-
PDF
- Split View
-
Views
-
Cite
Cite
Erika Segawa, Keiichi Jingu, Rei Umezawa, Takaya Yamamoto, Noriyoshi Takahashi, Noriyuki Kadoya, Ken Takeda, Cardiac impacts of postoperative radiotherapy for breast cancer in Japanese patients, Journal of Radiation Research, Volume 64, Issue 3, May 2023, Pages 569–573, https://doi.org/10.1093/jrr/rrad013
Close - Share Icon Share
Abstract
Radiotherapy for breast cancer has attracted attention in Western countries because radiation to the heart can cause cardiac events. The purposes of this study were to evaluate the relationship between radiotherapy after breast-conserving surgery and the frequency of cardiac events in Japanese patients and to investigate the risk factors of cardiac events after postoperative radiotherapy in those patients. Female patients who received postoperative radiotherapy following breast-conserving surgery between 2007 and 2012 at our hospital were evaluated. In this study, we estimated the cumulative incidence of cardiac events including angina pectoris, myocardial infarction, ischemic heart disease, heart failure and cardiomyopathy after radiotherapy. Of 311 eligible patients, 7.1% of the patients had a smoking history, 20.3% of the patients were obese and 22.2% of the patients had hypertension. The median follow-up period was 118 months (interquartile range, 102–132 months). Twelve patients (3.9%) experienced cardiac events after treatment. The mean time to cardiac events was 126 months. The 10-year cumulative incidences of cardiac events after treatment were 4.2% and 4.3% for patients with left-sided and right-sided breast cancer, respectively, without a significant difference. Multivariate analysis showed that only hypertension was a risk factor for cardiac events (hazard ratio = 16.67, P = 0.0003). In conclusion, postoperative radiotherapy for breast cancer did not increase the incidence of cardiac events. Since at least 2007, postoperative radiotherapy for breast cancer has been safely performed without effects on the heart.
BACKGROUND
Breast cancer treatments have shifted to less invasive treatments, and more than half of all breast cancer patients now receive breast-conserving therapy, including radiotherapy, as the initial treatment. The basic idea of breast-conserving therapy is surgical excision of gross lesions in the breast and radiation eradication of minimal residual disease. Radiotherapy following breast-conserving surgery has been shown in a meta-analysis of randomized controlled trials to reduce the risk of intra-breast recurrence by one-third and improve survival [1, 2]. However, although irradiation after breast-conserving surgery contributes to improvement in the survival rate, attention has been paid to the risk of cardiac events caused by irradiation of the heart. In a study using a long-term database of over 300 000 people in USA (SEER: Surveillance Epidemiology and End Results), it was found that mortality from heart disease in women diagnosed with breast cancer between 1973 and 1982 who received radiation therapy was higher in patients with left-sided breast cancer than in patients with right-sided breast cancer [3]. A systematic review by Taylor et al. [4], in which an irradiated group (194 957 patients) was compared with a non-irradiated group (180 250 patients) and in which patients treated by old irradiation techniques were included showed significantly more cardiac deaths in the irradiated group. Darby et al. [5] reported that cardiac deaths increase from a few years after to at least 20 years after treatment. It has also been shown that the incidence of cardiac adverse events increases linearly with increase in radiation dose, with a relative 7.4% increase in coronary events for each 1 Gy increase in mean dose of the whole heart. On the other hand, a study in which the relationship between radiation therapy and cardiac events was investigated in Korean patients showed that postoperative radiotherapy after breast-conserving surgery may have a low excess risk of cardiac events [6]. Chang et al. [7] conducted a similar survey for Korean patients and reported that there was no difference in the incidence of cardiac events after radiotherapy between patients with left-sided breast cancer and those with right-sided breast cancer. The purpose of this study was to evaluate the occurrence of cardiac events after radiotherapy following breast-conserving surgery for breast cancer in Japanese and to determine the risk factors, including dose parameters of the whole heart, left ventricle and left anterior descending coronary artery, of cardiac events after radiotherapy.
