-
PDF
- Split View
-
Views
-
Cite
Cite
Sujan Dawadi, Clifford S Sadof, Urban microclimate warming improves overwintering survival of evergreen bagworms, Journal of Urban Ecology, Volume 8, Issue 1, 2022, juac014, https://doi.org/10.1093/jue/juac014
Close - Share Icon Share
Abstract
In the northernmost latitude of North America, the evergreen bagworm, Thyridopteryx ephemeraeformis (Haworth), distribution is limited by overwintering temperatures. Urban impervious surfaces such as roads, buildings and parking lots can warm microclimates and create ecological temperature gradients that have the potential to increase the winter survival of insects. To test this hypothesis, we evaluated survival of bagworms over gradients of microclimatic conditions. Bagworms live within spindle-shaped bags constructed from fragments of foliage. In late summer, adult male bagworms fly to bags containing wingless adult females. Mated neotenous females lay eggs within their pupal case. These eggs hatch into larvae during the late spring of the following year and disperse to hosts by ballooning. A total of 2255 bagworm bags were collected from 119 sites in Indiana and Illinois prior to egg hatch in the spring of 2018 and 2019. The maximum temperature during the coldest days of winter was recorded at each site. Up to 25 bagworms were removed from each host plant to assess the overwintering survival of eggs. Survivorship rose as estimates of impervious surface within a 20-m radius increased. Specifically, 50% of bagworm eggs survived at maximum daily temperatures of −19.4°C, −20°C and −20.6°C when plants were surrounded by 25.7%, 48.39% and 50.75% impervious surface, respectively. Egg mortality was not buffered by impervious surfaces at temperatures at or below −21.67°C. Our findings provide insights about how impervious surface in urban areas can provide refugia for marginally hardy insects and improve their chances of surviving the cold of winter.
Introduction
Urbanization can create ecological gradients by changing physical environments (Grimm et al. 2008). As such, urban and peri-urban landscapes have been used to test hypotheses about how these gradients impact arthropod communities (e.g. Tooker and Hanks 2000; Raupp, Shrewsbury, and Herms 2010). Urban warming has the potential to promote herbivorous pests by inducing drought stress in plants during the growing season and changing phenology and fecundity (Meineke et al. 2013; Dale, Youngsteadt, and Frank 2016; Dale and Frank 2017). Urban microclimate warming is defined as a localized measure of the climate in the immediate vicinity of a plant and animal (Rosenberg et al. 1983) and is distinct from macroclimate which describes a larger area that extends beyond localized conditions.
The capacity of urban structures and impervious surfaces to increase temperature was first noticed in the 1830s (Howard 1833) and has long been considered to be a contributing factor to urban warming (Biswas and John 1900; Oke 1972; Oke 1981; Grimmond 2007; Lee and Kim 2008; Cui et al. 2016; Dale, Youngsteadt, and Frank 2016; Yan et al. 2016; Ren 2017). The recent development of a systematic pacing technique to measure impervious surfaces has made it possible to quantify the urban microhabitat in ways that are associated with increased temperature and water stress on plants in the southern and eastern USA (Dale, Youngsteadt, and Frank 2016; Just, Frank, and Dale 2018). Drought and warming act additively in cities to cause pest insect outbreaks by increasing the fecundity and rate of development on host plants (Dale and Frank 2017). However, such studies have only focused on sap feeders and their reproductive capacity. The effect of microclimate warming on the overwintering survival of insect pests has yet to be fully described.
Microclimate warming has been associated with the abundance of insects at the northern edge of their distribution where it can buffer cold temperatures that cause substantial winter mortality. Mimosa webworm (Homadaula anisocentra Meyrick) provides a notable example of this on honeylocust trees (Gleditsia triacanthos L.) in Iowa and Indiana (Miller 1984; Sperry et al. 2001). Sperry et al. (2001) related the abundance of mimosa webworm and its natural enemies to the amount of impervious surface within 20-m of its host plants. Since then, little work has been conducted to explore the effects of urban warming on other lepidopterans (Battisti et al. 2005; Robinet et al. 2012). The evergreen bagworm (Lepidoptera: Psychidae, Thyridopteryx ephemeraeformis Haworth) provides an opportunity to study the effects of microclimate warming in cities because this insect is widespread and its northern limit of distribution is limited by season length and extremes of low temperatures (Lynch et al. 2014).
