-
PDF
- Split View
-
Views
-
Cite
Cite
Kathryn M Cook, Jessica Gillon, Alison G Grisso, Ritu Banerjee, Natalia Jimenez-Truque, Elizabeth J Phillips, Sara L Van Driest, Incidence of Nephrotoxicity Among Pediatric Patients Receiving Vancomycin With Either Piperacillin–Tazobactam or Cefepime: A Cohort Study, Journal of the Pediatric Infectious Diseases Society, Volume 8, Issue 3, July 2019, Pages 221–227, https://doi.org/10.1093/jpids/piy030
Close - Share Icon Share
Abstract
Recent studies in adults have found an incidence of acute kidney injury (AKI) in patients treated with a combination of vancomycin and piperacillin–tazobactam (TZP) that is greater than that expected with either medication alone. The purpose of this study was to determine whether combination therapy with vancomycin and TZP is associated with an incidence of AKI in pediatric patients higher than that in those on combination therapy with vancomycin and cefepime.
We performed a retrospective single-center matched-cohort study of pediatric patients who received vancomycin in combination with TZP or cefepime between January 2015 and June 2016. The patients were matched according to chronic disease, age, sex, and number of concomitant nephrotoxic medications at the time of combination antibiotic therapy. The primary outcome was incidence of AKI. Secondary outcomes included differences between groups in time to AKI, resolution of AKI, and effect of vancomycin trough levels on the incidence of nephrotoxicity. Conditional logistic regression was used to compare categorical and continuous variables between treatment groups. Conditional Poisson regression was used to assess the association between AKI and treatment groups. Stratified log-rank tests and Cox proportional hazards models with shared frailty were used to compare the times to AKI according to treatment group.
Two hundred twenty-eight matched patients were included. AKI developed in 9 (7.9%) of 114 and 33 (28.9%) of 114 patients in the cefepime and TZP groups, respectively (P < .001). Type of combination therapy remained a significant predictor for AKI in multivariate conditional Poisson analysis in which adjustments were made for age, sex, use of concomitant nephrotoxins, and vancomycin dose (relative risk, 2.5 [95% confidence interval, 1.1–5.8]; P = .03). AKI developed almost 3 times sooner in the TZP group than in the cefepime group (hazard ratio, 2.9 [95% confidence interval, 1.3–6.1]; P = .006). Sensitivity analyses in which adjustment was made for antibiotic indication in addition to the aforementioned variables and excluding those with gastrointestinal infection revealed similar results.
Among hospitalized children at our institution, combination therapy with vancomycin and TZP was associated with an incidence of AKI higher than that associated with vancomycin and cefepime.
Adverse drug reactions are an unintended consequence of medication administration. In pediatric patients, antibiotics are one of the most commonly implicated drugs to cause adverse reactions [1]. Among antibiotics, β-lactams and vancomycin are the most common causes of adverse drug reactions in children [2].
Vancomycin-associated toxicities have been well described and include administration reactions, ototoxicity, and nephrotoxicity [3–5]. Risk factors for vancomycin-associated nephrotoxicity based on studies in adult patients include a total daily dose of ≥4 g, trough levels of ≥15 µg/mL, older age, longer durations of therapy (>7 days), preexisting renal dysfunction, and concomitant use of other nephrotoxins [6–8]. Data regarding risk factors for the development of vancomycin-associated nephrotoxicity in pediatric patients are limited, although 1 study identified vancomycin trough levels of ≥15 µg/mL, intensive care stay, and concomitant furosemide exposure as risk factors [9]. Avoiding concomitant nephrotoxic medications, such as nonsteroidal antiinflammatory drugs, contrast dyes, loop diuretics, amphotericin B, acyclovir, vasopressors, and medications that affect the renin–angiotensin system, during vancomycin therapy is recommended to decrease the incidence of acute kidney injury (AKI) caused by additive toxicity [8].
Patients with suspected sepsis are often treated with combination therapy (multiple antibiotics), which can increase drug-associated adverse events [10–12]. The nephrotoxic potential of antipseudomonal β-lactam antibiotics, such as piperacillin–tazobactam (TZP), has been well described [10]. However, many recent studies found a more significant increase in the incidence of nephrotoxicity in adults treated with a combination of vancomycin and TZP than was expected from vancomycin alone [11, 13]. Downes et al [14] recently published the results of a retrospective cohort study that used data from the Pediatric Health Information System (PHIS) database and found an increased incidence of AKI among pediatric patients who received vancomycin in combination with TZP over those who received other antipseudomonal β-lactam antibiotics. Although their study was large, the patients were not matched, and vancomycin trough data and comorbid conditions were not evaluated. Therefore, although the potential nephrotoxicity of TZP has been explored in adult and pediatric patients, robust studies have not been conducted to compare vancomycin–TZP combination therapy with another combination therapy in children [8, 11, 12, 14–17].