MATERIALS AND METHODS
We retrospectively analyzed 984 Japanese female patients diagnosed with breast cancer between January 2007 and December 2012 who received radiotherapy following breast-conserving surgery at our hospital. Of those patients, 455 patients with a follow-up period of <5 years, 55 patients diagnosed with bilateral breast cancer, 102 patients with distant metastases, 52 patients with a history of other malignancies and 11 patients with a history of heart disease at the time of breast cancer diagnosis were excluded, and a total of 311 patients were enrolled in this study. In almost all of the patients, four or six MV X-rays were used to irradiate the whole breast after breast-conserving surgery in a supine position with 2 Gy per fraction for a total of 50 Gy using tangential irradiation fields. Planning for all of the patients was conducted using a CT simulator. Some patients with a positive surgical margin received boost irradiation to the tumor bed by electron beams with 2 Gy per fraction for a total of 10 Gy. During this period, we did not do anything to protect the heart, though we used the field-in-field method with a multi-leaf collimator to eliminate as much as possible of the area irradiated with a prescription dose of 108% or higher (Fig. 1). All eligible patients received physical examinations every 3–6 months for 3 years after surgery and then every 6–12 months, and they received at least annual mammography and ultrasound examination. The cumulative incidence of cardiac events and risk factors for cardiac events after radiotherapy following breast-conserving surgery were evaluated. A cardiac event was defined as a diagnosis of angina pectoris, myocardial infarction, ischemic heart disease, heart failure or cardiomyopathy. The Kaplan–Meier method was used to calculate the cumulative incidence of cardiac events after radiotherapy following breast-conserving surgery from the date of initiation of radiotherapy to the occurrence of cardiac events, death or the date of the last hospital visit, and differences were evaluated by the log-rank test. For the analysis of risk factors for cardiac events after radiotherapy following breast-conserving surgery, the Cox proportional hazards regression model was used in multivariate analysis. Explanatory variables were age at the time of irradiation, body mass index (BMI), smoking, hypertension, diabetes, dyslipidemia, analgesics (Celecox, Nikesan, Brufen), anthracycline antitumor drugs, Herceptin and presence or absence of coronary artery calcification. To reveal the risk factors of cardiac events after radiotherapy following breast-conserving surgery, we investigated the mean doses of the whole heart, left ventricle and left anterior descending coronary artery in patients with left-sided breast cancer. In all patients with left-sided breast cancer, the whole heart, left ventricle and left anterior descending coronary artery were delineated with reference to the cardiac atlas written by Feng et al. [8].
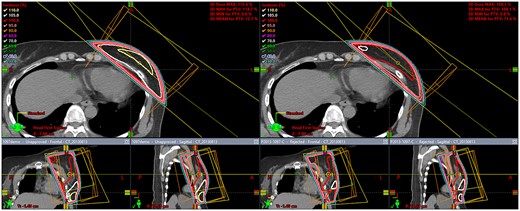
Comparison of dose distributions by tangential irradiation fields for the left breast with the field-in-field method (right) and without the field-in-field method (left).
All analyses were performed using JMP Pro Version 16.00 (SAS Institute, Cary, NC). A two-sided P-value < 0.05 was considered statistically significant. This study was conducted with the approval of the Ethics Committee.
RESULTS
Three hundred eleven patients were enrolled in this study. The patients’ characteristics are shown in Table 1. The median follow-up period was 118 months [interquartile range (IQR), 102–132 months]. Sixty-three patients (20.3%) were obese (BMI ≥ 25) and 22 patients (7.1%) had a history of smoking. Sixty-nine patients (22.2%), 27 patients (8.7%), 53 patients (17.0%), 17 patients (5.5%) and 8 patients (2.6%) had hypertension, diabetes, dyslipidemia, history of analgesic use and coronary artery calcification, respectively. Fifty-five patients (17.7%) had received anthracycline antineoplastic agents and 13 patients (4.2%) had received Herceptin. The total prescribed irradiation doses were 50 Gy in 213 patients (68.5%), 60 Gy in 97 patients (31.2%) and 52.56 Gy in 1 patient (0.3%).
