-
PDF
- Split View
-
Views
-
Cite
Cite
Ryosuke Matsuda, Tetsuro Tamamoto, Nobuyoshi Inooka, Shigeto Hontsu, Akihiro Doi, Ryosuke Maeoka, Tsutomu Nakazawa, Takayuki Morimoto, Kaori Yamaki, Sachiko Miura, Yudai Morisaki, Shohei Yokoyama, Masashi Kotsugi, Yasuhiro Takeshima, Fumiaki Isohashi, Ichiro Nakagawa, Systemic inflammation response index predicts overall survival in patients undergoing stereotactic radiosurgery for brain metastasis from non-small cell lung cancer, Journal of Radiation Research, Volume 66, Issue 2, March 2025, Pages 129–136, https://doi.org/10.1093/jrr/rrae099
Close - Share Icon Share
ABSTRACT
This study aimed to evaluate the prognostic value of pre-treatment blood cell counts in patients with brain metastasis (BM) from non-small cell lung cancer (NSCLC) who were treated using linear accelerator (linac)-based stereotactic radiosurgery (SRS) and fractionated stereotactic radiotherapy (fSRT) with a micro-multileaf collimator. Between January 2011 and November 2022, 271 consecutive patients underwent linac-based SRS/fSRT for BM from NSCLC. Thirty patients with insufficient blood test data during this period were excluded from this analysis. Thirty-five patients with steroid intake at the time point of the blood test and 18 patients with higher C-reactive protein were excluded. Thus, 188 patients were eventually enrolled in this study. The median follow-up period after SRS/fSRT was 21 months (range: 0–121 months), and the median survival time after SRS/fSRT was 19 months. Neutrophil–lymphocyte ratio ≥ 1.90, lymphocyte–monocyte ratio ≤ 1.67 and systemic inflammation response index (SIRI) ≥ 2.95 were unfavorable predictors of prognosis for patients who underwent SRS/fSRT for BM from NSCLC. Cox proportional-hazard multivariate analysis revealed that the SIRI was independent prognostic factors for increased risk of death. Thus, simple, less expensive, and routinely performed pre-treatment blood cell count measurements such as SIRI can predict the overall survival of patients treated with SRS/fSRT for BM from NSCLC.
INTRODUCTION
Brain metastasis (BM) is the most common neoplasm among brain tumors, and lung cancer is the most frequently observed primary lesion in patients showing BM [1]. Advances in chemotherapy and radiotherapy have yielded more options for treating BM, and stereotactic radiosurgery (SRS) and fractionated stereotactic radiotherapy (fSRT) for BM are associated with good tumor control and minimal complications [2–4]. A previous report evaluating the use of SRS/fSRT for BM, especially asymptomatic and smaller BMs, suggested that SRS could be the first-line treatment for such cases [4]. However, despite recent advancements in multimodality treatment for cancer, the prognosis for patients with BM requiring SRS/fSRT remains quite limited [2–4]. In this regard, pre-treatment prognostic assessments can provide important information to neurosurgeons, oncologists, and patients. One such prognostic assessment, the Diagnosis-specific graded prognostic assessment (DS-GPA), is a scoring system based on the Karnofsky Performance Status (KPS), age, extracranial metastases, and number of metastases, while the newly proposed lung-mol GPA adds driver mutation status of non-small lung cancer (NSCLC) to the DS-GPA to provide a more accurate scoring system [5–7].
Nevertheless, these pre-treatment scoring systems do not include hematological markers. Blood test data obtained before SRS/fSRT has been suggested to reflect the prognosis after treatment. In particular, the pre- and post-SRS/fSRT neutrophil–lymphocyte ratio (NLR) is a hematological biomarker that has been reported to reflect the prognosis after SRS/fSRT [8–11]. More accurate biomarkers obtained by combining several blood-based markers are also under investigation. The systemic immune-inflammation index (SII) is determined on the basis of neutrophil, lymphocyte, and platelet counts, while the systemic inflammation response index (SIRI) is based on neutrophil, lymphocyte, and monocyte counts. However, only a few studies have conducted comprehensive analyses of blood cell counts, including the SII and SIRI, in patients treated with SRS/fSRT for BM from NSCLC. Therefore, in the present study, we aimed to examine the relationship between the SII/SIRI and the prognosis after SRS/fSRT for BM from NSCLC.
