mn | 0.24 |
| Fe | 5.09 |
| Cu | 0.17 |
| Properties | Sand |
| pH (water) | 6.8 |
| Conductivity (dS m−1) | 0.012 |
| Organic carbon (g kg−1) | 0.1 |
| Nitrate-nitrogen (mg kg−1) | 1 |
| Ammonium (mg kg−1) | 1 |
| Phosphorus (mg kg−1) | 2 |
| Potassium (mg kg−1) | 10 |
| DTPA-extractable (mg kg−1)a | |
| Zn | 0.12 |
| Mn | 0.24 |
| Fe | 5.09 |
| Cu | 0.17 |
aDTPA, diethylene triamine penta-acetic acid.
| Properties | Sand |
| pH (water) | 6.8 |
| Conductivity (dS m−1) | 0.012 |
| Organic carbon (g kg−1) | 0.1 |
| Nitrate-nitrogen (mg kg−1) | 1 |
| Ammonium (mg kg−1) | 1 |
| Phosphorus (mg kg−1) | 2 |
| Potassium (mg kg−1) | 10 |
| DTPA-extractable (mg kg−1)a | |
| Zn | 0.12 |
| Mn | 0.24 |
| Fe | 5.09 |
| Cu | 0.17 |
| Properties | Sand |
| pH (water) | 6.8 |
| Conductivity (dS m−1) | 0.012 |
| Organic carbon (g kg−1) | 0.1 |
| Nitrate-nitrogen (mg kg−1) | 1 |
| Ammonium (mg kg−1) | 1 |
| Phosphorus (mg kg−1) | 2 |
| Potassium (mg kg−1) | 10 |
| DTPA-extractable (mg kg−1)a | |
| Zn | 0.12 |
| Mn | 0.24 |
| Fe | 5.09 |
| Cu | 0.17 |
aDTPA, diethylene triamine penta-acetic acid.
Solution culture:
The composition of nutrient solution was as follows (in μM); Ca(NO3)2, 1000; KNO3, 1000; MgSO4, 250; NH4H2PO4, 100; KCl, 50; H3BO3, 12.5; Fe-HEDTA, 10; MnSO4, 0.4; CuSO4, 0.1; H2MoO4, 12.5; ZnSO4, 0.5; NiSO4, 0.1; and MoO3, 0.1. There were three Zn treatments: severely deficient (0.005 μM), deficient (0.05 μM), and adequate (0.5 μM). All nutrient stock solutions were prepared separately, and mixed together in black containers containing double-deionized water (18 MΩ cm−1 resistivity). Concentrations of macronutrients and micronutrients were maintained at half and full strength, respectively until 8 d after transplanting (DAT), and all nutrients at full strength thereafter. The nutrient solution was aerated continuously and replaced at 8, 12, and 15 DAT. The pH of the solutions was buffered at 6.0 by 2 mM MES (2-[N-morpholino]ethane-sulphonic acid)–0.218 M KOH.
The seeds of both Pallas and brb were surface-sterilized [soaked in 70% ethanol for 1 min then in 5% sodium hypochlorite for 5 min (v/v)] and pregerminated on filter papers in Petri dishes for 48 h at room temperature. In soil culture, four pregerminated seeds of each genotype were transplanted into each pot and thinned to two per pot at 4 DAT. Plants were watered daily with double de-ionized water to 12% (w/w) moisture content throughout the experiment. In solution culture, two seeds of each genotype with radicle emerged were transferred into polyvinylchloride cups tightly fitting over 1.0 l black polyethylene containers (one seed per cup; two cups per container), which coincided with plant emergence in soil culture. Both experiments were conducted in a growth chamber set at 20/15 °C day/night temperature and 14/10 h day/night photoperiod with 300 μmol m−2 s−1 photon flux intensity at the plant level.