| Patients’characteristics . | . | All patients (n = 311) . | Right-sided (n = 160) . | Left-sided (n = 151) . | |||
|---|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . | |
| Age | <55 | 174 | 55.9% | 87 | 54.4% | 87 | 57.6% |
| ≥55 | 137 | 44.1% | 73 | 45.6% | 64 | 42.4% | |
| Clinical stage (UICC 7th) | 0 | 48 | 15.4% | 21 | 13.1% | 27 | 17.9% |
| I | 164 | 52.7% | 82 | 51.3% | 82 | 54.3% | |
| II | 49 | 15.8% | 27 | 16.9% | 22 | 14.6% | |
| III | 17 | 5.5% | 9 | 5.6% | 8 | 5.3% | |
| Unknown | 33 | 10.6% | 21 | 13.1% | 12 | 7.9% | |
| T stage | Tis | 19 | 6.1% | 7 | 4.4% | 12 | 7.9% |
| 0 | 23 | 7.4% | 14 | 8.8% | 9 | 6% | |
| 1 | 190 | 61.1% | 92 | 57.5% | 98 | 64.9% | |
| 2 | 37 | 11.9% | 22 | 13.8% | 15 | 9.9% | |
| 3 | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Unknown | 41 | 13.2% | 24 | 15% | 17 | 11.3% | |
| N stage | 0 | 237 | 76.2% | 116 | 72.5% | 121 | 80.1% |
| 1 | 36 | 11.6% | 21 | 13.1% | 15 | 9.9% | |
| 2 | 4 | 1.3% | 2 | 1.3% | 2 | 1.3% | |
| 3 | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Unknown | 33 | 10.6% | 20 | 12.5% | 13 | 8.6% | |
| BMI | <25 | 248 | 79.7% | 132 | 82.5% | 116 | 76.8% |
| 25≤ | 63 | 20.3% | 28 | 17.5% | 35 | 23.2% | |
| Smoking history | No | 192 | 61.7% | 99 | 61.9% | 93 | 61.6% |
| Yes | 22 | 7.1% | 8 | 5% | 14 | 9.3% | |
| NA | 97 | 31.2% | 53 | 33.1% | 44 | 29.1% | |
| Hypertension | No | 242 | 77.8% | 127 | 79.4% | 115 | 76.2% |
| Yes | 69 | 22.2% | 33 | 20.6% | 36 | 23.8% | |
| Diabetes | No | 284 | 91.3% | 146 | 91.3% | 138 | 91.4% |
| Yes | 27 | 8.7% | 14 | 8.8% | 13 | 8.6% | |
| Dyslipidemia | No | 258 | 83% | 131 | 81.9% | 127 | 84.1% |
| Yes | 53 | 17% | 29 | 18.1% | 24 | 15.9% | |
| Chemotherapy | No | 235 | 75.6% | 119 | 74.4% | 116 | 76.8% |
| Yes | 76 | 24.4% | 41 | 25.6% | 35 | 23.2% | |
| Hormone therapy | No | 127 | 40.8% | 70 | 43.8% | 57 | 37.7% |
| Yes | 184 | 59.2% | 90 | 56.3% | 94 | 62.3% | |
| Painkiller | No | 294 | 94.5% | 151 | 94.4% | 143 | 94.7% |
| Yes | 17 | 5.5% | 9 | 5.6% | 8 | 5.3% | |
| Anthracycline antitumor drug | No | 256 | 82.3% | 129 | 80.6% | 127 | 84.1% |
| Yes | 55 | 17.7% | 31 | 19.4% | 24 | 15.9% | |
| Herceptin | No | 295 | 94.9% | 151 | 94.4% | 144 | 95.4% |
| Yes | 13 | 4.2% | 7 | 4.4% | 6 | 4% | |
| NA | 3 | 1% | 2 | 1.3% | 1 | 0.7% | |
| Coronary artery calcification | No | 303 | 97.4% | 157 | 98.1% | 146 | 96.7% |
| Yes | 8 | 2.6% | 3 | 1.9% | 5 | 3.3% | |
| Total irradiation dose | 50 Gy | 213 | 68.5% | 112 | 70% | 100 | 66.2% |
| 60 Gy | 97 | 31.2% | 47 | 29.4% | 51 | 33.8% | |