MATERIALS AND METHODS
Patient characteristics
This retrospective study was approved by the ethics committee of our hospital (No. 3333). The patients’ medical records were reviewed to extract clinical information, including information related to sex, age, pre-treatment KPS score, control of primary cancer, extracranial metastasis, number of metastases, hemoglobin, neutrophil count, lymphocyte count, monocyte count, platelet count, driver mutation of NSCLC, the expression of programmed death-ligand 1 (PD-L1), systemic chemotherapy prior to SRS/fSRT and overall survival (OS) data. Between January 2011 and November 2021, 271 consecutive patients were treated with SRS and fSRT for BM from NSCLC at our hospital. Patients who underwent SRS and fSRT for surgically resected sites were not included in this study. The inclusion criterion was the availability of full blood count data before SRS and fSRT for analysis. Therefore, 30 patients without adequate blood test data within 1 month of the initial SRS/fSRT were excluded. Thirty-five patients with steroid intake at the time point of the blood test and 18 patients with higher C-reactive protein were excluded. Thus, 188 patients were analyzed in this study. The characteristics of all patients with BMs from NSCLC who underwent SRS and fSRT are summarized in Table 1.
Characteristics of the patients with brain metastasis from non-small cell lung cancer who underwent stereotactic radiosurgery/fractionated stereotactic radiotherapy
| Characteristic . | n = 188 . |
|---|---|
| Sex | |
| Female | 70 |
| Male | 118 |
| Age(years) | 70.3 ± 10.3 |
| Pretreatment KPS | |
| <80 | 37 |
| ≥80 | 151 |
| Mutation status in lung cancer | |
| EGFR/ALK negative | 115 |
| EGFR positive | 64 |
| ALK positive | 9 |
| PD-L1 TPS | |
| low | 58 |
| high | 24 |
| NA | 106 |
| Systemic chemotherapy prior to SRS/fSRT | |
| yes | 89 |
| no | 99 |
| Tumor number | |
| median/average/range | 2/2.35/1–11 |
| single | 93 |
| multiple | 95 |
| Tumor volume(cc) | |
| median/average/range | 1.09/3.40/0.04–42.3 |
| Control of primary tumor | |
| yes | 96 |
| no | 92 |
| Extracranial metastasis | |
| yes | 69 |
| no | 119 |
| RPA class | |
| I | 13 |
| II | 144 |
| III | 31 |
| DS-GPA | |
| 0–1 | 36 |
| 1.5–2 | 61 |
| 2.5–3 | 79 |
| 3.5–4 | 12 |
| Hemoglobin | |
| <13.2 | 131 |
| ≥13.2 | 57 |
| NLR | |
| <1.90 | 53 |
| ≥1.90 | 135 |
| PLR | |
| <202.2 | 123 |
| ≥202.2 | 65 |
| LMR | |
| <1.67 | 36 |
| ≥1.67 | 152 |
| SII | |
| <1095 | 144 |
| ≥1095 | 44 |
| SIRI | |
| <2.95 | 157 |
| ≥2.95 | 31 |
| Characteristic . | n = 188 . |
|---|---|
| Sex | |
| Female | 70 |
| Male | 118 |
| Age(years) | 70.3 ± 10.3 |
| Pretreatment KPS | |
| <80 | 37 |
| ≥80 | 151 |
| Mutation status in lung cancer | |
| EGFR/ALK negative | 115 |
| EGFR positive | 64 |
| ALK positive | 9 |
| PD-L1 TPS | |
| low | 58 |
| high | 24 |
| NA | 106 |
| Systemic chemotherapy prior to SRS/fSRT | |
| yes | 89 |
| no | 99 |
| Tumor number | |
| median/average/range | 2/2.35/1–11 |
| single | 93 |
| multiple | 95 |
| Tumor volume(cc) | |
| median/average/range | 1.09/3.40/0.04–42.3 |
| Control of primary tumor | |
| yes | 96 |
| no | 92 |
