-
PDF
- Split View
-
Views
-
Cite
Cite
Brian Thomas, Light signals and flowering, Journal of Experimental Botany, Volume 57, Issue 13, October 2006, Pages 3387–3393, https://doi.org/10.1093/jxb/erl071
Close - Share Icon Share
Abstract
Physiological studies over a long period have shown that light acts to regulate flowering through the three main variables of quality, quantity, and duration. Intensive molecular genetic and genomic studies with the model plant Arabidopsis have given considerable insight into the mechanisms involved, particularly with regard to quality and photoperiod. For photoperiodism light, acting through phytochromes and cryptochromes, the main photomorphogenetic photoreceptors, acts to entrain and interact with a circadian rhythm of CONSTANS (CO) expression leading to transcription of the mobile floral integrator, FLOWERING LOCUS T (FT). The action of phytochromes and cryptochromes in photoperiodism is augmented by ZEITLUPE (ZTL) and FLAVIN-BINDING, KELCH REPEAT, F-BOX (FKF1) acting as accessory photoreceptors on entrainment and interaction, respectively. Light quality acts independently of the circadian system through Phytochromes B, D, and E to regulate FT. Light quantity effects, on the other hand, are still incompletely understood but are likely to be linked either directly or indirectly to patterns of assimilate partitioning and resource utilization within the plant.
Introduction
As with almost all aspects of plant development, the flowering behaviour of higher plants is modified by environmental signals. Light is foremost among these environmental signals and can modify flowering in several different ways. This is not particularly surprising as plants need to adapt to precise ecological niches defined both spatially and temporally in terms of a favourable environmental envelope. In this review the action of light is considered both with regard to light acting directly through photomorphogenetic mechanisms, or alterations in photosynthetic assimilation or as part of the more complex regulatory mechanisms of photoperiodism. Much of the body of literature on physiological studies will be assumed although it is worth noting that many of the revelations from recent molecular genetic studies were presaged by careful whole plant studies. Thus, concepts of photoperiodic control of flowering, based on physiological studies, envisaged an external coincidence circadian model with multiple photoreceptors acting in the leaf to generate a complex mobile flowering signal (Thomas and Vince-Prue, 1997). The explosion of knowledge and resources in Arabidopsis thaliana has resulted in much of the fine detail beneath the model being teased out to confirm and extend this basic concept. For light to affect a particular biological process it must be detected through the excitation of a light-absorbing pigment (or photoreceptor) which then passes on the light as energy or as a signal through highly refined regulatory pathways or networks. A consideration of light signalling is therefore inseparable from a consideration of the roles of photoreceptors in the process of flowering.
Light and photoperiodism
Daylength is a major regulator of flowering time and allows sexual reproduction to take place at an appropriate time or times of the year and helps ensure that flowering is co-ordinated to enable cross-pollination where this forms part of the plant's adaptive strategy. Studies on Arabidopsis have enabled the framework of understanding, based on physiological studies over more than half a century, to be supported by detailed information on the cellular and molecular genetic processes that give rise to the occurrence of photoperiodism. It is worth remembering that Arabidopsis is typical of a particular photoperiodic class, namely a facultative cruciferous, light-dominant, long-day plant (LDP). This is only one of a range of patterns of response that plants show to daylength (Thomas and Vince-Prue, 1997). Resolving the relationship of the Arabidopsis model to species with contrasting physiological responses, for example, short-day plants (SDP) is an area that is currently being addressed by a range of research groups. As these other species almost always lack the exquisite combination of genetic properties and resources that make Arabidopsis such a powerful model, resolving their photoperiodic mechanisms is a considerable challenge, but will almost certainly add levels of subtlety and complexity to our understanding.
Light and clock entrainment
As a starting point for examining the role of light, daylength measurement will be taken to result from an external coincidence model based on a circadian clock (Carre, 2001; Yanovsky and Kay, 2002). In this model, light has two roles. Firstly, it acts to entrain the circadian clock and, secondly, it interacts with an output from the clock at critical phases to allow or prevent flowering. Reconciliation of earlier physiological and more recent genetic models has been achieved through the findings that the clock acts to establish a rhythm of the CONSTANS (CO) gene expression, at least partially mediated by the flowering time gene GIGANTEA (GI) (Mizoguchi et al., 2005). CO levels are high towards the end of a long-day (LD) and the interaction with light results in the transcriptional activation of FLOWERING LOCUS T (FT) (Kardailsky et al., 1999; Samach et al., 2000; Yanovsky and Kay, 2002). The regulation of FT takes place in leaves from which FT mRNA travels to the apex to interact with FD and initiate floral development (Huang et al., 2005; Abe et al., 2005; Wigge et al., 2005).