Our institution experienced a severe cefepime shortage from June 15 to August 6, 2015, and again from May 12 to June 30, 2016, which prompted prescribers to change from cefepime to TZP as the drug of choice for empiric therapy for suspected infection because of its similar, although not identical, coverage. Examples of empiric regimens that were altered from vancomycin and cefepime to vancomycin and TZP include those for fever with congenital heart disease, cystic fibrosis pulmonary exacerbations, febrile neutropenia, and severe sepsis. Because this prescribing change was implemented hospital-wide through guidance from the antimicrobial stewardship program and was not influenced by individual clinician preference, it provided an opportunity to compare outcomes using the 2 antibiotic combinations in more well-balanced patient groups that otherwise would not have been feasible in a retrospective study. Therefore, the purpose of our study was to determine if treatment with the combination of vancomycin and TZP in children leads to a greater incidence of nephrotoxicity than does the combination of vancomycin and cefepime. To this end, we conducted a single-center cohort study to compare the outcomes of pediatric patients who received 1 of these 2 antibiotic combinations.
METHODS
Population and Data Collection
We performed a retrospective single-center matched-cohort study of pediatric patients who received vancomycin in combination with either TZP (TZP group) or cefepime (cefepime group) for >48 hours between January 1, 2015, and June 30, 2016. Patients were included if they were ≤18 years old, underwent a minimum of 48 hours of combination antibiotic therapy, had at least 1 vancomycin trough measurement at steady state, and had a baseline serum creatinine measurement within the 2 months that preceded the antibiotic therapy. Patients were excluded from the study if they were undergoing extracorporeal membrane oxygenation during the vancomycin course, had a history of structural kidney disease, were undergoing dialysis at antibiotic initiation, or were given vancomycin orally. In an effort to include only patients with normal kidney function at the beginning of the study, patients with preexisting renal dysfunction and with AKI at admission were not included. Each eligible patient who was exposed to vancomycin and TZP was matched 1:1 to a patient who was exposed to vancomycin and cefepime according to their chronic disease (oncologic disease, cystic fibrosis, congenital heart disease, or none of the above), age (±1 year for patients aged ≥1 year and ±3 months for those aged <1 year), sex, and number of concomitant nephrotoxic medications, defined as the maximum number of concomitant nephrotoxic medications the patient received during the time of combination antibiotic therapy. This study was approved by the Vanderbilt University Medical Center institutional review board.
The following baseline characteristics were obtained from the electronic health record: age, sex, weight, baseline and subsequent serum creatinine measurements, location in the hospital at the time of antibiotic initiation, chronic disease, indication for antibiotic therapy, daily weight-adjusted vancomycin dose at the time troughs were measured during concomitant antibiotic administration, vancomycin troughs measured correctly at steady state (approximately 30 minutes before the fourth dose on the regimen), and concomitant nephrotoxins (see Supplementary Table 1 for list). Medication administration times were assumed to be documented accurately. Data were stored in a secure password-protected online database [18].
Statistical Analyses
The primary end point for this study was the difference in incidences of AKI between the 2 treatment groups. AKI was defined according to the serum creatinine criteria set in the Kidney Disease Improving Global Outcomes (KDIGO) guideline, namely, an increase of ≥0.3 mg/dL over the baseline serum creatinine level or an increase to ≥1.5 times the patient’s baseline level during combination antibiotic administration [15]. KDIGO time windows were not enforced, but time from the initiation of combination antibiotics to the elevated serum creatinine level was assessed in each patient. Secondary outcomes included time to AKI, resolution of AKI, and length of hospital stay.
Bivariate analyses using conditional logistic regression were conducted to compare categorical or continuous variables according to treatment group. Conditional Poisson regression was used to assess the association between AKI and the treatment groups while accounting for the matched pairs. The stratified log-rank test and Cox proportional hazards models, with shared frailty for the matched pairs, were used to compare the times to AKI and lengths of stay according to treatment group. Shared-frailty models are used to account for observations that are not independent of each other and can be thought of as a Cox model with random effects. Observations within a group (matched pairs in this case) are correlated (not independent) because they share the same frailty (their own predisposition to develop AKI). For time-to-event models, baseline (day 0 of treatment) was defined as the first date on which the patient was being given both antibiotics (eg, the first date on which both vancomycin and TZP were administered). The time to AKI was defined as the difference in days between the start of combination antibiotics and the highest serum creatinine level measured during combination antibiotic therapy; if a patient’s highest serum creatinine level met the KDIGO guidelines mentioned earlier, he or she was classified as having AKI. When sample size allowed, multivariate models were adjusted by potential confounders selected a priori. Potential confounders included age (years, continuous variable), sex (male or female, dichotomous variable), number of nephrotoxic drugs (tally of total, ordinal variable), and median daily dose of vancomycin over the course of therapy (in milligrams per kilogram per day, continuous variable). Sensitivity analyses were conducted for the time-to-AKI models after adjustment for antibiotic indication and the exclusion of patients with a gastrointestinal infection, because many providers at our institution prescribe TZP for the treatment of gastrointestinal infection. Cox proportional hazards model analysis for length of stay was repeated excluding patients in the neonatal intensive care unit, given their long length of stay. Additional stratified analyses according to chronic disease, as defined above, were attempted to assess whether there was a modification of the effect of combination antibiotic therapy on the incidence of AKI according to disease group. Analyses were conducted using Stata 14 (StataCorp, LP, College Station, Texas).