| 52.56 Gy | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Mean dose of whole heart | Median (range) | 3.15 Gy (0.86–10.85) | |||||
| Mean dose of left ventricle | Median (range) | 5.08 Gy (1.26–14.19) | |||||
| Mean dose of left anterior descending coronary artery | Median (range) | 29.94 Gy (2.23–53.33) | |||||
| Patients’characteristics . | . | All patients (n = 311) . | Right-sided (n = 160) . | Left-sided (n = 151) . | |||
|---|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . | |
| Age | <55 | 174 | 55.9% | 87 | 54.4% | 87 | 57.6% |
| ≥55 | 137 | 44.1% | 73 | 45.6% | 64 | 42.4% | |
| Clinical stage (UICC 7th) | 0 | 48 | 15.4% | 21 | 13.1% | 27 | 17.9% |
| I | 164 | 52.7% | 82 | 51.3% | 82 | 54.3% | |
| II | 49 | 15.8% | 27 | 16.9% | 22 | 14.6% | |
| III | 17 | 5.5% | 9 | 5.6% | 8 | 5.3% | |
| Unknown | 33 | 10.6% | 21 | 13.1% | 12 | 7.9% | |
| T stage | Tis | 19 | 6.1% | 7 | 4.4% | 12 | 7.9% |
| 0 | 23 | 7.4% | 14 | 8.8% | 9 | 6% | |
| 1 | 190 | 61.1% | 92 | 57.5% | 98 | 64.9% | |
| 2 | 37 | 11.9% | 22 | 13.8% | 15 | 9.9% | |
| 3 | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Unknown | 41 | 13.2% | 24 | 15% | 17 | 11.3% | |
| N stage | 0 | 237 | 76.2% | 116 | 72.5% | 121 | 80.1% |
| 1 | 36 | 11.6% | 21 | 13.1% | 15 | 9.9% | |
| 2 | 4 | 1.3% | 2 | 1.3% | 2 | 1.3% | |
| 3 | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Unknown | 33 | 10.6% | 20 | 12.5% | 13 | 8.6% | |
| BMI | <25 | 248 | 79.7% | 132 | 82.5% | 116 | 76.8% |
| 25≤ | 63 | 20.3% | 28 | 17.5% | 35 | 23.2% | |
| Smoking history | No | 192 | 61.7% | 99 | 61.9% | 93 | 61.6% |
| Yes | 22 | 7.1% | 8 | 5% | 14 | 9.3% | |
| NA | 97 | 31.2% | 53 | 33.1% | 44 | 29.1% | |
| Hypertension | No | 242 | 77.8% | 127 | 79.4% | 115 | 76.2% |
| Yes | 69 | 22.2% | 33 | 20.6% | 36 | 23.8% | |
| Diabetes | No | 284 | 91.3% | 146 | 91.3% | 138 | 91.4% |
| Yes | 27 | 8.7% | 14 | 8.8% | 13 | 8.6% | |
| Dyslipidemia | No | 258 | 83% | 131 | 81.9% | 127 | 84.1% |
| Yes | 53 | 17% | 29 | 18.1% | 24 | 15.9% | |
| Chemotherapy | No | 235 | 75.6% | 119 | 74.4% | 116 | 76.8% |
| Yes | 76 | 24.4% | 41 | 25.6% | 35 | 23.2% | |
| Hormone therapy | No | 127 | 40.8% | 70 | 43.8% | 57 | 37.7% |
| Yes | 184 | 59.2% | 90 | 56.3% | 94 | 62.3% | |
| Painkiller | No | 294 | 94.5% | 151 | 94.4% | 143 | 94.7% |
| Yes | 17 | 5.5% | 9 | 5.6% | 8 | 5.3% | |
| Anthracycline antitumor drug | No | 256 | 82.3% | 129 | 80.6% | 127 | 84.1% |
| Yes | 55 | 17.7% | 31 | 19.4% | 24 | 15.9% | |
| Herceptin | No | 295 | 94.9% | 151 | 94.4% | 144 | 95.4% |
| Yes | 13 | 4.2% | 7 | 4.4% | 6 | 4% | |