The evergreen bagworm is a common defoliating pest of ornamental trees and shrubs in eastern USA (Neal and Santamour 1990; Ellis et al. 2005; Rhainds et al. 2013). It has a broad host range (Johnson and Lyon 1991) but commonly feeds on evergreens Juniperus, Picea and Pinus and Thuja species (Ghent 1999; Moore and Hanks 2004; Ellis et al. 2005) as well as deciduous trees and shrubs like Acer, Quercus, Ulmus and Gleditizia. They are called bagworms because their worm-like larvae are within spindle-shaped bags they construct from silken threads and host foliage (Davis 1964; Moore and Hanks 2004). During molting and pupation, they attach these bags to the host branch with a band of silk. In late summer, winged males mate with wingless adult females, after which the female lays eggs within its pupal case (Rhainds et al. 2013). Eggs overwinter within the female pupal case and remain covered by silken bags until they hatch in late spring of the following year (Ellis et al. 2005). Bagworms have only one generation a year (Morden and Waldbauer 1971). They disperse as first instar larvae by ballooning and may be carried on the wind to other hosts or locations (Moore and Hanks 2004).
In North America, the bagworm can be found as far north as Michigan and into Ontario, Canada (Rhainds et al. 2013). The winter survival along the northern edge of its range has been explained by season length and winter temperatures. Specifically, exposing bagworms to temperature of −18°C for 24 h killed ∼50% of the eggs in laboratory experiments (Rhainds et al. 2013). Though broad climatic factors have played a significant role in the northward expansion of the evergreen bagworm, microclimatic effects on temperature may be crucial to overwintering survival near the northern limits of its range. The potential impacts of urban microclimate warming on overwintering egg survival have not been studied.
In this study, we explore the extent to which urban microclimate warming affects the overwintering survival of bagworms. In addition, we also predict that the pace to plant technique for measuring impervious surface is sensitive enough to predict the warming effect. We hypothesize that increasing areas of impervious surfaces will elevate temperatures and improve bagworm overwintering survival in the Midwest.
Materials and methods
Site selection
Sites for bagworm collection were selected based on the availability of infested plants and the distribution of days of potentially lethal cold temperatures (at or near −18°C for 24 h, Rhainds et al. 2013) during the winters of 2017–8 and 2018–9 in Indiana and Illinois. A total of 72 sites were selected in eight cities in 2018 and a total of 47 sites from six cities in 2019. Sites were selected randomly to include gradients of the impervious surface around host trees. For example, trees selected from park-like settings had little or no impervious surface while those in parking lots, streets and sidewalks were surrounded by the most impervious surface. 1 January was the coldest day in 2018 and 2 February was the coldest day in 2019. The maximum temperature on extreme cold days ranged from −20°C to −17.22°C at 72 sites in 2018 and from −22.22°C to −17.22°C at 47 sites in 2019.
Infested host selection and bagworm collection
Bagworm-infested trees and shrubs were identified, collected and GPS coordinates were recorded with an iPhone-6 during 12–17 March 2018 and during 11–16 March 2019. A total of 1303 bagworms were collected from 72 sites in Indiana and Illinois with latitudes ranging from 39.93° to 40.50° in 2018. Similarly, a total of 952 bagworms were collected from 47 sites with latitudes ranging from 39.93° to 41.47° in 2019. However, only 58 sites from 2018 were included in our study because bagworms in the remaining 14 sites had no intact pupal cases inside overwintering bags. Depending on the existing infestation level, up to 25 female bagworms (at least six in 2018 and at least eight in 2019) were removed from each host plant and placed into locking plastic bags. Bags were brought into the laboratory and kept at room temperature (25°C) for 1 week before assessing the overwintering survival of eggs, and the capacity of each clutch of bagworm eggs to produce live larvae.