Much of the basic information on the circadian clock underlying daylength measurement derives from transgenic plants using reporters of circadian regulated photosynthetic genes fused to a luciferase reporter. It is assumed that essentially the same clock elements will form the basis of the photoperiodic clock. In general, this is supported by the fact that under the right conditions mutations that alter expression of components of the circadian clock alter flowering time (Somers et al., 1998a; Doyle et al., 2002; Matsushika et al., 2002; Sato et al., 2002; Yamamoto et al., 2003). For example, the EARLY FLOWERING 3 mutation ELF3 exhibits light-dependent arrhythmia of the clock and the gene product is thought to modulate the action of photoreceptors on the input to the clock. Entrainment of the circadian clock to cycles of light and darkness involves the action of multiple photoreceptors. There are five members of the phytochrome gene family in Arabidopsis and equivalent gene families in other species (Izawa et al., 2002; Kendrick and Weller, 2004). In Arabidopsis, at least four of the five PHYTOCHROMES (PHYA, B, D, and E) and both CRYPTROCHROMES (CRY1,2) have been shown to contribute input to the clock (Somers et al., 1998b; Devlin and Kay, 2000). A suite of photoreceptors acting singly or together enables the clock to be entrained by light at a range of wavelengths and irradiances. PHYB, PHYD, and PHYE are primarily responsible for mediating red light at high irradiances while CRY1 and CRY2 mediate high irradiance blue light. Perception of low irradiance red and blue light is mediated by PHYA. Devlin and Kay (2000) also provided evidence that cryptochrome may act downstream of PHYA on the input to the clock. Light affects components of the circadian oscillator at several levels. First, light promotes transcription of the LATE ELONGATED HYPOCOTYL (LHY), and CIRCADIAN CLOCK-ASSOCIATED 1 (CCA1) genes in the early part of the day (Martinez-Garcia et al., 2000; Kim et al., 2003). Light also acts through through the effect of ZEITLUPE (ZTL) to modulate the accumulation of the TIMING OF CAB1 (TOC1) protein (Somers et al., 2000; Mas et al., 2003). ZTL containins a LOV domain which is highly similar to the flavin chromophore binding site in PHOTOTROPINS, which act as blue light photoreceptors for phototropism and other orientation responses. ZTL is also an F-box protein that acts to target specific molecules, for example, TOC1 for degradation via the 26S proteasome.
Light and the clock output
The multiple effects of light on the timing mechanism that act to set the phase of the photoperiodic response rhythm and the separate effects of light acting downstream of the clock to mediate floral response can be distinguished. As with light input to the clock, phytochromes, cryptochromes, and potentially novel photoreceptors all appear to have a role. All five phytochromes contain identical tetrapyrrole chromophores (Lagarias and Rapoport, 1980; Kendrick and Weller, 2004). Mutants or transgenic plants in which chromophore biosynthesis is impaired, will be incapacitated with regard to all of the functional phytochromes. Such plants show altered daylength responses, flowering earlier than wild types in both LDP and SDP ( Montgomery et al., 2001; Izawa et al., 2002; Sawers et al., 2002) although, as different phytochromes may have opposing actions, it is difficult to draw any real significance from this.
PHYA typically acts to detect light rich in far-red in de-etiolating seedlings (Whitelam et al., 1993; Weller et al., 2001). Low irradiance treatments with far-red rich incandescent lamps are routinely given as photoperiod extension treatments in daylength experiments and found to be highly effective, implying a potential role for PHYA in daylength sensing. Supporting evidence for a role for PHYA in promoting flowering in LDP came from the action spectrum for flowering in wheat which shows a far-red peak similar to the far-red high irradiance response in dark-grown seedlings and which is mediated by PHYA (Carr-Smith et al., 1989, 1994). This has been confirmed and extended by more recent genetic studies. PHYA is required for normal perception of long days in Arabidopsis and in the LDP, pea (Johnson et al., 1994; Neff and Chory, 1998; Weller et al., 2001). PHYA acts principally through the photoperiod pathway as it has no effect on flowering time under short days. Arabidopsis mutants deficient in PHYB flower earlier than the wild type in both SD and LD, but retain sensitivity to daylength (Reed et al., 1994). PHYB, along with PHYD and PHYE mediates early flowering in Arabidopsis in response to low red to far-red ratios (Franklin et al., 2003; Halliday et al., 2003). This response plays a role in shade avoidance and is distinct from the role of PHYB in photoperiodism (see section on Light Quality and Flowering below). By contrast, PHYB is essential for daylength perception in barley since the BMDR-1 mutant of Barley, which contains a defective PHYB is insensitive to photoperiod (Hanumappa et al., 1999) and similarly the PHYB mutant of pea is aphotoperiodic (Weller et al., 2001).