RESULTS
A total of 340 eligible patients were reviewed for inclusion. Of those patients, 112 met the exclusion criteria or were excluded because no match in the cefepime group was found. Overall, 114 patients in the TZP group and 114 matched patients in the cefepime group (n = 228 total) were included in our study (Table 1). The groups were well balanced in terms of ICU admission (67.5% in the cefepime group vs 70.2% in the TZP group), numbers of concomitant nephrotoxins (median, 1 [interquartile range (IQR), 1–2] in the cefepime group vs 2 [IQR, 1–2] in the TZP group), and baseline serum creatinine levels (median, 0.43 mg/dL [IQR, 0.37–0.57 mg/dL] in the cefepime group vs 0.45 mg/dL [IQR, 0.39–0.56 mg/dL] in the TZP group). The groups were comparable also with respect to vancomycin dosing at the time of the highest measured vancomycin trough at steady state (median, 45 mg/kg per day [IQR, 30–59.7 mg/kg per day] in the cefepime group vs 43.9 mg/kg per day [IQR, 30–56.6 mg/kg per day] in the TZP group) and highest vancomycin trough levels measured correctly at steady state (median, 12 µg/mL [IQR, 8–16 µg/mL] in the cefepime group vs 13 µg/mL [IQR, 8–19 µg/mL] in the TZP group). The median ages at admission were statistically different between groups (0.44 years [IQR, 0–6.6 years] in the cefepime group vs 0.22 years [IQR, 0–6.3 years] in the TZP group; P = .004).
| Characteristic . | Vancomycin–TZP (n = 114)a . | Vancomycin–Cefepime (n = 114)a . | P . |
|---|---|---|---|
| Indication for Antibiotics . | . | . | . |
| Age (years) | 0.22 (0–6.3) | 0.44 (0–6.6) | .004b |
| Male sex | 52 (45.6) | 57 (50) | .30b |
| Weight (kg) | 5.7 (3.1–18.4) | 6 (3.5–20.9) | .09b |
| Baseline creatinine level (mg/dL) | 0.45 (0.39–0.56) | 0.43 (0.37–0.57) | .16b |
| Critical care location at antibiotic initiation | 80 (70.2) | 77 (67.5) | .49b |
| Chronic diseases | NAc | ||
| CHD | 48 (42.1) | 48 (42.1) | |
| CF | 13 (11.4) | 13 (11.4) | |
| Cancer | 9 (7.9) | 9 (7.9) | |
| None of the above | 44 (38.6) | 44 (38.6) | |
| Indication for antibioticsd | |||
| Empiric therapy | 41 (36.0) | 38 (33) | <.001b |
| Prophylaxis | 5 (4.4) | 21 (18.4) | |
| CNS disease | 0 (0) | 3 (2.6) | |
| Bacteremia | 4 (3.5) | 6 (5.3) | |
| Cardiovascular disease | 0 (0) | 1 (0.9) | |
| Respiratory disease | 24 (21.1) | 19 (16.7) | |
| GU/GI disease | 20 (17.5) | 1 (0.9) | |
| SSTI | 7 (6.1) | 14 (12.3) | |
| Febrile neutropenia | 7 (6.1) | 5 (4.4) | |
| Sepsis | 7 (6.1) | 7 (6.1) | |
| Hospital day at start of therapy (days) | 11 (1–26) | 3 (0–25.5) | .31b |
| Concomitant nephrotoxins | 2 (1–2) | 1 (1–2) | .56b |
| Weight-based vancomycin dosing at highest trough (mg/kg per day) | 43.9 (30–56.6) | 45 (30–59.7) | .10b |
| Highest vancomycin trough (µg/mL) | 13 (8–19) | 12 (8–16) | .07b |
| Characteristic . | Vancomycin–TZP (n = 114)a . | Vancomycin–Cefepime (n = 114)a . | P . |
|---|---|---|---|
| Indication for Antibiotics . | . | . | . |
| Age (years) | 0.22 (0–6.3) | 0.44 (0–6.6) | .004b |
| Male sex | 52 (45.6) | 57 (50) | .30b |
| Weight (kg) | 5.7 (3.1–18.4) | 6 (3.5–20.9) | .09b |
| Baseline creatinine level (mg/dL) | 0.45 (0.39–0.56) | 0.43 (0.37–0.57) | .16b |
| Critical care location at antibiotic initiation | 80 (70.2) | 77 (67.5) | .49b |
| Chronic diseases | NAc | ||
| CHD | 48 (42.1) | 48 (42.1) | |
| CF | 13 (11.4) | 13 (11.4) | |
| Cancer | 9 (7.9) | 9 (7.9) | |
| None of the above | 44 (38.6) | 44 (38.6) | |