| Extracranial metastasis | |
| yes | 69 |
| no | 119 |
| RPA class | |
| I | 13 |
| II | 144 |
| III | 31 |
| DS-GPA | |
| 0–1 | 36 |
| 1.5–2 | 61 |
| 2.5–3 | 79 |
| 3.5–4 | 12 |
| Hemoglobin | |
| <13.2 | 131 |
| ≥13.2 | 57 |
| NLR | |
| <1.90 | 53 |
| ≥1.90 | 135 |
| PLR | |
| <202.2 | 123 |
| ≥202.2 | 65 |
| LMR | |
| <1.67 | 36 |
| ≥1.67 | 152 |
| SII | |
| <1095 | 144 |
| ≥1095 | 44 |
| SIRI | |
| <2.95 | 157 |
| ≥2.95 | 31 |
Characteristics of the patients with brain metastasis from non-small cell lung cancer who underwent stereotactic radiosurgery/fractionated stereotactic radiotherapy
| Characteristic . | n = 188 . |
|---|---|
| Sex | |
| Female | 70 |
| Male | 118 |
| Age(years) | 70.3 ± 10.3 |
| Pretreatment KPS | |
| <80 | 37 |
| ≥80 | 151 |
| Mutation status in lung cancer | |
| EGFR/ALK negative | 115 |
| EGFR positive | 64 |
| ALK positive | 9 |
| PD-L1 TPS | |
| low | 58 |
| high | 24 |
| NA | 106 |
| Systemic chemotherapy prior to SRS/fSRT | |
| yes | 89 |
| no | 99 |
| Tumor number | |
| median/average/range | 2/2.35/1–11 |
| single | 93 |
| multiple | 95 |
| Tumor volume(cc) | |
| median/average/range | 1.09/3.40/0.04–42.3 |
| Control of primary tumor | |
| yes | 96 |
| no | 92 |
| Extracranial metastasis | |
| yes | 69 |
| no | 119 |
| RPA class | |
| I | 13 |
| II | 144 |
| III | 31 |
| DS-GPA | |
| 0–1 | 36 |
| 1.5–2 | 61 |
| 2.5–3 | 79 |
| 3.5–4 | 12 |
| Hemoglobin | |
| <13.2 | 131 |
| ≥13.2 | 57 |
| NLR | |
| <1.90 | 53 |
| ≥1.90 | 135 |
| PLR | |
| <202.2 | 123 |
| ≥202.2 | 65 |
| LMR | |
| <1.67 | 36 |
| ≥1.67 | 152 |
| SII | |
| <1095 | 144 |
| ≥1095 | 44 |
| SIRI | |
| <2.95 | 157 |
| ≥2.95 | 31 |
| Characteristic . | n = 188 . |
|---|---|
| Sex | |
| Female | 70 |
| Male | 118 |
| Age(years) | 70.3 ± 10.3 |
| Pretreatment KPS | |
| <80 | 37 |
| ≥80 | 151 |
| Mutation status in lung cancer | |
| EGFR/ALK negative | 115 |
| EGFR positive | 64 |
| ALK positive | 9 |
| PD-L1 TPS | |
| low | 58 |
| high | 24 |
| NA | 106 |
| Systemic chemotherapy prior to SRS/fSRT | |
| yes | 89 |
| no | 99 |
| Tumor number | |
| median/average/range | 2/2.35/1–11 |
| single | 93 |
| multiple | 95 |
| Tumor volume(cc) | |
| median/average/range | 1.09/3.40/0.04–42.3 |
| Control of primary tumor | |
| yes | 96 |
| no | 92 |
| Extracranial metastasis | |
| yes | 69 |
| no | 119 |
| RPA class | |
| I | 13 |
| II | 144 |
| III | 31 |
| DS-GPA | |
| 0–1 | 36 |
| 1.5–2 | 61 |
| 2.5–3 | 79 |
| 3.5–4 | 12 |
| Hemoglobin | |
| <13.2 | 131 |
| ≥13.2 | 57 |
| NLR | |
| <1.90 | 53 |
| ≥1.90 | 135 |
| PLR | |
| <202.2 | 123 |
| ≥202.2 | 65 |
| LMR | |
| <1.67 | 36 |
| ≥1.67 | 152 |
| SII | |
| <1095 | 144 |
| ≥1095 | 44 |
| SIRI | |
| <2.95 | 157 |
| ≥2.95 | 31 |
The individual blood cell count data were extracted from the full blood count. The hematological counts were used to calculate several combined variables: NLR; neutrophil/lymphocyte ratio, platelet–lymphocyte ratio (PLR; platelet count/lymphocyte count), lymphocyte–monocyte ratio (LMR; lymphocyte count/monocyte count), SII ([platelet count × neutrophil count]/lymphocyte count), and SIRI ([neutrophil count × monocyte count]/lymphocyte count).