| NA | 3 | 1% | 2 | 1.3% | 1 | 0.7% | |
| Coronary artery calcification | No | 303 | 97.4% | 157 | 98.1% | 146 | 96.7% |
| Yes | 8 | 2.6% | 3 | 1.9% | 5 | 3.3% | |
| Total irradiation dose | 50 Gy | 213 | 68.5% | 112 | 70% | 100 | 66.2% |
| 60 Gy | 97 | 31.2% | 47 | 29.4% | 51 | 33.8% | |
| 52.56 Gy | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Mean dose of whole heart | Median (range) | 3.15 Gy (0.86–10.85) | |||||
| Mean dose of left ventricle | Median (range) | 5.08 Gy (1.26–14.19) | |||||
| Mean dose of left anterior descending coronary artery | Median (range) | 29.94 Gy (2.23–53.33) | |||||
| Patients’characteristics . | . | All patients (n = 311) . | Right-sided (n = 160) . | Left-sided (n = 151) . | |||
|---|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . | |
| Age | <55 | 174 | 55.9% | 87 | 54.4% | 87 | 57.6% |
| ≥55 | 137 | 44.1% | 73 | 45.6% | 64 | 42.4% | |
| Clinical stage (UICC 7th) | 0 | 48 | 15.4% | 21 | 13.1% | 27 | 17.9% |
| I | 164 | 52.7% | 82 | 51.3% | 82 | 54.3% | |
| II | 49 | 15.8% | 27 | 16.9% | 22 | 14.6% | |
| III | 17 | 5.5% | 9 | 5.6% | 8 | 5.3% | |
| Unknown | 33 | 10.6% | 21 | 13.1% | 12 | 7.9% | |
| T stage | Tis | 19 | 6.1% | 7 | 4.4% | 12 | 7.9% |
| 0 | 23 | 7.4% | 14 | 8.8% | 9 | 6% | |
| 1 | 190 | 61.1% | 92 | 57.5% | 98 | 64.9% | |
| 2 | 37 | 11.9% | 22 | 13.8% | 15 | 9.9% | |
| 3 | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Unknown | 41 | 13.2% | 24 | 15% | 17 | 11.3% | |
| N stage | 0 | 237 | 76.2% | 116 | 72.5% | 121 | 80.1% |
| 1 | 36 | 11.6% | 21 | 13.1% | 15 | 9.9% | |
| 2 | 4 | 1.3% | 2 | 1.3% | 2 | 1.3% | |
| 3 | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Unknown | 33 | 10.6% | 20 | 12.5% | 13 | 8.6% | |
| BMI | <25 | 248 | 79.7% | 132 | 82.5% | 116 | 76.8% |
| 25≤ | 63 | 20.3% | 28 | 17.5% | 35 | 23.2% | |
| Smoking history | No | 192 | 61.7% | 99 | 61.9% | 93 | 61.6% |
| Yes | 22 | 7.1% | 8 | 5% | 14 | 9.3% | |
| NA | 97 | 31.2% | 53 | 33.1% | 44 | 29.1% | |
| Hypertension | No | 242 | 77.8% | 127 | 79.4% | 115 | 76.2% |
| Yes | 69 | 22.2% | 33 | 20.6% | 36 | 23.8% | |
| Diabetes | No | 284 | 91.3% | 146 | 91.3% | 138 | 91.4% |
| Yes | 27 | 8.7% | 14 | 8.8% | 13 | 8.6% | |
| Dyslipidemia | No | 258 | 83% | 131 | 81.9% | 127 | 84.1% |
| Yes | 53 | 17% | 29 | 18.1% | 24 | 15.9% | |
| Chemotherapy | No | 235 | 75.6% | 119 | 74.4% | 116 | 76.8% |
| Yes | 76 | 24.4% | 41 | 25.6% | 35 | 23.2% | |
| Hormone therapy | No | 127 | 40.8% | 70 | 43.8% | 57 | 37.7% |
| Yes | 184 | 59.2% | 90 | 56.3% | 94 | 62.3% | |
| Painkiller | No | 294 | 94.5% | 151 | 94.4% | 143 | 94.7% |