| Indication for antibioticsd | |||
| Empiric therapy | 41 (36.0) | 38 (33) | <.001b |
| Prophylaxis | 5 (4.4) | 21 (18.4) | |
| CNS disease | 0 (0) | 3 (2.6) | |
| Bacteremia | 4 (3.5) | 6 (5.3) | |
| Cardiovascular disease | 0 (0) | 1 (0.9) | |
| Respiratory disease | 24 (21.1) | 19 (16.7) | |
| GU/GI disease | 20 (17.5) | 1 (0.9) | |
| SSTI | 7 (6.1) | 14 (12.3) | |
| Febrile neutropenia | 7 (6.1) | 5 (4.4) | |
| Sepsis | 7 (6.1) | 7 (6.1) | |
| Hospital day at start of therapy (days) | 11 (1–26) | 3 (0–25.5) | .31b |
| Concomitant nephrotoxins | 2 (1–2) | 1 (1–2) | .56b |
| Weight-based vancomycin dosing at highest trough (mg/kg per day) | 43.9 (30–56.6) | 45 (30–59.7) | .10b |
| Highest vancomycin trough (µg/mL) | 13 (8–19) | 12 (8–16) | .07b |
Abbreviations: CF, cystic fibrosis; CHD, congenital heart disease; CNS, central nervous system; GU/GI, genitourinary/gastrointestinal; SSTI, skin or soft-tissue infection; TZP, piperacillin–tazobactam.
aValues shown are median (interquartile range) or number (percentage).
bConditional logistic regression.
cNot applicable (no estimate possible, no difference within groups).
dMultiple indications for antibiotic therapy were allowed for each patient.
| Characteristic . | Vancomycin–TZP (n = 114)a . | Vancomycin–Cefepime (n = 114)a . | P . |
|---|---|---|---|
| Indication for Antibiotics . | . | . | . |
| Age (years) | 0.22 (0–6.3) | 0.44 (0–6.6) | .004b |
| Male sex | 52 (45.6) | 57 (50) | .30b |
| Weight (kg) | 5.7 (3.1–18.4) | 6 (3.5–20.9) | .09b |
| Baseline creatinine level (mg/dL) | 0.45 (0.39–0.56) | 0.43 (0.37–0.57) | .16b |
| Critical care location at antibiotic initiation | 80 (70.2) | 77 (67.5) | .49b |
| Chronic diseases | NAc | ||
| CHD | 48 (42.1) | 48 (42.1) | |
| CF | 13 (11.4) | 13 (11.4) | |
| Cancer | 9 (7.9) | 9 (7.9) | |
| None of the above | 44 (38.6) | 44 (38.6) | |
| Indication for antibioticsd | |||
| Empiric therapy | 41 (36.0) | 38 (33) | <.001b |
| Prophylaxis | 5 (4.4) | 21 (18.4) | |
| CNS disease | 0 (0) | 3 (2.6) | |
| Bacteremia | 4 (3.5) | 6 (5.3) | |
| Cardiovascular disease | 0 (0) | 1 (0.9) | |
| Respiratory disease | 24 (21.1) | 19 (16.7) | |
| GU/GI disease | 20 (17.5) | 1 (0.9) | |
| SSTI | 7 (6.1) | 14 (12.3) | |
| Febrile neutropenia | 7 (6.1) | 5 (4.4) | |
| Sepsis | 7 (6.1) | 7 (6.1) | |
| Hospital day at start of therapy (days) | 11 (1–26) | 3 (0–25.5) | .31b |
| Concomitant nephrotoxins | 2 (1–2) | 1 (1–2) | .56b |
| Weight-based vancomycin dosing at highest trough (mg/kg per day) | 43.9 (30–56.6) | 45 (30–59.7) | .10b |
| Highest vancomycin trough (µg/mL) | 13 (8–19) | 12 (8–16) | .07b |
| Characteristic . | Vancomycin–TZP (n = 114)a . | Vancomycin–Cefepime (n = 114)a . | P . |
|---|---|---|---|
| Indication for Antibiotics . | . | . | . |
| Age (years) | 0.22 (0–6.3) | 0.44 (0–6.6) | .004b |
| Male sex | 52 (45.6) | 57 (50) | .30b |
| Weight (kg) | 5.7 (3.1–18.4) | 6 (3.5–20.9) | .09b |
| Baseline creatinine level (mg/dL) | 0.45 (0.39–0.56) | 0.43 (0.37–0.57) | .16b |
| Critical care location at antibiotic initiation | 80 (70.2) | 77 (67.5) | .49b |
| Chronic diseases | NAc | ||
| CHD | 48 (42.1) | 48 (42.1) | |
| CF | 13 (11.4) | 13 (11.4) | |
| Cancer | 9 (7.9) | 9 (7.9) | |
| None of the above | 44 (38.6) | 44 (38.6) | |
| Indication for antibioticsd | |||
| Empiric therapy | 41 (36.0) | 38 (33) | <.001b |
| Prophylaxis | 5 (4.4) | 21 (18.4) | |
| CNS disease | 0 (0) | 3 (2.6) | |