Stereotactic radiosurgery and fractionated stereotactic radiotherapy
SRS/fSRT planning was based on computed tomography (CT) scans with a slice thickness of 1 mm. All patients were immobilized using a thermoplastic mask (BRAINLAB AG, Munich, Germany). The gross tumor volume (GTV) for each lesion was delineated on magnetic resonance imaging scans with a slice thickness of 1 mm. The planning target volume (PTV) was defined as GTV plus 1–2 mm for all dimensions. Treatment was provided within 1 week after planning the CT scan. Treatment planning was performed using BrainSCAN or iPlan RT (BRAINLAB AG). Dose calculations were performed using a pencil beam algorithm. SRS and fSRT were performed using linac with a micro-multileaf collimator: Novalis® (BRAINLAB AG) with a collimator width of 3 mm or TrueBeam STx (Varian Medical Systems, Palo Alto, USA) with a collimator width of 2.5 mm. Novalis was used for patients treated until November 2017, while TrueBeam STx was used for patients treated since November 2017. Every patient was treated using X-rays with beam energy of 6 MV.
All patients were treated using Novalis® or TrueBeam STx with non-coplanar multi-beams, non-coplanar multi-arcs, or both. The treatment methods in SRS or fSRT employed conformal beams, dynamic conformal arcs, intensity-modulated radiotherapy (IMRT), or hybrid arcs. Hybrid arcs are a novel treatment technique that blends aperture-enhanced optimized arcs with discrete IMRT elements, allowing arc selection with a set of static IMRT beams [12].
Patient positioning and verification were performed using BrainLab ExacTrac® (BRAINLAB AG). This device consisted of two infrared cameras and two dual diagnostic kV X-ray tubes, which can be moved automatically into the treatment position to minimize setup errors [13, 14]. Additionally, cone-beam CT was used to reduce setup errors in TrueBesm STx. Isocenter prescription method was used for conformal beams and dynamic conformal arcs, and D50 prescription method (dose prescribed to >50% of the PTV) was used for hybrid arc and IMRT cases. The treatment plan was designed to ensure uniform dose within the PTV, and the marginal dose was ~90%. Patients with neurological symptoms, those with brain metastases >20 mm in size, and those with brain metastases in the eloquent area, including the basal ganglia and primary motor cortex, underwent fSRT with a dose of 35 Gy in five fractions [15]. Patients with brainstem metastasis were treated using doses of 24–40 Gy in 7–13 fractions [3]. Asymptomatic patients with brain metastases smaller than 20 mm were treated with SRS with doses of 21–22.5 Gy in a single fraction, and the decision was based on tumor size, location, surrounding edema, and other factors [16].
Clinical and radiologic follow-up
Follow-up contrast-enhanced MRI was performed every 3 months after the end of SRS/fSRT if possible. Tumor volumes were evaluated before and after SRS/fSRT by using BrainSCAN or iPlan RT. Once disease progression was detected, MRI was performed at intervals of 1–2 months. The decision of additional treatment for disease progression was based on evidence of clinical deterioration and associated imaging progression judged by the tumor board review on brain tumors. Local failure was defined as either a pathologically proven recurrence within the SRS/fSRT treatment field. Imaging progression that triggered additional treatment (surgery for symptomatic radiation necrosis, SRS/fSRT, or medication including steroid) was counted as local failure without pathologic confirmation. Distant failure was defined as intracranial failure at a site not previously treated with SRS/fSRT.
Statistical analysis
The median survival time (MST) after SRS/fSRT was calculated using the Kaplan–Meier method. The log-rank test was used for univariate analyses. The prognostic factors analyzed in this study included age, sex, number of metastasis (single versus multiple), extracranial metastasis, control of the primary cancer, driver mutation, the expression of PD-L1 (tumor proportion score 1–49% vs 50% or higher), systemic chemotherapy prior to SRS/fSRT and pre-treatment KPS score (≥80 vs <80). Cox proportional-hazard analysis was used to identify the factors associated with survival at the univariate and multivariate levels. Competing risk survival analysis for local failure after SRS/fSRT was performed. A receiver operating characteristic (ROC) curve was generated and the area under the curve (AUC) was calculated to evaluate the prognostic power of the hematological markers for OS. C-reactive protein (CRP) value outliers were calculated using the Smirnov-Grubbs test. All analyses were performed with the EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [17], and a P-value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics and survival factors
Overall, a total of 188 patients were analyzed in this study. The median follow-up period after SRS/fSRT was 21 months (range: 0–121 months), and the MST after SRS/fSRT was 19 months (Fig. 1A). During the study period, 141 patients died due to worsening primary cancer and 8 patients died due to BM. In the entire cohort, female sex (P = 0.004), age < 70 years (P = 0.005), good pre-treatment KPS score (P < 0.001), and control of primary cancer (P < 0.001) were significantly favorable prognostic factors for OS. The expression of PD-L1, extracranial metastasis, number of metastasis (single vs multiple), EGFR/ALK mutated SCLC (P = 0.09), and systemic chemotherapy prior to SRS/fSRT did not affect OS. The Cox proportional-hazard univariate analysis revealed that sex, pre-treatment KPS score, and control of primary cancer were independent prognostic factors for increased risk of death (Table 2). Freedom from distant failure at 6 months and 1 and 2 years was 73.8%, 58.5%, and 48.2%, respectively (Fig. 2A). Freedom from local failure at 6 months and 1 and 2 years was 98.4%, 95.2%, and 89.1%, respectively (Fig. 2B).