| Yes | 17 | 5.5% | 9 | 5.6% | 8 | 5.3% | |
| Anthracycline antitumor drug | No | 256 | 82.3% | 129 | 80.6% | 127 | 84.1% |
| Yes | 55 | 17.7% | 31 | 19.4% | 24 | 15.9% | |
| Herceptin | No | 295 | 94.9% | 151 | 94.4% | 144 | 95.4% |
| Yes | 13 | 4.2% | 7 | 4.4% | 6 | 4% | |
| NA | 3 | 1% | 2 | 1.3% | 1 | 0.7% | |
| Coronary artery calcification | No | 303 | 97.4% | 157 | 98.1% | 146 | 96.7% |
| Yes | 8 | 2.6% | 3 | 1.9% | 5 | 3.3% | |
| Total irradiation dose | 50 Gy | 213 | 68.5% | 112 | 70% | 100 | 66.2% |
| 60 Gy | 97 | 31.2% | 47 | 29.4% | 51 | 33.8% | |
| 52.56 Gy | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Mean dose of whole heart | Median (range) | 3.15 Gy (0.86–10.85) | |||||
| Mean dose of left ventricle | Median (range) | 5.08 Gy (1.26–14.19) | |||||
| Mean dose of left anterior descending coronary artery | Median (range) | 29.94 Gy (2.23–53.33) | |||||
| Patients’characteristics . | . | All patients (n = 311) . | Right-sided (n = 160) . | Left-sided (n = 151) . | |||
|---|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . | |
| Age | <55 | 174 | 55.9% | 87 | 54.4% | 87 | 57.6% |
| ≥55 | 137 | 44.1% | 73 | 45.6% | 64 | 42.4% | |
| Clinical stage (UICC 7th) | 0 | 48 | 15.4% | 21 | 13.1% | 27 | 17.9% |
| I | 164 | 52.7% | 82 | 51.3% | 82 | 54.3% | |
| II | 49 | 15.8% | 27 | 16.9% | 22 | 14.6% | |
| III | 17 | 5.5% | 9 | 5.6% | 8 | 5.3% | |
| Unknown | 33 | 10.6% | 21 | 13.1% | 12 | 7.9% | |
| T stage | Tis | 19 | 6.1% | 7 | 4.4% | 12 | 7.9% |
| 0 | 23 | 7.4% | 14 | 8.8% | 9 | 6% | |
| 1 | 190 | 61.1% | 92 | 57.5% | 98 | 64.9% | |
| 2 | 37 | 11.9% | 22 | 13.8% | 15 | 9.9% | |
| 3 | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Unknown | 41 | 13.2% | 24 | 15% | 17 | 11.3% | |
| N stage | 0 | 237 | 76.2% | 116 | 72.5% | 121 | 80.1% |
| 1 | 36 | 11.6% | 21 | 13.1% | 15 | 9.9% | |
| 2 | 4 | 1.3% | 2 | 1.3% | 2 | 1.3% | |
| 3 | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Unknown | 33 | 10.6% | 20 | 12.5% | 13 | 8.6% | |
| BMI | <25 | 248 | 79.7% | 132 | 82.5% | 116 | 76.8% |
| 25≤ | 63 | 20.3% | 28 | 17.5% | 35 | 23.2% | |
| Smoking history | No | 192 | 61.7% | 99 | 61.9% | 93 | 61.6% |
| Yes | 22 | 7.1% | 8 | 5% | 14 | 9.3% | |
| NA | 97 | 31.2% | 53 | 33.1% | 44 | 29.1% | |
| Hypertension | No | 242 | 77.8% | 127 | 79.4% | 115 | 76.2% |
| Yes | 69 | 22.2% | 33 | 20.6% | 36 | 23.8% | |
| Diabetes | No | 284 | 91.3% | 146 | 91.3% | 138 | 91.4% |
| Yes | 27 | 8.7% | 14 | 8.8% | 13 | 8.6% | |
| Dyslipidemia | No | 258 | 83% | 131 | 81.9% | 127 | 84.1% |
| Yes | 53 | 17% | 29 | 18.1% | 24 | 15.9% | |