| Bacteremia | 4 (3.5) | 6 (5.3) | |
| Cardiovascular disease | 0 (0) | 1 (0.9) | |
| Respiratory disease | 24 (21.1) | 19 (16.7) | |
| GU/GI disease | 20 (17.5) | 1 (0.9) | |
| SSTI | 7 (6.1) | 14 (12.3) | |
| Febrile neutropenia | 7 (6.1) | 5 (4.4) | |
| Sepsis | 7 (6.1) | 7 (6.1) | |
| Hospital day at start of therapy (days) | 11 (1–26) | 3 (0–25.5) | .31b |
| Concomitant nephrotoxins | 2 (1–2) | 1 (1–2) | .56b |
| Weight-based vancomycin dosing at highest trough (mg/kg per day) | 43.9 (30–56.6) | 45 (30–59.7) | .10b |
| Highest vancomycin trough (µg/mL) | 13 (8–19) | 12 (8–16) | .07b |
Abbreviations: CF, cystic fibrosis; CHD, congenital heart disease; CNS, central nervous system; GU/GI, genitourinary/gastrointestinal; SSTI, skin or soft-tissue infection; TZP, piperacillin–tazobactam.
aValues shown are median (interquartile range) or number (percentage).
bConditional logistic regression.
cNot applicable (no estimate possible, no difference within groups).
dMultiple indications for antibiotic therapy were allowed for each patient.
AKI According to Antibiotic Combination Group
AKI developed in 9 (7.9%) of 114 (95% confidence interval [CI], 3.7%–14.4%) patients in the cefepime group and 33 (28.9%) of 114 (95% CI, 20.8%–38.2%) patients in the TZP group (unadjusted conditional Poisson relative risk [RR], 3.7 [95% CI, 1.8–7.7]; P = .001), resulting in a number needed to harm of 4.8. Of the 42 patients who experienced AKI, all but 5 had a 150% increase in their serum creatinine level over that at baseline within the first 7 days of combination antibiotic therapy. One patient met our criteria for AKI on the day of initiation of combination antibiotics, and 10 patients met AKI criteria 1 calendar day after starting combination antibiotics; the median among those who experienced AKI was 3 days (range, 0–16 days). On the basis of a multivariate conditional Poisson regression model, in which we adjusted for age, sex, number of concomitant nephrotoxic medications, and weight-adjusted daily vancomycin dose, treatment group remained a significant predictor for AKI; the TZP group had 2.5 times the risk of AKI than those in the cefepime group (RR, 2.5 [95% CI, 1.1–5.8]; P = .03) (Table 2).
Multivariate Conditional Poisson Regression Analysis of the Occurrence of AKI According to Antibiotic Combination Group
| Antibiotic Combination Group . | RR . | 95% CI . | P . |
|---|---|---|---|
| Vancomycin–cefepime | Reference | — | — |
| Vancomycin–TZPa | 2.5 | 1.1–5.8 | .03 |
| Antibiotic Combination Group . | RR . | 95% CI . | P . |
|---|---|---|---|
| Vancomycin–cefepime | Reference | — | — |
| Vancomycin–TZPa | 2.5 | 1.1–5.8 | .03 |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; RR, relative risk; TZP, piperacillin–tazobactam.
aAdjusted for age, sex, concomitant nephrotoxins, and vancomycin dose.
Multivariate Conditional Poisson Regression Analysis of the Occurrence of AKI According to Antibiotic Combination Group
| Antibiotic Combination Group . | RR . | 95% CI . | P . |
|---|---|---|---|
| Vancomycin–cefepime | Reference | — | — |
| Vancomycin–TZPa | 2.5 | 1.1–5.8 | .03 |
| Antibiotic Combination Group . | RR . | 95% CI . | P . |
|---|---|---|---|
| Vancomycin–cefepime | Reference | — | — |
| Vancomycin–TZPa | 2.5 | 1.1–5.8 | .03 |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; RR, relative risk; TZP, piperacillin–tazobactam.
aAdjusted for age, sex, concomitant nephrotoxins, and vancomycin dose.