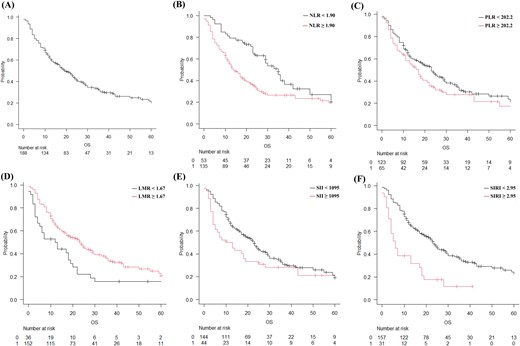
Post-radiosurgery overall survival (OS) of the patients with brain metastasis estimated using the Kaplan–Meier method considering each parameter. (A) OS in the entire cohort, (B) neutrophil–lymphocyte ratio (NLR; black line: NLR < 1.90; red line, NLR ≥ 1.90), (C) platelet–lymphocyte ratio (PLR; black line: PLR < 202.2; red line: PLR ≥ 202.2), (D) lymphocyte–monocyte ratio (LMR; black line: LMR < 1.67; red line: LMR ≥ 1.67), (E) systemic immune-inflammation index (SII; black line: SII < 1095; red line: SII ≥ 1095), (F) systemic inflammation response index (SIRI; black line: SIRI <2.95; red line: SIRI ≥2.95).
Univariate and multivariate analysis of the prognostic factors affecting overall survival after stereotactic radiosurgery/ fractionated stereotactic radiotherapy
| . | Univariate analysis . | Multivariate analysisa . | ||||
|---|---|---|---|---|---|---|
| Factor . | HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . |
| Sex | 1.67 | 1.16–2.40 | 0.006 | |||
| Age (≥70 vs. <70) | 1.65 | 1.15–2.36 | 0.006 | |||
| Pretreatment KPS (≥80 vs. <80) | 0.24 | 0.16–0.36 | <0.001 | |||
| Number of metastasis (single vs. multiple) | 1.12 | 0.80–1.58 | 0.51 | |||
| Control of primary cancer | 1.79 | 1.27–2.52 | <0.001 | |||
| Extracranial metastasis | 0.87 | 0.61–1.24 | 0.44 | |||
| driver mutation | 0.74 | 0.52–1.06 | 0.098 | |||
| Hemoglobin | 0.96 | 0.66–1.38 | 0.81 | |||
| NLR | 1.82 | 1.22–2.72 | 0.003 | 1.50 | 0.98–2.29 | 0.06 |
| PLR | 1.28 | 0.90–1.83 | 0.17 | |||
| LMR | 0.56 | 0.37–0.83 | 0.004 | 0.61 | 0.40–0.91 | 0.017 |
| SII | 1.35 | 0.91–2.00 | 0.13 | |||
| SIRI | 2.57 | 1.66–3.98 | <0.001 | 1.86 | 1.18–2.93 | 0.008 |
| . | Univariate analysis . | Multivariate analysisa . | ||||
|---|---|---|---|---|---|---|
| Factor . | HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . |
| Sex | 1.67 | 1.16–2.40 | 0.006 | |||
| Age (≥70 vs. <70) | 1.65 | 1.15–2.36 | 0.006 | |||
| Pretreatment KPS (≥80 vs. <80) | 0.24 | 0.16–0.36 | <0.001 | |||
| Number of metastasis (single vs. multiple) | 1.12 | 0.80–1.58 | 0.51 | |||
| Control of primary cancer | 1.79 | 1.27–2.52 | <0.001 | |||
| Extracranial metastasis | 0.87 | 0.61–1.24 | 0.44 | |||
| driver mutation | 0.74 | 0.52–1.06 | 0.098 | |||
| Hemoglobin | 0.96 | 0.66–1.38 | 0.81 | |||
| NLR | 1.82 | 1.22–2.72 | 0.003 | 1.50 | 0.98–2.29 | 0.06 |
| PLR | 1.28 | 0.90–1.83 | 0.17 | |||
| LMR | 0.56 | 0.37–0.83 | 0.004 | 0.61 | 0.40–0.91 | 0.017 |
| SII | 1.35 | 0.91–2.00 | 0.13 | |||