To avoid complications associated with longer growth periods (e.g. root binding and damaging roots in the process of removing the sand particles and separating individual roots), plants were harvested at 16 d after emergence (DAE) in the soil experiment or 16 DAT in solution culture (Figs 1, 2). At harvest, one of the two plants in each pot was sampled for biomass analysis (shoot and root dry matter, and root:shoot dry matter ratios), and the other was sampled for analysis of root morphological traits and nutrients in the shoots. For nutrient analysis, shoots were separated from roots, and rinsed with double-deionized water then oven-dried at 65 °C for 48 h for dry weight and subsequent nutrient analysis by an inductively coupled plasma emission spectrometer (Zarcinas et al., 1987).
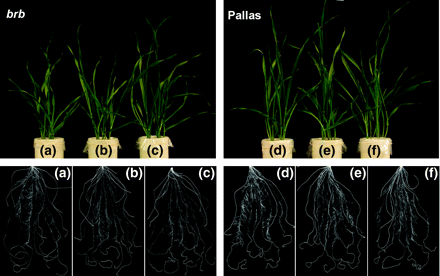
Shoot and root growth in barley genotypes grown at different Zn levels in soil culture at 16 DAE. (a) Zn0, (b) Zn0.1, and (c) Zn1 in brb; (d) Zn0, (e) Zn0.1, and (f) Zn1 in Pallas.
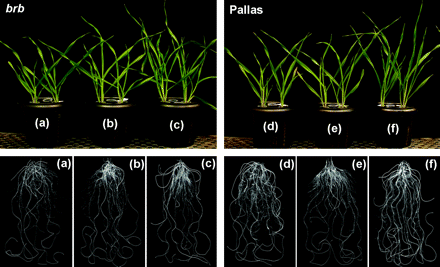
Shoot and root growth in barley genotypes grown at different Zn levels in solution culture at 16 DAT. (a) Zn0.005, (b) Zn0.05, and (c) Zn0.5 in brb; (d) Zn0.005, (e) Zn0.05, and (f) Zn0.5 in Pallas.
Measurements
Root morphological traits (root length, diameter, and surface area) were measured using the WinRHIZOPro Image Analysis system (Reagent Instruments Inc., Canada). The entire root system rather than a subsample of the root was scanned to prevent errors associated with root subsampling. To maintain a density of roots required for accurate measurement, the entire root system was divided into two or three portions, and each portion was placed into the WinRHIZOPro root positioning system (20×30 cm tray) filled with water. Roots were carefully cut out along the main axis and spread out evenly to minimize overlapping. The scanning resolution was set at 600 dpi and the threshold was optimized at 205. The root density was in the range of 0.15–0.25 and 0.15–0.35 mm length per mm2 for soil-, and solution-grown plants, respectively. These densities are less than the recommended density of 0.5 mm root per mm2 surface (Bouma et al., 2000).
As Pallas and brb are genetically similar except for the root hair trait, it is possible to quantify directly the contribution of root hairs to Zn uptake. This was done in the present study by expressing shoot Zn uptake on a root surface area basis (Zn uptake efficiency). Differences in Zn uptake efficiency between Pallas and brb would point to involvement of root hairs.
Root:shoot dry matter ratio was based on the plants set aside for dry matter analysis.
Statistical analysis
The experimental set-up was a completely randomized block design with four and five replications for solution and soil culture, respectively. The soil experiment was repeated twice with similar results. Therefore, herein the data from one of the two soil experiments are presented. The results were analysed using the Genstat Statistical Program. The least significant difference (LSD) at P=0.05 was used in pairwise comparisons of means.
Results
Soil culture
Dry matter, shoot Zn concentration, and content:
Shoot dry matter was affected by both Zn supply and genotype. Pallas had greater shoot dry matter than brb when Zn supply was deficient (28%) or moderate (12%), while both genotypes had a similar shoot dry matter at adequate Zn supply (Fig. 3). In contrast, root dry matter was similar in both genotypes at all Zn levels (Fig. 3). brb allocated more biomass to the roots at deficient and moderate Zn supply compared with that at adequate Zn supply, while Pallas had a similar allocation of biomass between roots and shoots at all Zn levels.