Analysis of Time to Development of AKI
The overall median time to AKI for the full cohort was 12 days. Fifteen patients were excluded from the time-to-AKI analyses because their highest creatinine level was on the same day they started combination therapy. The median time to AKI was shorter, although not statistically significantly different so, in the TZP group than in the cefepime group (7 vs >15 days, respectively; P = .06, stratified log-rank test) (Figure 1). On the basis of an adjusted Cox proportional hazards analysis with shared frailty for the matched pairs, AKI occurred almost 3 times sooner in the TZP group than in the cefepime group (hazard ratio [HR], 2.9 [95% CI, 1.3–6.1]; P = .006) (Table 3). Similar results were observed in sensitivity analyses in which we adjusted for antibiotic indication in addition to the aforementioned variables (HR, 3.4 [95% CI, 1.4–7.8]; P = .005) (Table 3), and excluding those with a gastrointestinal infection revealed similar results (HR, 3.0 [95% CI, 1.4–6.6]; P = .005) (Table 3).
Cox Proportional Hazards Models With Shared Frailty to Compare Times to AKI According to Antibiotic Combination Group
| Antibiotic Combination Group . | HR (95% CI) . | P . |
|---|---|---|
| Vancomycin–cefepime | Reference | — |
| Vancomycin–TZP | 2.9 (1.3–6.1)a | .006 |
| 3.4 (1.4–7.8)b | .005 | |
| 3.0 (1.4–6.6)c | .005 |
| Antibiotic Combination Group . | HR (95% CI) . | P . |
|---|---|---|
| Vancomycin–cefepime | Reference | — |
| Vancomycin–TZP | 2.9 (1.3–6.1)a | .006 |
| 3.4 (1.4–7.8)b | .005 | |
| 3.0 (1.4–6.6)c | .005 |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; HR, hazard ratio; TZP, piperacillin-tazobactam.
aAdjusted for age, sex, concomitant nephrotoxins, and vancomycin dose.
bAdjused for age, sex, concomitant nephrotoxins, vancomycin dose, and antibiotic indication.
cAdjusted for age, sex, concomitant nephrotoxins, and vancomycin dose and excluding gastrointestinal infection indication.
Cox Proportional Hazards Models With Shared Frailty to Compare Times to AKI According to Antibiotic Combination Group
| Antibiotic Combination Group . | HR (95% CI) . | P . |
|---|---|---|
| Vancomycin–cefepime | Reference | — |
| Vancomycin–TZP | 2.9 (1.3–6.1)a | .006 |
| 3.4 (1.4–7.8)b | .005 | |
| 3.0 (1.4–6.6)c | .005 |
| Antibiotic Combination Group . | HR (95% CI) . | P . |
|---|---|---|
| Vancomycin–cefepime | Reference | — |
| Vancomycin–TZP | 2.9 (1.3–6.1)a | .006 |
| 3.4 (1.4–7.8)b | .005 | |
| 3.0 (1.4–6.6)c | .005 |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; HR, hazard ratio; TZP, piperacillin-tazobactam.
aAdjusted for age, sex, concomitant nephrotoxins, and vancomycin dose.
bAdjused for age, sex, concomitant nephrotoxins, vancomycin dose, and antibiotic indication.
cAdjusted for age, sex, concomitant nephrotoxins, and vancomycin dose and excluding gastrointestinal infection indication.
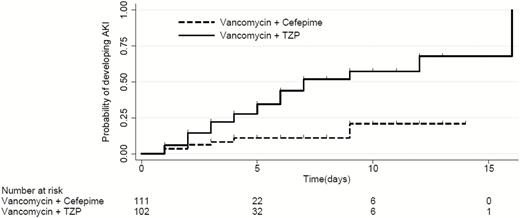
Kaplan-Meier curve representing the median time to development of acute kidney injury (AKI). The overall median time to development of AKI was 12 days. The time to AKI was shorter, although not statistically significantly so, in those receiving piperacillin–tazobactam (TZP) and vancomycin than in those receiving cefepime and vancomycin (7 vs >15 days, respectively; P = .059, log-rank test). The number at risk includes all patients who, at that time point, had not developed AKI but remained on combination antibiotic therapy.