Impervious surface area estimation
We used a pacing technique (pace to plant; Dale, Youngsteadt, and Frank 2016) to estimate impervious surface area within ∼20-m radius of the host plant. ‘Pace to plant’ is a tool developed to measure the amount of the impervious surface area that surrounds the tree or specific site. Briefly, this was accomplished by identifying the nearest impervious surface to the host tree and having the same observer use a step technique. The first 25-step transect was walked along a 45° angle from the tree base location toward the nearest impervious surface edge and counting the number of steps that touched the impervious surface. Then three more transects were taken at 90° angles to complete the circle. The percentage of impervious surface area around each host plant was calculated as the total number of steps that touched an impervious surface. The average distance (19.81 ± 0.15 m) covered by the single observer who counted these steps was estimated by measuring the distance covered at 15 random landscape locations in West Lafayette, IN.
Overwintering survival of eggs
Adult females surrounded by their intact pupal covering were extracted from each bagworm bag and weighed to the nearest 0.001 gm. The body length of each pupa-encased female was also measured to the nearest 0.001 mm. Two females of near average weight were sampled from each site to estimate the percentage of overwintering eggs that survived. Each of the two females was dissected so that the first 50 eggs could be examined to estimate the percentage of viable eggs (Rhainds et al. 2013). Live and dead eggs were classified based on their color following Rhainds and Sadof (2009) where white eggs were counted as live and black eggs as dead.
Bags producing viable larvae
Bags that were not used for examining the overwintering survival of eggs were kept in the cups in the laboratory at room temperature for 12 weeks and monitored until larvae hatched (Rhainds et al. 2013). Overwintering bags containing a clutch of eggs that produced at least one live larva were counted as viable. Bags that did not produce at least one live larva were dissected to make sure whether there were larvae inside or only dead eggs. Finally, bags with at least one larva were converted to percentage viable bags out of the total bags collected at each site.
Relationship between temperature and impervious surface
It was impossible for us to monitor the effects of impervious surfaces on temperatures at each of our study sites because we had to wait until the end of winter to find sites with the coldest temperatures of the season. As such, measurements of winter temperatures in 2020 were used to determine the extent to which impervious surfaces affected tree canopy temperatures in our study region. The temperature of the bagworm host trees and shrubs was monitored in 20 sites from West Lafayette, and Lafayette, IN was selected from 15 December 2019 through 15 April 2020. A total of 20 iButton® Thermochron® DS1921G (Embedded Data Systems, LLC, Lawrenceburg, KY, USA) temperature loggers were individually kept inside an inverted 60-ml translucent plastic cup (Dart container cooperation, Mason, MI, USA) to protect them from precipitation. Cups were affixed to trees and shrubs at breast height (1.37-m from soil line) using plastic zip ties. The temperature loggers were set to record at 2-h intervals. To determine the buffering effect of the impervious surface during cold snaps, we focused our analysis on days when the maximum T was at or below the freezing temperature of water, ≤0°C. On these days at each time point, we assessed the differences between the average measured air temperature at the sites with no impervious surface and the air temperature along a gradient of impervious surface cover (maximum = 97%). The ability of the impervious surface to influence this temperature was determined via simple linear regression.
Statistical analysis
Each year, sites were grouped by the maximum temperature during the coldest day to the nearest °F. The first analysis was performed to determine if impervious surface, weight or lengths of overwintering females affected one of two estimates of bagworm survival; overwintering survival of eggs and the percentage of bags producing viable larvae. This analysis was accomplished using forward stepwise regression (PROC REG, SAS 9.4) for all sites that were in the same temperature grouping. The model starts with a null model and adds or removes predictor variables based on the capacity to improve the P-value of each model by 0.05. The proportion of overwintering egg survival (out of 50 eggs) and viable bags were transformed using Arcsine square root transformation for analysis. Original values were presented in graphs. In regression analyses, vif (variance inflation factor) and tol (tolerance) diagnostic options were added to the model to check for collinearities between the predictors. In addition, other criteria such as R2 (coefficient of determination), adjusted R2, Mallow’s Cp, and Akaike’s Information Criterion (AIC) were used in the model to support the results.