Cryptochromes are flavoproteins that act as blue light photoreceptors. The participation of cryptochromes in the control of flowering in Arabidopsis is consistent with physiological studies that have shown that blue light has a promotive effect on flowering for LDP of the Cruciferae although this is not necessarily true for other families (Thomas and Vince-Prue, 1997; Runkle and Heins, 2001). Two members of the cryptochrome gene family (CRY1 and CRY2) are present in Arabidopsis (Lin and Shalitin, 2003). CRY2 is thought to be the major blue photoreceptor for flowering in Arabidopsis (Guo et al., 1998), although cry1 cry2 double mutants flowered earlier in blue light than the single mutants, indicating that both cryptochromes play a role to promote flowering (Mockler et al., 1999). CRY1 and CRY 2 act along with PHYA to stabilize CO protein synthesized towards the end of a LD treatment (Valverde et al., 2004), which provides a mechanism for an external coincidence model of day length regulation. There is evidence that allelic diversity in cryptochromes contribute to the natural variation in the response of Arabidopsis to daylength. A QTL for flowering time was accounted for by an allele of CRY2 (El-Assal et al., 2001) indicating that the blue light photoreceptor has a significant role in daylength sensing under natural conditions. The early flowering phenotype resulted from a single amino acid substitution that reduces the light-induced turnover of the CRY2 protein under short photoperiods. There is as yet no physiological evidence for a specific role for blue light, and by inference, cryptochromes, in SDP, but this remains to be confirmed in genetic and comparative genomic studies.
A further novel photoreceptor may play an additional role in the Arabidopsis photoperiodic mechanism. A FLAVIN-BINDING, KELCH REPEAT, F-BOX (FKF1) (Nelson et al., 2000) protein is required to generate peak the of CO transcription observed towards the end of a long day. The mRNA is under circadian control and the protein binds flavin mononucleotide and is photoactive. In addition, FKF1's action to regulate CO requires light and it may, therefore, act as an auxiliary blue light photoreceptor (Imaizumi et al., 2003). It has been recently shown that FKF1 controls daily CO expression in part by degrading CDF1, a repressor of CO transcription, to boost CO production at the critical point in the photoperiod for long day sensing (Imaizumi et al., 2005).
Light and short-day plants
The bulk of published information on photoperiodic mechanisms is derived from Arabidopsis, a facultative LDP. There is accumulating evidence that elements of the LD-sensing model also form part of the mechanism in SDP (Liu et al., 2001; Searle and Coupland, 2004). Genetic analysis has identified that in species that act as SDPs, common components to those found in Arabidopsis are involved in the photoperiodic response. In rice, a SDP, Hd1, an orthologue of CO, and Hd3a, an orthologue of FT, are required for flowering in response to short-days (Yano et al., 2000; Hayama et al., 2003). The relationship between the CO and FT orthologues is, however, reversed from that found in Arabidopsis. In rice Hd1 is under circadian regulation and Hd1 mRNA accumulates towards the end of the day in LD. Coincidental exposure to light at this time acts to suppress transcription of Hd3a (Hayama and Coupland, 2004) and consequently inhibits flowering in LD. Orthologues of CO have been isolated from the SDP Pharbitis nil and show diurnal regulation of expression (Liu et al., 2001), consistent with a general mechanism for daylength regulation.
A consequence of these opposite actions of CO and orthologues in different species is that mutants lacking CO default to late flowering in LDP and early flowering in SDP. Mutants of FT on the other hand would be expected always to be late flowering, irrespective of response type. If it is further assumed that FT is the mobile florigenic signal, this reversible relationship between CO and FT provides an explanation for the observations from grafting experiments that the flowering stimulus is interchangeable between response types as well as between species and in some cases, genera (Thomas and Vince-Prue, 1997).
Light quality and flowering
Plants grown under canopy shade conditions or in the proximity of other plants show a range of responses to the change in red (R) to far-red (FR) ratio of the ambient light. This response, known as the shade-avoidance or near neighbour detection response is characterized by increased internode extension, reduced leaf area and acceleration of flowering (Halliday et al., 1994). Many of the characteristics of the shade avoidance response can be mimicked by a short end-of-day FR treatment, implicating light-stable phytochrome as the photoregulator. PHYB is a major player in this response, but there is redundancy with PHYD and PHYE such that double or triple mutants show increasingly extreme phenotypes (Halliday et al., 1994; Aukerman et al., 1997; Devlin et al., 1998, 1999). Mutants in PHYB in Arabidopsis flower earlier than WT in both LD and SD but retain a differential response to daylength indicating a light-dependent non-photoperiodic regulatory pathway. Both light-dependent pathways, photoperiodic and light quality, act through the regulation of FT. Regulation of FT by PHYB occurs through the nuclear protein, PHYTOCHROME AND FLOWERING TIME 1 (PFT1) (Cerdan and Chory, 2003). Another characteristic of this pathway is that the early flowering phenotype in the absence of PHYB is temperature sensitive (Halliday et al., 2003). As other aspects of the shade-avoidance response do not show the same response to temperature, sensitivity lies in a flowering-specific branch of the PHYB signalling pathway.