Length of Stay
The median length of stay for the entire cohort was 37 days and was longer in the TZP group than in the cefepime group (median, 45 days [IQR, 16–108 days] vs 23 days [IQR, 12–70 days], respectively; P = .01, stratified log-rank test) (Table 4). On the basis of a Cox proportional hazards analysis in which we adjusted for age, sex, number of concomitant nephrotoxic medications, and weight-adjusted daily vancomycin dose, the HR in a comparison of those who received TZP with those who received cefepime was 0.67 (95% CI, 0.5–0.9; P = .003). Sensitivity analyses in which we excluded patients admitted to the neonatology service and adjusted only for age and weight-adjusted daily vancomycin dose to avoid overfitting revealed similar results; the median lengths of stay were 36 days in the TZP group and 20 days in the cefepime group (P = .037 for stratified log rank test; HR, 0.7 [95% CI, 0.5-0.9]; P = .02 for Cox proportional hazards analysis.)
| Outcome . | Vancomycin–TZP (n = 114) . | Vancomycin–Cefepime (n = 114) . | P . |
|---|---|---|---|
| Actual time to AKI (median [IQR]) (days) | 7 (4–16) | >15 (NA) | .06a |
| No. (%) without resolution of AKIb | 3 (9.7) | 0 (0) | NAc |
| Length of stay (median [IQR]) (days) | 45 (16–108) | 23 (12–70) | .01a |
| Outcome . | Vancomycin–TZP (n = 114) . | Vancomycin–Cefepime (n = 114) . | P . |
|---|---|---|---|
| Actual time to AKI (median [IQR]) (days) | 7 (4–16) | >15 (NA) | .06a |
| No. (%) without resolution of AKIb | 3 (9.7) | 0 (0) | NAc |
| Length of stay (median [IQR]) (days) | 45 (16–108) | 23 (12–70) | .01a |
Abbreviations: AKI, acute kidney injury; IQR, interquartile range; NA, not applicable; TZP, piperacillin-tazobactam.
aLog-rank test stratified according to matched pairs.
bData available for 40 patients, 31 in the TZP–vancomycin group and 9 in the cefepime–vancomycin group.
cNo estimation possible; resolution of AKI did not vary within groups.
| Outcome . | Vancomycin–TZP (n = 114) . | Vancomycin–Cefepime (n = 114) . | P . |
|---|---|---|---|
| Actual time to AKI (median [IQR]) (days) | 7 (4–16) | >15 (NA) | .06a |
| No. (%) without resolution of AKIb | 3 (9.7) | 0 (0) | NAc |
| Length of stay (median [IQR]) (days) | 45 (16–108) | 23 (12–70) | .01a |
| Outcome . | Vancomycin–TZP (n = 114) . | Vancomycin–Cefepime (n = 114) . | P . |
|---|---|---|---|
| Actual time to AKI (median [IQR]) (days) | 7 (4–16) | >15 (NA) | .06a |
| No. (%) without resolution of AKIb | 3 (9.7) | 0 (0) | NAc |
| Length of stay (median [IQR]) (days) | 45 (16–108) | 23 (12–70) | .01a |
Abbreviations: AKI, acute kidney injury; IQR, interquartile range; NA, not applicable; TZP, piperacillin-tazobactam.
aLog-rank test stratified according to matched pairs.
bData available for 40 patients, 31 in the TZP–vancomycin group and 9 in the cefepime–vancomycin group.
cNo estimation possible; resolution of AKI did not vary within groups.
Other Analyses
Three patients, all in the TZP group, had unresolved AKI within 2 months after the discontinuation of antibiotics, although it should be noted that each of those patients died relatively quickly after AKI occurred as a result of sequelae of their chronic disease. Overall, we found no difference between groups in terms of proportions with resolution of AKI (Table 4).
We performed a subgroup analysis within each of our 3 chronic disease (oncologic disease, cystic fibrosis, and congenital heart disease) groups (Supplementary Table 2). Because of the small sample sizes, it was not possible to estimate the association between treatment group and AKI for patients with cystic fibrosis or oncologic disease. In contrast, among those with congenital heart disease, the risk of AKI was 3 times higher in the TZP group than in the cefepime group (RR, 3.3 [95% CI, 1.4–7.7]; P = .006). In patients with none of the 3 aforementioned chronic conditions, the risk of AKI was borderline statistically significantly higher in the TZP group than in the cefepime group (RR, 8.0 [95% CI, 1.0–64.0]; P = .05).
DISCUSSION
In this large retrospective matched-cohort study of hospitalized children, exposure to vancomycin and TZP was associated with a 2.5-fold greater risk of AKI than was exposure to vancomycin and cefepime (multivariate conditional Poisson RR, 2.5 [95% CI, 1.1–5.8]; P = .03). To our knowledge, this study provides the first evidence from a matched-cohort study of a difference in the incidences of AKI among a diverse pediatric population treated with these antibiotic combinations. We found the incidence of nephrotoxicity in patients treated with vancomycin–TZP to be 21% higher than that associated with vancomycin–cefepime combination therapy, which resulted in a number needed to harm of 4.8. It should be noted that our institution previously published data regarding the baseline incidence of AKI in our critical care and non–critical care patient populations. The incidence of AKI in patients under critical care was found to be at least 28%, whereas the incidence of AKI in our non–critically ill patients was found to be at least 5% [19, 20].