| SIRI | 2.57 | 1.66–3.98 | <0.001 | 1.86 | 1.18–2.93 | 0.008 |
HR: hazard ratio; CI:confidence interval
aAdjusted by sex, age, pretreatment KPS, and control of primary cancer
Univariate and multivariate analysis of the prognostic factors affecting overall survival after stereotactic radiosurgery/ fractionated stereotactic radiotherapy
| . | Univariate analysis . | Multivariate analysisa . | ||||
|---|---|---|---|---|---|---|
| Factor . | HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . |
| Sex | 1.67 | 1.16–2.40 | 0.006 | |||
| Age (≥70 vs. <70) | 1.65 | 1.15–2.36 | 0.006 | |||
| Pretreatment KPS (≥80 vs. <80) | 0.24 | 0.16–0.36 | <0.001 | |||
| Number of metastasis (single vs. multiple) | 1.12 | 0.80–1.58 | 0.51 | |||
| Control of primary cancer | 1.79 | 1.27–2.52 | <0.001 | |||
| Extracranial metastasis | 0.87 | 0.61–1.24 | 0.44 | |||
| driver mutation | 0.74 | 0.52–1.06 | 0.098 | |||
| Hemoglobin | 0.96 | 0.66–1.38 | 0.81 | |||
| NLR | 1.82 | 1.22–2.72 | 0.003 | 1.50 | 0.98–2.29 | 0.06 |
| PLR | 1.28 | 0.90–1.83 | 0.17 | |||
| LMR | 0.56 | 0.37–0.83 | 0.004 | 0.61 | 0.40–0.91 | 0.017 |
| SII | 1.35 | 0.91–2.00 | 0.13 | |||
| SIRI | 2.57 | 1.66–3.98 | <0.001 | 1.86 | 1.18–2.93 | 0.008 |
| . | Univariate analysis . | Multivariate analysisa . | ||||
|---|---|---|---|---|---|---|
| Factor . | HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . |
| Sex | 1.67 | 1.16–2.40 | 0.006 | |||
| Age (≥70 vs. <70) | 1.65 | 1.15–2.36 | 0.006 | |||
| Pretreatment KPS (≥80 vs. <80) | 0.24 | 0.16–0.36 | <0.001 | |||
| Number of metastasis (single vs. multiple) | 1.12 | 0.80–1.58 | 0.51 | |||
| Control of primary cancer | 1.79 | 1.27–2.52 | <0.001 | |||
| Extracranial metastasis | 0.87 | 0.61–1.24 | 0.44 | |||
| driver mutation | 0.74 | 0.52–1.06 | 0.098 | |||
| Hemoglobin | 0.96 | 0.66–1.38 | 0.81 | |||
| NLR | 1.82 | 1.22–2.72 | 0.003 | 1.50 | 0.98–2.29 | 0.06 |
| PLR | 1.28 | 0.90–1.83 | 0.17 | |||
| LMR | 0.56 | 0.37–0.83 | 0.004 | 0.61 | 0.40–0.91 | 0.017 |
| SII | 1.35 | 0.91–2.00 | 0.13 | |||
| SIRI | 2.57 | 1.66–3.98 | <0.001 | 1.86 | 1.18–2.93 | 0.008 |
HR: hazard ratio; CI:confidence interval
aAdjusted by sex, age, pretreatment KPS, and control of primary cancer
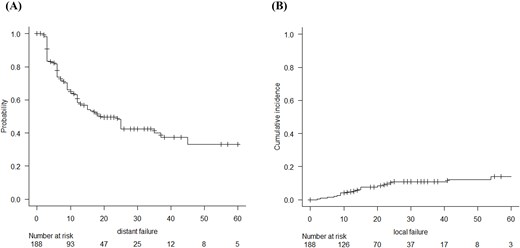
Freedom of distant failure of the patients with brain metastasis from NSCLC who underwent SRS/fSRT estimated using the Kaplan–Meier method (A) and freedom of local failure of the patients estimated using competing risk survival analysis (B).