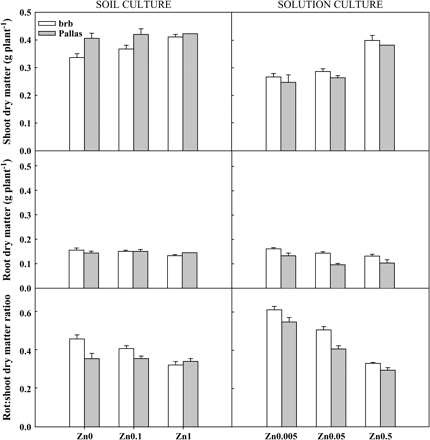
Shoot dry matter, root dry matter, and root:shoot dry matter ratios in brb and Pallas grown in a Zn-deficient soil (left) and a nutrient solution (right) supplied with different levels of Zn. The vertical bars represent standard errors based on five and four replicates in soil and solution culture, respectively.
Zn concentration in the shoots was also influenced by Zn supply and genotype (Fig. 4). At deficient Zn supply, Pallas and brb had shoot Zn concentrations of 12.0 mg and 8.5 mg kg−1 dry weight (DW), respectively. These concentrations are below the critical deficiency concentration of 15 mg Zn kg−1 DW in this study. The critical deficiency concentration is associated with 90% of maximum growth (Ulrich and Hills, 1993). The critical concentration of 15 mg Zn kg−1 DW in the present study serves as a guide as it was based on a small number of observations (e.g. 12 mg Zn kg−1 DW and 14 mg Zn kg−1 DW was associated with 89% and 87% of maximum growth in Pallas and brb, respectively). At moderate Zn supply, the Zn concentration of Pallas (21 mg kg−1 DW) was well above the critical level, while that of brb (14 mg kg−1 DW) was approaching the critical level. At adequate Zn supply, both Pallas and brb had Zn concentrations (37–41 mg kg−1 DW) above the critical level. It is interesting to note that Pallas had higher Zn concentration than brb at all Zn levels, and the difference between the two genotypes was largest at moderate Zn supply (Fig. 4). This amounted to approximately 40, 50, and 10% higher shoot Zn concentration in Pallas at deficient, moderate, and adequate Zn supply, respectively. Shoot Zn content showed similar patterns to shoot Zn concentration. Pallas accumulated greater amounts of Zn than brb at all levels of Zn supply (82, 70, and 15% higher shoot Zn content in Pallas at deficient, moderate, and adequate Zn supply, respectively) (Fig. 4).
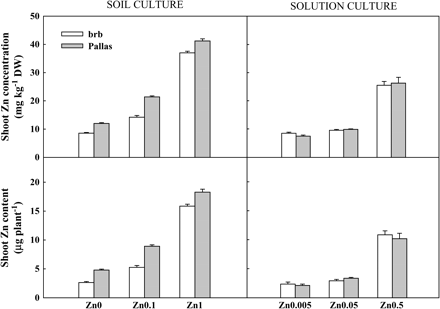
Shoot Zn concentration and Zn content in brb and Pallas grown in a Zn-deficient soil (left) and a nutrient solution (right) supplied with different levels of Zn. The vertical bars represent standard errors based on five and four replicates in soil and solution culture, respectively.
Root morphology (length, diameter, surface area, and root hairs):
Total root length was affected by Zn supply only (Fig. 5). Plants grown at deficient and moderate Zn supply developed longer roots than those grown at adequate Zn supply (on average 10% and 5% longer roots at deficient and moderate Zn supply, respectively). The percentage of nodal, primary, and lateral roots in the total root length was also calculated using nodal and primary root lengths measured manually before scanning and the total root length obtained by WinRHIZOPro. The results demonstrated that lateral roots made up the majority of total root length (87%), and this was followed by primary roots (8%) and nodule roots (5%).