Logistic regression and probit analysis have the potential for quantifying the relationship between continuous predictor variables and the binomial response of living organisms. For example, the toxicity of pesticides to living organisms is frequently characterized by an LD50, or the lethal dose required to kill 50% of the population (Rozman, Doull, and Hayes 2010). Similarly, we used a probit analysis (PROC PROBIT, SAS 9.4) to determine the amount of impervious surface required for 50% overwintering egg survival (IS50) for each group of sites with the same maximum daily temperature. In this procedure, the predictor variable, impervious surface, was log-transformed and the response variable, percentage mortality was transformed to its probit value prior to calculating the regression equation.
Results
Overwintering survival of eggs
The maximum temperature recorded on the coldest days ranged from −20°C to −17.2°C in 2018 and −22.2°C to −17.2°C in 2019, respectively. The stepwise model used three different variables to explain the overwintering survival of eggs. These included the impervious surface around the host plant, weight and length of the overwintering female bagworms. Results with R2, adjusted R2, Mallow’s Cp and AIC showed this three-predictor model to give the best fit. For example, R2 was highest in the three-predictor model and Mallow’s Cp was 4 in 2018. Similarly, R2 was highest in the three-predictor model, Mallow’s Cp was 4 and AIC was lower than the two-predictor model in 2019. The linear relationship between % impervious surface and % egg survival was significant when temperature was at or below −18.9°C in 2018 [at −20°C: F(1,4) = 7.17, P = 0.05; at −19.4°C: F(1,18) = 11.16, P = 0.004; at −18.9°C: F(1,4) = 17.68, P = 0.025; Fig. 1A] and when the temperature was at or below −20°C in 2019 [at −22.2°C: F(1,8) = 15.50, P = 0.004; at −21.7°C: F(1,6) = 18.12, P = 0.005; at −20.6°C: F(1,4) = 11.73, P = 0.019; Fig. 1B]. Neither variable, weight nor length contributed enough to the model (criteria for entry or exit was P = 0.05) to be included in the analysis. As a general rule of thumb, a vif higher than 10 and tol lower than 0.1 indicates potential collinearities between predictor variables (Marcoulides and Raykov 2019). Results were within these criteria and as such that there were no collinearities between predictors.
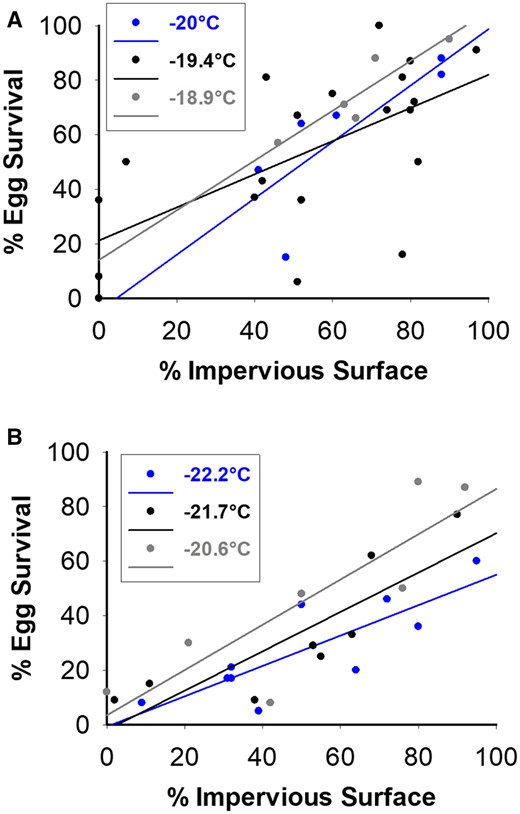
Effect of the impervious surface around 20-m radius of infested-host tree on overwintering survival of evergreen bagworm eggs in 2018 (A) and 2019 (B). Overwintering survival is based on the first 50 eggs counted. Temperatures present in the legend are the maximum temperature during the coldest day to the nearest °C
The probit analyses estimated the amount of impervious surface required for 50% of bagworm eggs to survive at sites with different maximum daily temperatures (Fig. 2). Fifty percent of bagworm eggs survived at maximum daily temperatures of −19.4°C, −20°C and −20.6°C when plants were surrounded by 25.7%, 48.39% and 50.75% of impervious surface, respectively. Egg mortality was not buffered by impervious surfaces at temperatures at or below −21.67°C.