Hemoglobin
An ROC curve was generated, and the AUC was calculated to evaluate the prognostic power of the hemoglobin for OS. The cutoff hemoglobin value for OS was 13.2. The MST after SRS/fSRT of patients with hemoglobin ≥13.2 (n = 57) and hemoglobin <13.2 (n = 131) was 23 and 18 months, respectively (P = 0.81).
Neutrophil–lymphocyte ratio
An ROC curve was generated, and the AUC was calculated to evaluate the prognostic power of the NLR for OS. The cutoff NLR value for OS was 1.90. The MST after SRS/fSRT of patients with NLR ≥ 1.90 (n = 135) and NLR < 1.90 (n = 53) was 14 and 35 months, respectively (P = 0.002) (Fig. 1B). The Cox proportional-hazard multivariate analysis that included sex, KPS score, control of primary cancer, and the NLR as continuous values revealed that sex (hazard ratio [HR]: 1.51; 95% confidence interval [CI] = 1.05–2.19; P = 0.028), control of primary cancer (HR: 1.42; 95% CI = 0.99–2.03; P = 0.053), KPS (HR: 0.31; 95%CI = 0.20–0.48; P < 0.001), and age (HR: 1.51; 95% CI = 1.04–2.19 P = 0.029) were independent prognostic factors for OS. The Cox proportional-hazard multivariate analysis revealed that the NLR (HR: 1.50; 95% CI = 0.98–2.29; P = 0.059) was not an independent prognostic factor for OS.
Platelet–lymphocyte ratio
The cutoff PLR value for OS was 202.2. The MST after SRS/fSRT of patients with PLR < 202.2 (n = 123) and PLR ≥ 202.2 (n = 65) was 23 and 17 months, respectively (P = 0.16) (Fig. 1C). The Cox proportional-hazard univariate analysis with the PLR as a continuous value revealed that the PLR (HR: 1.28; 95% CI =0.90–1.83; P = 0.17) was not an independent prognostic factor for OS.
Lymphocyte–monocyte ratio
The cutoff LMR value for OS was 1.67. The MST after SRS/fSRT of patients with LMR ≥ 1.67 (n = 152) and LMR < 1.67 (n = 36) was 23 and 12 months, respectively (P = 0.003) (Fig. 1D).
The Cox proportional-hazard multivariate analysis that included sex, KPS score, control of primary cancer, and the LMR as continuous values revealed that LMR (HR: 0.61;95%CI = 0.40–0.91; P = 0.018), sex (HR: 1.55; 95% CI = 1.07–2.23; P = 0.02), control of primary cancer (HR: 1.53; 95% CI = 1.07–2.19; P = 0.019), KPS (HR: 0.31; 95%CI = 0.20–0.47; P < 0.001), and age (HR: 1.49; 95% CI = 1.03–2.15 P = 0.033) were independent prognostic factors for OS.
Systemic immune-inflammation index
The cutoff SII value for OS was 1095. The MST after SRS/fSRT of patients with SII ≥ 1095 (n = 44) and SII < 1095 (n = 144) was 11.5 and 23 months, respectively (P = 0.127) (Fig. 1E). The Cox proportional-hazard univariate analysis with the SII as a continuous value revealed that the SII (HR: 1.35; 95% CI =0.91–2.0; P = 0.13) was not an independent prognostic factor for OS.
Systemic inflammation response index
The cutoff SIRI value for OS was 2.95. The MST after SRS/fSRT of patients with SIRI ≥2.95 (n = 31) and SIRI <2.95 (n = 157) was 6 and 23 months, respectively (P < 0.001) (Fig. 1F). The Cox proportional-hazard multivariate analysis that included sex, KPS score, control of primary cancer, age, and the SIRI as continuous values revealed that SIRI (HR: 1.86; 95% CI =1.18–2.93; P = 0.008), sex (HR: 1.52; 95% CI = 1.05–2.20; P = 0.027), KPS score (HR: 0.32; 95% CI =0.21–0.49; P < 0.001), control of primary cancer (HR: 1.47; 95% CI = 1.03–2.09; P = 0.034, and age (HR: 1.49; 95% CI = 1.03–2.14; P = 0.033) were independent prognostic factors for OS.