| Chemotherapy | No | 235 | 75.6% | 119 | 74.4% | 116 | 76.8% |
| Yes | 76 | 24.4% | 41 | 25.6% | 35 | 23.2% | |
| Hormone therapy | No | 127 | 40.8% | 70 | 43.8% | 57 | 37.7% |
| Yes | 184 | 59.2% | 90 | 56.3% | 94 | 62.3% | |
| Painkiller | No | 294 | 94.5% | 151 | 94.4% | 143 | 94.7% |
| Yes | 17 | 5.5% | 9 | 5.6% | 8 | 5.3% | |
| Anthracycline antitumor drug | No | 256 | 82.3% | 129 | 80.6% | 127 | 84.1% |
| Yes | 55 | 17.7% | 31 | 19.4% | 24 | 15.9% | |
| Herceptin | No | 295 | 94.9% | 151 | 94.4% | 144 | 95.4% |
| Yes | 13 | 4.2% | 7 | 4.4% | 6 | 4% | |
| NA | 3 | 1% | 2 | 1.3% | 1 | 0.7% | |
| Coronary artery calcification | No | 303 | 97.4% | 157 | 98.1% | 146 | 96.7% |
| Yes | 8 | 2.6% | 3 | 1.9% | 5 | 3.3% | |
| Total irradiation dose | 50 Gy | 213 | 68.5% | 112 | 70% | 100 | 66.2% |
| 60 Gy | 97 | 31.2% | 47 | 29.4% | 51 | 33.8% | |
| 52.56 Gy | 1 | 0.3% | 1 | 0.6% | 0 | 0% | |
| Mean dose of whole heart | Median (range) | 3.15 Gy (0.86–10.85) | |||||
| Mean dose of left ventricle | Median (range) | 5.08 Gy (1.26–14.19) | |||||
| Mean dose of left anterior descending coronary artery | Median (range) | 29.94 Gy (2.23–53.33) | |||||
Twelve patients (3.9%) experienced cardiac events during the follow-up period. The mean time to cardiac events was 126 months (range: 14–128 months). The 10-year cumulative incidences of cardiac events were 4.2 and 4.3% for patients with left-sided and right-sided breast cancer, respectively. There was no significant difference in the cumulative incidence of cardiac events between patients with right-sided breast cancer and patients with left-sided breast cancer (HR = 0.76, 95% CI: 0.24–2.38, P = 0.63) (Fig. 2). Mean doses of the whole heart, left ventricle and left anterior descending coronary artery in patients with left-sided breast cancer were 3.15 Gy (range: 0.86–10.85 Gy), 5.08 Gy (range: 1.26–14.19 Gy) and 29.94 Gy (range: 2.23–53.33 Gy), respectively. There were no correlations between those parameters and cardiac events.
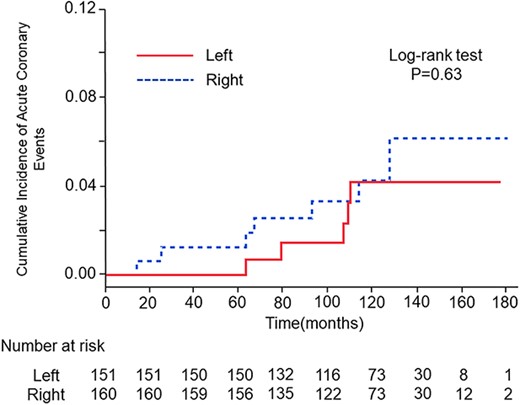
Cumulative incidences of cardiac events after radiotherapy in patients with left-sided breast cancer (red line) and patients with right-sided breast cancer (blue line).
In univariate analysis, BMI, hypertension, dyslipidemia and presence or absence of diabetes were selected as risk factors; however, only hypertension was a risk factor of cardiac events after radiotherapy following breast-conserving surgery for early breast cancer in multivariate analysis (HR = 16.67, 95% CI: 3.65–76.17, P = 0.0003).