PHYB acts through FT, implying that the light quality pathway is leaf-based. This is supported by experiments with enhancer trap lines that showed suppression of FT expression by PHYB expressed in mesophyll cells (Endo et al., 2005). In pea, however, the inhibitory effect of PHYB on flowering was found not to be graft-transmissible, in contrast to the photoperiodic effect of PHYA (Weller et al., 1997). One difference between pea and Arabidopsis appears to be that, in pea, a transmissible inhibitor is linked to the effect of PHYA whereas in Arabidopsis, control by events in the leaf is mediated entirely through FT, a floral promoter.
Light quantity responses
While increased light levels are usually accepted as having a positive effect on flowering, the plant response to light quantity for flowering is very variable. In a study of 41 herbaceous species, 10 were found to have a facultative irradiance response while 28 were unaffected (Mattson and Erwin, 2005). Interestingly, three species in this study had a negative response to increasing irradiance. In general, light quantity responses are assumed to be linked to photosynthesis and the availability of assimilates, the photoreceptors in this case being chlorophylls and other photosynthetic pigments. This idea is supported by experiments in which a strong irradiance dependence on flowering in Brassica campestris could be eliminated by supplying sucrose (Friend, 1984). Bagnall and King (2001) found that flowering was delayed under low irradiance in PHYA mutants, but not at higher irradiances, and suggested that part of PHYA action was mediated indirectly through photosynthesis although it is not clear how such a mechanism might operate.
Light quantity seems to be particularly important during early development, particularly with herbaceous species that show a clear juvenile phase (Perilleux and Bernier, 2002). It is proposed that the inability to flower during this juvenile period is because of a foliar inability to produce floral signals and/or of the competence of the apex to respond (Zeevaart, 1985; McDaniel, 1996; McDaniel et al., 1996). The length of the juvenile phase in photoperiod-sensitive plants can be revealed by reciprocal transfers between permissive and non-permissive daylengths. Such experiments with Petunia showed that the length of the juvenile phase is prolonged at lower irradiances (Adams, 1999).
What is not clear is the precise molecular mechanisms by which irradiance, if acting through photosynthetic assimilation, can modify the length of the juvenile phase. It may well be that assimilates themselves act as part of a complex flowering signal (Perilleux and Bernier, 2002; Bernier and Perilleux, 2005) or it may be that the delivery of the mobile flowering signals such as FT is dependent upon a sufficient mass flow of assimilates. One factor inhibiting a resolution of this issue is that the juvenile phase is very short in Arabidopsis and experimental separation of source–sink relationships in very young seedlings is difficult to achieve in a manner that is comparable to the extended juvenile phase of many herbaceous species.
Conclusions
Physiological studies over a long period have shown that light acts to regulate flowering through the three main variables of quality, quantity, and duration (Fig. 1). Intensive molecular genetic and genomic studies with the model plant Arabidopsis have given considerable insight into the mechanisms involved, particularly with regard to quality and duration. The Arabidopsis model is now being extended to other plants, including crops and different response types and a range of variations can be expected to emerge as more studies are undertaken. Light quantity effects, on the other hand, are still incompletely understood but are likely to be linked either directly or indirectly to patterns of assimilate partitioning and resource utilization within the plant.
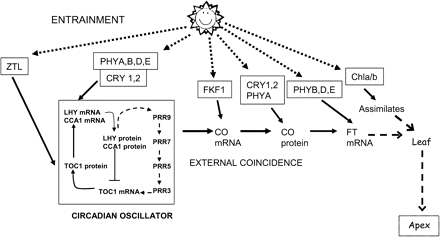
Multiple actions of light affect flowering. The diagram indicates the main targets of light and the photoreceptors involved. Photoperiodic control involves the entrainment of the circadian rhythm and interaction with the output, primarily through CO to regulate FT. Light acts directly through phytochromes in the leaf via FT. In addition, light acts indirectly through chlorophyll (Chla/b) via photosynthesis and the availability of assimilates which are translocated to the apex with FT. (Modified from Thomas B, Carré I, Jackson SD. 2006. Photoperiodism and flowering. In: Jordan BR, ed. The molecular biology and biotechnology of flowering, 2nd edn. CAB International, 3–25, and reproduced by kind permission of CAB International.)
The author would like to acknowledge support from the Department for Environment, Food and Rural Affairs (Defra) in preparing this review.




Comments