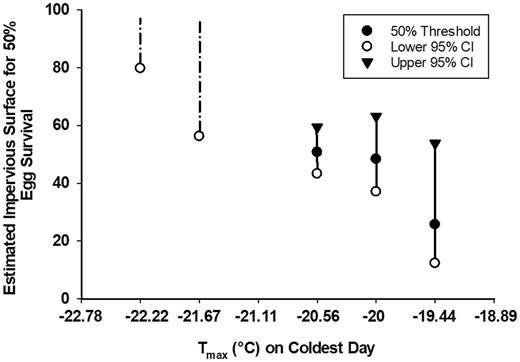
Estimated impervious surface area (IS50) required for 50% survival of evergreen bagworm eggs at sites grouped by maximum daily temperature on the coldest day of winter (Tmax) around 20-m radius of bagworm-infested host tree during winters of 2018 and 2019. Imaginary probit values of the impervious surface area beyond 100% were estimated from the regression procedure and displayed to reveal lower confidence limits within the realistic probit range. Solid lines represent, lower and upper 95% confidence interval (CI) of realistic IS50 (<100%). Dotted lines represent lower 95% CI when the estimated IS50 was imaginary
Capacity of bagworms to produce viable larvae
Of the three different variables (Impervious surface, weight and length of females) examined in the stepwise regression procedure, only impervious surface met the criteria for inclusion in the model that predicted the capacity of overwintering bags to produce larvae in 2018 and 2019. The linear relationship between % impervious surface and % viable bags was significant when the temperature was at or below −18.9°C in 2018 [at −20°C: F(1,4) = 12.41, P = 0.024; at −19.4°C: F(1,18) = 12.16, P = 0.002; at −18.9°C: F(1,4) = 12.59, P = 0.023; Fig. 3A] and when the temperature was below −18.9°C in 2019 [at −22.2°C: F(1,8) = 6.93, P = 0.03; at −21.7°C: F(1,6) = 9.55, P = 0.021; at −20.6°C: F(1,4) = 7.70, P = 0.039; Fig. 3B]. At temperatures above −18.9°C, there was no effect of impervious surface. There were no collinearities between predictor variables.
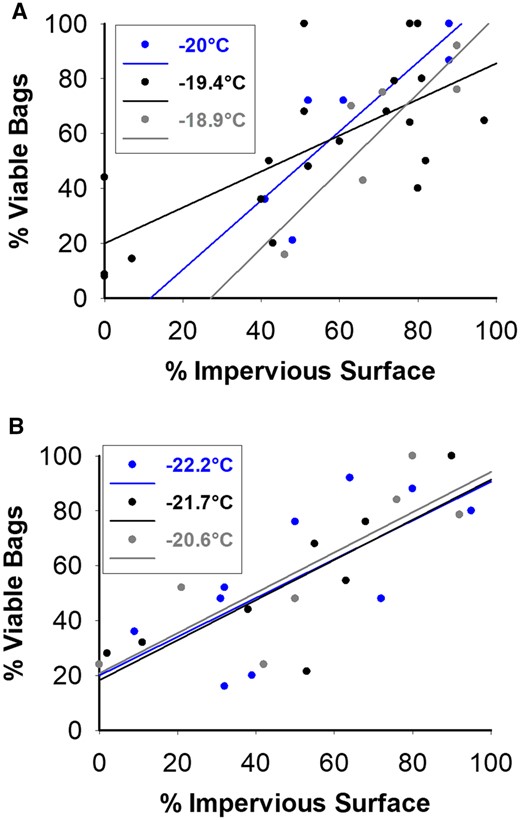
Effect of impervious surface on the production of viable bags (evergreen bagworm bags producing live larvae) in 2018 (A) and 2019 (B), respectively. Viable bags produced at least one larva for each temperature location. Temperatures present in the legend are the maximum temperature during the coldest day to the nearest °C
Determining the relationship between temperature and impervious surface
There were only 5 days in winter 2019–20 when the maximum daily temperature was ≤0°C (Fig. 4). Therefore, only 5 days were analyzed to explore the effect of impervious surface on an increase in host canopy temperature. The impervious surface around each infested host had a small, but significant effect on host canopy temperature [F(1,478) = 5.02, P = 0.025]. The site with 97% impervious surface was 1.3°C warmer than the site with no impervious surface.