DISCUSSION
To the best of our knowledge, this study is the first study in which the correlation between radiotherapy following breast-conserving surgery and cardiac events after treatment was investigated in Japanese patients with breast cancer with a relatively long observation period. In this study, there was no significant difference in the cumulative incidence of cardiac events between patients with left-sided breast cancer and patients with right-sided breast cancer. The results suggested that postoperative radiotherapy did not have a cardiac impact in patients with early breast cancer. Boero et al. [9], who performed analysis using the SEER database between 2000 and 2010, also reported that the effect of tumor laterality on cardiac events was not statistically significant. Their results are consistent with our results. However, Darby et al. [5] showed that the incidence of major coronary events in patients with left-sided breast cancer was significantly higher than that in patients with right-sided breast cancer in Sweden and Denmark. However, studies conducted in South Korea and USA showed results similar to our results [7, 9]. Asian women’s smoking and obesity rates are known to be lower than those in Westerns, and vascular condition in Asians might be relatively healthy. The prevalences of obesity among women in Japan, South Korea, Sweden and USA were 3.7, 5.3, 12.3 and 38.2%, respectively [10]. Although Americans have a higher rate of obesity than Swedes, a study showed that the prevalence of coronary artery calcification in Germans was 67%, which was much higher than the prevalence of 32.5% in Americans [11]. Differences in the coronary artery condition might cause the difference in the frequency of cardiac events after radiotherapy following breast-conserving surgery. Boero et al. [9] also showed that the effect of tumor laterality was limited to the high cardiac risk subgroup. However, coronary artery calcification was not selected as a risk factor in multivariate analysis in this study. The main reason why only Europe did poorly may be that that the study was carried out in an era in which old radiotherapy techniques were used.
In this study, there were no significant correlations between cardiac events and mean doses of the whole heart, left ventricle and left anterior descending coronary artery. Darby et al. [5] reported that the risk of major coronary events increased linearly with increase in the mean dose to the heart. On the other hand, Chang et al. reported that no significant relationship was found between cardiac dose and cardiac events. It is thought that the effects of radiation on the heart became minor because the mean cardiac dose was 3.15 ± 1.57 Gy in patients with left-sided breast cancer in this study, although it was 6.6 ± 4.9 Gy in patients with left-sided breast cancer in the study by Darby et al. We used the field-in-field method with three-dimensional conformal radiation therapy (3D-CRT) to reduce the area irradiated with a higher dose in some cases, which enabled the cardiac dose to be reduced [12]. In patients enrolled in this study in whom the field-in-field method was used, the mean dose to the left anterior descending artery was reduced by an average of 0.5 Gy and a maximum of 3.5 Gy. Therefore, it is possible that the heart, left ventricle and left anterior descending coronary artery were not irradiated with a sufficient dose to affect the heart. Furthermore, in recent years, techniques for performing breast-conserving postoperative irradiation with higher accuracy than that of 3D-CRT while protecting the heart have begun to be used clinically [13, 14]. In the future, as radiation therapy technology advances, the relationship between cardiac dose and cardiac events may become weaker.
This study had several limitations. First, it was a retrospective study. We might not have been able to detect cardiac events. Second, our patients were younger than those in past studies. The median age at the time of irradiation for breast cancer in this study was 52 years, which is younger than the ages of patients in past studies. Third, the follow-up period of 118 months might be too short for detecting cardiac events. Fourth, the dose of boost irradiation was not considered for the mean dose of each organ because the dose-calculation accuracy of electron beams at that time was low and the effect of boost irradiation using electron beams on the heart was small. Finally, there was a lack of detailed information on smoking and severity of coronary artery calcification.
CONCLUSION
In this study, it was shown that the cardiac impacts of postoperative radiotherapy following breast-conserving surgery using the 3D-CRT method were minimal. Hypertension was selected in multivariate analysis as a risk factor of cardiac events after postoperative radiotherapy for early breast cancer. Therefore, it may be necessary to reduce the irradiation dose to the heart, especially in patients with risk factors.
CONFLICT OF INTEREST
K.J. received personal fees from Varian Medical Systems, Inc, Elekta K.K. and AstraZeneca K.K. and T.Y. received personal fees from AstraZeneca K.K.
Funding
Not applicable.