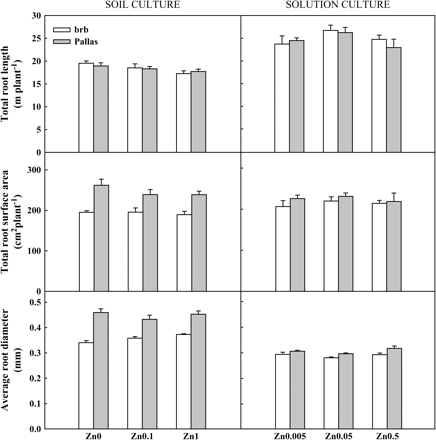
Total root length, root surface area, and average root diameter in brb and Pallas grown in a Zn-deficient soil (left) and a nutrient solution (right) supplied with different levels of Zn. The vertical bars represent standard errors based on five and four replicates in soil and solution culture, respectively.
Average root diameter varied with genotype only (Fig. 5): it was larger in Pallas than in brb at all Zn levels. These differences in average root diameter between the two genotypes resulted in significant differences in root surface area of nodal, primary, and lateral roots (Fig. 5). Pallas (246 cm2 per plant) had greater surface area than brb (193 cm2 per plant). When the contribution of different types of roots to the total root surface area was estimated using diameter data obtained by WinRHIZOPro, lateral roots made the largest contribution (57–61%), followed by primary roots (24–25%) and nodule roots (15–18%).
Root hair length and density in Pallas did not appear to be influenced by Zn supply (Fig. 6).
![Root hair growth on primary and lateral roots of Pallas grown at different Zn levels in soil [(a) Zn0, (b) Zn0.1, and (c) Zn1] and solution culture [d) Zn0.005, (e) Zn0.05, and (f) Zn0.5]. Root sections of primary roots were observed under a microscope [Leica MZFL III (10×)].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/58/11/10.1093/jxb/erm142/2/m_jexboterm142f06_ht.gif?Expires=1747904823&Signature=kSL14p46vdi36js6-J5r0JLXdLE3sF6QWt3N8NhuWCJtOIpW1RJ0yErnIrbQdC~9wKFJSGsnsP2FBKeSGNIRT37bLrNYPX0nEoJ-HL8gYMYoSoVkMerd-zuGmldCRJEDsZ4cnKzPT24vFKq-Duc1jmFOfqDfJ-3-L8umuEUUb59AuqR0GtCyjgrC0x1oYuceXSssRED1QqJChxLwI0ZnKMvHa8CR1M54DMlBvZM~iKRYIV09NhJ33rMl0wIQSTfed4H15xtCiqz5q0uiK2iqZuHbwCiBQgHkq946flAk9Vl5zP7ybgUUmLnTgVXQN7DzWzeDnicRot2y5ffBI98Rpg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Root hair growth on primary and lateral roots of Pallas grown at different Zn levels in soil [(a) Zn0, (b) Zn0.1, and (c) Zn1] and solution culture [d) Zn0.005, (e) Zn0.05, and (f) Zn0.5]. Root sections of primary roots were observed under a microscope [Leica MZFL III (10×)].
Shoot Zn uptake per cm2 root surface area:
Pallas had greater shoot Zn uptake per cm2 root surface area than brb at deficient and moderate Zn supply (27% and 28% higher shoot Zn uptake at deficient and moderate Zn supply, respectively) (Fig. 7).
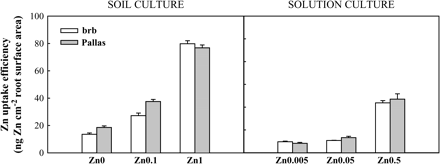
Zn uptake efficiency (ng Zn cm−2 root surface area) in brb and Pallas grown in a Zn-deficient soil (left) and a nutrient solution (right) supplied with different levels of Zn. The vertical bars represent standard errors based on five and four replicates in soil and solution culture, respectively.
Solution culture
Dry matter, shoot Zn concentration, and content:
There were no differences between Pallas and brb in shoot dry matter, Zn concentration, and content at any levels of Zn supply (Figs 3, 4). As Zn supply increased, shoot dry matter, shoot Zn concentration, and shoot Zn content increased dramatically (7% and 42%, 22% and 226%, and 41% and 375% increase at Zn0.05 and Zn0.5 for these three parameters, respectively). Root dry matter was higher at Zn0.005 compared with Zn0.05 and Zn0.5 in brb than in Pallas (Fig. 1). Root:shoot ratios increased with Zn deficiency, and was higher in brb than in Pallas (Fig. 3).