DISCUSSION
In this study, we examined the association between pre-treatment blood cell counts and the outcomes after SRS/fSRT for BM from NSCLC. The NLR can be easily measured using a blood test, and it has been studied as a potential biomarker in various cancers, cardiovascular disease, and autoimmune disorders. In particular, the NLR is a promising marker of a systemic inflammatory response, and higher NLR values have been shown to be a poor prognostic factor for many malignancies, including lung, colon, prostate, kidney, bladder and breast cancers [18–23]. Furthermore, an elevated NLR is independently associated with an increased risk of BM development in patients with NSCLC [24]. Although our data were analyzed in patients with NSCLC, the cutoff value for the NLR was 1.90, which is similar to that reported previously. A meta-analysis of the NLR and BM from NSCLC that included 11 articles showed a statistically significant association between the NLR and OS [25]. The pre-treatment NLR has been reported to affect the OS after SRS for BM [8, 26]. Similar to the pre-treatment NLR, the post-treatment NLR has also been reported to influence OS after SRS for BM [10]. In this study, NLR values correlated with prognosis in univariate analysis but did not affect prognosis in multivariate analysis. Many reports on NLR and metastatic brain tumors did not exclude cases where the patient was treated with oral steroids or had a high CRP level. We used stricter criteria in our data analysis, excluding such cases; this may have affected the NLR results.
In addition to the NLR, the PLR and LMR have been reported to affect OS after SRS for BM. However, while all reports of the NLR have shown an effect on OS, the predictive abilities of the PLR and LMR differ among reports [8, 26, 27].
Several mechanisms have been proposed to explain the effects of neutrophils, monocytes, and platelets on the prognosis of cancer patients. Circulating neutrophils in the peripheral blood contain and secrete vascular endothelial growth factor (VEGF), tumor necrosis factor, and other cytokines that contribute to cancer progression [28]. Increased levels of neutrophils may release matrix metalloproteinase 9, oxygen radicals, and other inflammatory mediators, exacerbating BBB damage and further increasing the permeability of the BBB. Monocytes are innate immune cells that are crucial in cancer progression, invasion, and metastasis and can be classified as macrophages and myeloid-derived suppressor cells. In this study, SIRI values and the LMR, including monocytes, strongly correlated with prognosis. In the prognosis after SRS/fSRT for BM from NSCLC, lymphocyte, neutrophil, and monocyte counts should be noted. Platelets are an essential source of cytokines, such as VEGF, transforming growth factor β, and platelet-derived growth factor, which exacerbate angiogenesis and cell invasion [29].
More accurate biomarkers obtained by combining several blood-based markers have also been investigated. The SII is determined by measuring the neutrophil, lymphocyte, and platelet counts, while the SIRI is determined by measuring neutrophil, lymphocyte, and monocyte counts. Both of these indices have been reported to be effective prognostic biomarkers in many malignancies [30–40]. Zhang et al. reported that a higher SII was a poor prognostic factor for PFS and OS after SRS for BM from NSCLC [41]. On the other hand, Wang et al. measured the SII before and after radiotherapy for BM from EGFR-mutated NSCLC and reported that the pre-treatment SII and the amount of change influenced the OS [42]. Thus, in comparison with other systemic immune-inflammation scores, the SII may be a more objective valid surrogate that reflects the balance between host immune and inflammatory status [41].
The SIRI has not been studied extensively, and to the best of our knowledge, evaluation of the SIRI for BM has not yet been reported. One report examining whether the SIRI predicts prognosis in patients with EGFR-mutated lung adenocarcinoma treated with first-generation TKIs reported a more sensitive response to the SIRI than to the NLR [41]. The present study also proves that the SIRI more accurately reflected the OS after SRS/fSRT than the NLR and SII. The SIRI value before SRS/fSRT is an important predictor of prognosis after treatment. Because of the poor prognosis in the high SIRI value group, the indication for SRS/fSRT should be treated with greater caution. However, patients with low SIRI value should be treated more aggressively and followed up with imaging because of the possibility of long-term survival.
LIMITATIONS
The present study had several limitations. First, this was a single-center retrospective analysis, which may have caused analytical bias. The lack of pre-treatment blood cell count data in the excluded patients may have influenced the results of the analysis.
CONCLUSION
In this study, we present the results obtained for patients showing BM from NSCLC who were treated with SRS/fSRT. The findings indicate that the SIRI obtained before radiosurgery was accurate prognostic factors for the OS of patients treated with SRS/fSRT for BM from NSCLC.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
FUNDING
None declared.