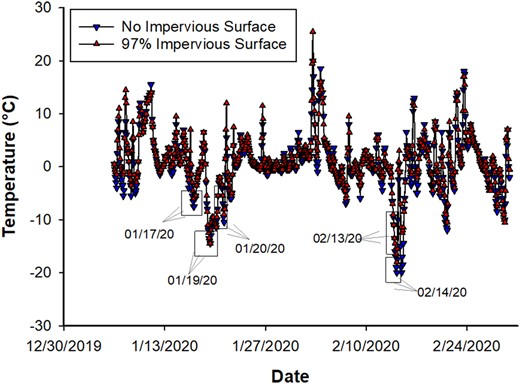
The daily host canopy temperatures from 6 January 2020 to 29 February 2020 of evergreen bagworm-infested plant in Lafayette and West Lafayette, IN. A solid line with down-pointing triangle represents the temperature recorded from infested host having no impervious surface whereas a solid line with up-pointing triangle represents the temperature recorded from the infested host having 97% impervious surface around 20-m of plant. The five rectangles and arrow with date in main graphs represent the coldest recorded (maximum temperature ≤0°C) days during winter of 2019–20
Discussion
The effect of an impervious surface within 20-m of the host plant has the capacity to warm the microclimate and increase the survival of evergreen bagworm during the winter in Indiana and Illinois when the temperature is above −21.7°C. The other two predictor variables, bagworm female weight and length, did not significantly alter bagworm survival. In addition, the pace to plant method to quantify impervious surface area was sensitive enough to predict increases in bagworm survival. Although we found the impervious surface increased the temperature of the microclimate, its capacity to insulate bagworms from the lethal effects of cold had its limits. When temperatures fell to −21.7°C and below, the impervious surface around the bagworm-infested host had no effect on survival.
Winter temperatures in 2020 in our study region suggest the extent to which impervious surfaces affected tree canopy temperatures. We found that trees completely surrounded by impervious surface (97%) experienced temperatures that were about 1.3°C warmer compared to hosts not surrounded by impervious surface (0%) during the coldest day of 2020. The magnitude of the increase in temperature caused by impervious surfaces is similar to that reported by other investigators. For example, Dale and Frank (2014) found the 2°C increase in summer temperature due to the impervious surface in urban areas was enough to allow scale insects to survive, reproduce and damage maple trees. Our findings are also consistent with more general studies which demonstrate that impervious surface is capable of augmenting temperatures year-round (Myint et al. 2013).
Our results and observations indicate that the percentage of surviving eggs was more sensitive to temperature and impervious surface for two reasons. First, calculating the mortality from a set number of eggs held for each overwintering female provided a consistent sample from every tree. In contrast, the number of bags collected at each site ranged between 4 and 25 which mathematically altered the possible proportions of live bagworms at each site. Furthermore, the live larval method does not distinguish between bags with one or many survivors. Overwintering survival of eggs has been used by other researchers to examine effects of latitude, and weight of overwintering survival of bagworm (Rhainds et al. 2013). For these reasons, our discussion of how microclimate affects bagworm will focus on egg survival.
We observed bagworms surviving at lower maximum daily air temperatures than reported by others. Rhainds et al. (2013) found 50% of bagworm eggs to survive when the temperature fell to −18°C and only about 1% survival when the temperature reached about −19.5°C. This freeze threshold is mostly calculated from rural environments which lacked the impervious surfaces that we studied. Rhainds et al. (2013) also assessed egg survival across 5° of latitude (36.5–41.5°N). Weights of egg clutches varied enough within this range to alter egg survival. In our study, we reduced the variation in survival caused by differences in the weight of egg clutches by collecting bags within a smaller latitudinal range of 1.44° (39.93–41.47°N). This leaves the effects of impervious surfaces on air temperature as the most likely factor to explain variation in the observed level of egg survival.