Root length, diameter, surface area, and root hairs:
Similar to soil culture, both genotypes developed longer roots at deficient Zn (Zn0.05 only) than sufficient Zn supply (Zn0.5) (Fig. 5). When the entire root system was divided into nodal, primary, and lateral roots using manually measured nodal and primary root lengths and total root length determined by WinRHIZOPro, lateral roots again made up the majority of total root length (86%), followed by primary (11%) and nodal roots (3%). Unlike soil culture (Fig. 5), there was no effect of genotype or Zn supply on either average root diameter or total surface area (Fig. 5).
Compared with soil culture, root hairs were considerably shorter and thinner in solution culture, but their length and density did not appear to be affected by Zn supply (Fig. 6).
Shoot Zn uptake per cm2 root surface area:
In contrast to soil culture, there was no difference in shoot Zn uptake per cm2 root surface area between Pallas and brb, irrespective of Zn supply (Fig. 7). Both genotypes had significantly higher shoot Zn uptake at adequate Zn than at deficient Zn supply (4.4-fold increase).
Discussion
Significance of root hairs in plant growth and Zn uptake in soil with low available Zn
Genetic and molecular studies demonstrated that the root-hairless mutant brb was a result of a single recessive gene mutation from wild-type Pallas, and both Pallas and brb were similar in their genomes (Gahoonia et al., 2001). Morphological studies also showed that root anatomy was similar between Pallas and brb, except for root hairs (Gahoonia and Nielsen, 2003). The gene underlying this mutation, β-expansin (HvEXPB1), has recently been isolated, and is responsible for the hairless phenotype (Kwasniewski and Szarejko, 2006). For these reasons, Pallas and brb are ideal genetic material to determine directly the contribution of root hairs to plant growth and Zn uptake in barley in the present study. The results show that the greater plant growth and Zn uptake in Pallas over the brb mutant in soil with low available Zn are due solely to the presence of root hairs in Pallas. Pallas had approximately 30% higher Zn uptake efficiency based on root surface area, indicating that the higher Zn uptake efficiency was not due to the root surface area of all three types of roots (nodal, primary, and lateral), but to the surface area of root hairs present in Pallas. This conclusion is further supported by the results from solution culture in which Pallas developed very few root hairs, and there were no differences in plant growth and Zn uptake between Pallas and brb. This indicates that mutation in brb does not affect Zn uptake. It has been reported that root hairs can increase root surface area in barley by up to 3-fold (Gahoonia et al., 1997). Therefore, it is evident that the effective surface area of root hairs is the most crucial root trait in terms of nutrient uptake, especially for diffusion-limiting nutrients in soils with low availability.
No notable changes in root hairs under Zn-deficient conditions
To our knowledge, at present, it is not possible to quantify the density and length of root hairs for the entire root system. In addition, root hair length can vary depending on the root type and the section of the root. For these reasons, the present study was limited to visual assessment of root hairs from representative samples under the microscope. Microscopic images suggested that root hair length and/or density were not altered by plant or soil Zn status (Fig. 6). However, in solution culture, root hairs were very few and short, but the effect of Zn supply on root hair growth was not notable. The results essentially agree with those of Ma et al. (2001), who demonstrated that root hair density of A. thaliana grown in agar growth medium was uniquely sensitive to P deficiency, but to a smaller extent to other nutrients including Zn. Compared with the control treatment, root hair density in P-deficient treatment increased by 5-fold, while Zn deficiency caused only a slight increase of 0.7-fold. Also, a study in barley with low P supply reported that root hairs were longer in soil-, than in solution-grown plants, but the difference between the media was non-significant (Gahoonia and Nielsen, 1997).