Landscape designers seeking to identify sites where plants are more likely to survive can benefit by being able to recognize threshold values of landscape characteristics associated with increased pest abundance and decreased plant health. Researchers were able to predict that more than 50% of red maples planted across the Southeastern USA were more likely to be considered in good condition, when surrounded by 36% or less impervious cover (Just, Frank, and Dale 2018). Similarly, we identify impervious surface thresholds associated with 50% mortality of bagworm eggs in years the coldest day had a maximum temperature at or above −20.6°C. While our findings support our hypothesis that impervious surfaces alter the overwintering survival of bagworms, microclimate warming is insufficient to protect bagworms when the temperature dips down below a critical limit. Thus, the capacity to use the impervious surface to predict the overwintering mortality of bagworms can only be applied within specific temperature ranges.
Our study contributes to a growing body of literature on how cold temperatures affect lepidopteran survival. It also demonstrates the capacity of the impervious surface to alter the temperature in ways that can affect insect survival. The urban landscape provides a natural laboratory to test the effect of urban settings on biological entities. Few studies have examined the effect on the overwintering survival of lepidopteran pests in urban conditions. For example, the urban environment has a positive impact on mimosa webworm pupae overwintering (Miller and Hart 1987) and it has increased the population of mimosa webworm and damage to host plants (Sperry et al. 2001). Other investigations into the effect of cold temperatures on overwintering caterpillars have mainly focused on cold hardiness and effect on body size and weight (Pullin and Bale 1989), spring emergence (Mironidis, Stamopoulos, and Savopoulou-Soultani 2010) and survival of overwintering host plants (Liu et al. 2007). Our study provides insight into how urban conditions can create refugia from lethal temperatures during the extreme cold of winter.
The relationship we have found between overwintering survival of bagworm and impervious surfaces complements research on how these surfaces affect the abundance of arthropods feeding on plants during the growing season (Coulson et al. 1993; Bale et al. 2002; Meineke et al. 2013; Just, Frank, and Dale 2018). In particular, sap-feeding arthropods are widely studied in urban conditions (Koricheva, Larsson, and Haukioja 1998; Herms 2002; Huberty and Denno 2004; Dale and Frank 2017). Studies on gloomy scale (Melanaspis tenebricosa; Dale and Frank 2014; Just, Frank, and Dale 2018) and the oak lecanium scale (Parthenolecanium quercifex; Meineke et al. 2013; Meineke, Dunn, and Frank 2014) in the southern and eastern USA showed the higher abundance of overwintering female population in hotter part of the cities compared to cooler areas. The abundance of horsechestnut scale (Pulvinaria regalis; Speight et al. 1998), gall midges and spider mites (Frankie et al. 1987) and lace bugs (Cregg and Dix 2001) abundances have also been reported to be elevated in highly disturbed urban areas.
Conclusion and future recommendations
Small changes in temperature associated impervious surfaces in urban areas can profoundly affect pest abundance. In this way, increased pest abundance caused by the elevated temperatures of impervious surfaces may be indicative of how climate change could affect pest problems. Increasing urban greenspace is one of many strategies to reduce the effects of urban microclimate warming. However, impervious surface responsible for warming and increased pest abundance remains an integral component of city infrastructure. Studies like ours that elucidate the relationship between impervious surface and the survival of overwintering pests complement other studies that focus on changes in the pest populations during the growing season. Armed with a simple method to quantify impervious surface, pest managers can predict which pest problems are likely to be more abundant due to overwintering survival. Conversely, landscape designers should consider using this tool to design landscapes that are resistant to insect pests by reducing impervious surfaces around host plants susceptible to pests that benefit from warmer winter temperatures.
Acknowledgements
We thank Dr Elizabeth Barnes and Sara Stack for help with bagworm collection and data measurement. We also thank Darrel Light of Berry’s Garden Center, Danville, IL for helping us to find research sites. Special thanks to Dr Elizabeth Barnes for help with making graphical abstract.
Funding
The research was conducted in partial fulfillment of the requirements for PhD to S.D. who was supported by USDA APHIS Cooperative Agreement 20-8218-0520-CA awarded to C.S.S.
Conflict of interest statement. None declared.
Data Availability
Data are available upon request to the corresponding author.