Zn deficiency increases biomass partitioning to roots
brb allocated more biomass to the root at deficient Zn supply compared with that at adequate Zn supply, while allocation of biomass to the root in Pallas remained unaffected by Zn supply. The greater partitioning of biomass into the roots of brb can be explained by the fact that brb was more Zn deficient than Pallas. This preferential allocation of biomass into roots under Zn deficiency has been previously demonstrated in a study with 54 barley genotypes (Genc et al., 2002) and in other plant species such as wheat (Cumbus, 1985) and chickpeas (Khan et al., 1998). However, higher root:shoot ratios of biomass do not necessarily mean better growth and/or Zn uptake (Genc et al., 2002), as effective surface area could be different.
Fundamental differences in root morphological traits between soil and solution culture
Both Pallas and brb developed longer and thinner roots when grown in solution compared with soil culture. It is also interesting to note that Pallas developed longer and denser root hairs in soil than in solution culture (Fig. 6). These changes in root morphology can lead to differences in plant nutrient uptake between the two culture systems. There may be several explanations for differential root morphology in soil and solution culture: (i) different mechanical resistance to which roots are subjected (Dong et al., 1995); (ii) stimulation of root hair growth under low moisture conditions (Mackay and Barber, 1985); and (iii) regulation of root hair growth being more specific to nutrient deficiencies such as P (Ma et al., 2001). The present results suggest that future studies on genetic variation in root hair length and density, and Zn uptake should consider soil rather than solution culture as the competitive advantage of root hairs in soil culture diminishes in solution culture.
It is also worthwhile to note that both average root diameter and root surface area contributed by nodal, primary, and lateral roots were greater in Pallas than in brb in soil culture, but not in solution culture (Fig. 5). The root dry weight of Pallas was similar to that of brb at all Zn levels (Fig. 3) and the total root length was also similar in both genotypes (Fig. 5). Therefore, the observed differences in the average root diameter and root surface area are probably due to errors introduced by the WinRHIZOPro software during the root analysis. It turns out that WinRHIZOPro software cannot clearly distinguish between roots (nodal, primary, and lateral) and root hairs, and thus overestimates root diameter and root surface area in Pallas due to shading caused by denser root hairs. This overestimation was not evident in solution culture as Pallas developed fewer root hairs compared with soil culture. Therefore, no genotypic differences were observed in solution culture. The analytical errors associated with estimating root diameter and root surface area can lead to an increase in these parameters (potentially up to 25% overestimation in root surface area in the present study) when comparisons are made between genotypes differing particularly in root hair density. In the present study, root hairs contributed significantly to growth and Zn uptake despite the likelihood of overestimated surface area of nodal, primary, and lateral roots; however, such overestimation may have significant impact in other studies.
In conclusion, the results demonstrate that root hairs could improve plant growth and Zn uptake in barley under Zn-deficient conditions. As soil-grown plants developed longer and denser root hairs compared with solution-grown plants, the contribution of root hairs to plant growth and Zn uptake would be best assessed in the soil rather than in solution. However, there is a need to determine root surface area of soil-grown plants accurately. In contrast to P, root hair formation does not appear to be regulated by plant or soil Zn status. While this ‘proof of concept’ study utilized barley genotype Pallas and its root hair-defective mutant brb to demonstrate the contribution of root hairs to growth and Zn uptake, it should be borne in mind that with the exception of a few mutants, almost all barley accessions or cultivars would naturally possess root hairs. Therefore, the question of how much variation exists in root hair length and density within barley germplasm and their likely contribution to growth or Zn uptake needs to be investigated in future studies.
We wish to thank Mrs Teresa Fowles and Mr Lyndon Palmer for technical assistance with ICP analysis, Drs Iver Jakobsen for providing the seed of Pallas and brb, and Dr Ursula Langridge for the seed propagation. We would also like to thank Dr Keith Gatford for his assistance with microscopy. We greatly appreciate constructive comments from the editor and anonymous reviewers. This work was supported by the Grain Research and Development Corporation (CYH, PL), the Australian Research Council (CYH, PL), the South Australian Government (CYH, PL), the Molecular Plant Breeding Cooperative Research Centre (YG), and the University of Adelaide (CYH, PL, YG).




Comments