Our secondary analysis included the patients with congenital heart disease, malignancy, or cystic fibrosis. An increased rate of AKI was seen in patients with congenital heart disease treated with vancomycin and TZP over that seen in those treated with vancomycin and cefepime (47.9% vs 14.6%, respectively). It is possible that these patients had an underlying renal insult caused by decreased renal perfusion that was exacerbated by the vancomycin–TZP antibiotic combination, because their rates of AKI were higher in both antibiotic combination groups than those in our overall study population. We could not assess differences in the patients with cystic fibrosis or malignancy because of the small sample sizes.
A recent study compared the incidences of AKI among children with cystic fibrosis treated with vancomycin and tobramycin in combination with either TZP or cefepime and found a higher incidence of AKI in the TZP group than in the cefepime group (55% vs 13%, respectively; P < .0001) [21]. As previously mentioned, Downes et al [14] published the results of a retrospective cohort study that compared the incidences of AKI among pediatric patients receiving vancomycin in combination with either TZP or another antipseudomonal β-lactam antibiotic. These authors found the risk of AKI with the coadministration of vancomycin and TZP (11.7%) to be higher than that with vancomycin in combination with another antipseudomonal β-lactam antibiotic (4.4%), which echoes the findings of our study. Another recent pediatric study found a higher risk of nephrotoxicity among patients treated with vancomycin and TZP than among those treated with vancomycin only (23.6% vs 3.8%, respectively) [13]. However, because of a difference in indications for the antibiotic between the treatment groups, whether the difference in the incidences of AKI was a result of drug exposure or the underlying illness was unclear. Our study confirming these findings was facilitated by a cefepime shortage, which required the use of TZP for many indications (eg, febrile neutropenia, sepsis rule-out in the pediatric cardiac intensive care unit, and cystic fibrosis exacerbations) for which cefepime has historically been the preferred agent. This therapeutic substitution resulted in patients with similar underlying conditions having different drug exposures, which reduced confounding according to indication and provided additional evidence that vancomycin–TZP is a more nephrotoxic combination than is vancomycin–cefepime.
In our study, the TZP and cefepime treatment groups were similar in terms of their weight-adjusted daily vancomycin doses (median, 43.9 vs 45 mg/kg per day, respectively) and highest vancomycin trough levels during treatment (median, 13 vs 12 µg/mL, respectively). Despite these similarities, the rate of AKI in the TZP group was significantly higher than that in the cefepime group (28.9% vs 7.9%, respectively; P < .001). The lengths of stay were also longer in the TZP group than in the cefepime group (median, 45 vs 24 days, respectively; P = .001), which might be a result of increased AKI rates among those treated with vancomycin and TZP, more severe illness among those treated with vancomycin and TZP (despite matching for chronic disease), or the combination of these factors. In this study, AKI was reversible within 2 months of the discontinuation of combination therapy with vancomycin and TZP, although the actual times to renal function normalization were not captured.
The results of this study highlight the adverse consequences of antibiotic use. Although the combination of vancomycin and TZP is widely prescribed for hospitalized patients and might be indicated in those with a suspected drug-resistant, severe, or complex infection, it can cause harm in those who have minimal risk for resistant or polymicrobial infections. The significant increase in the risk of AKI with this combination should weigh heavily in the selection of empiric antibiotics. In those patients receiving vancomycin–TZP therapy, our institution recommends close monitoring of renal function and measuring vancomycin trough levels every 3 days instead of once per week. These data support the activities of clinicians and antimicrobial stewardship programs in pursuing prompt deescalation of antibiotics and thoughtful empiric antibiotic selection because of the collateral damage associated with this antibiotic combination.
A secondary finding of our study is that antibiotic shortages can lead to substantial changes in antibiotic prescribing that, in turn, can be harmful to patients. Gross et al [22] recently found that the nationwide TZP shortage was associated with increased Clostridium difficile infection rates in adults. The unintended consequences of antibiotic shortages are difficult to predict and warrant additional study. Having data that quantify the harms of medication shortages might motivate and assist regulatory agencies in formulating policies and working with drug manufacturers to minimize medication shortages.
The mechanism through which TZP causes more nephrotoxicity than cefepime is not understood. We suspect that TZP might preferentially lead to AKI in patients with underlying renal disease, as it did in our cohort; the majority of our patients weighed ≤20 kg, had a history of congenital heart disease or recent surgical correction, or were at risk for decreased renal perfusion. The mechanism through which vancomycin causes nephrotoxicity is better understood, because it is known to alter mitochondrial function and cause proliferation of proximal tubular cells, thereby increasing oxidative stress as a means of causing nephrotoxicity [23]. The median vancomycin trough concentrations found in our study population were not supratherapeutic for either treatment group, so we do not believe that high trough concentrations contributed to the nephrotoxicity seen with TZP treatment. Interstitial nephritis is a commonly recognized adverse effect of TZP, other β-lactam antibiotics, and vancomycin, typically in the setting of vancomycin-associated drug reaction with eosinophilia and systemic symptoms (DRESS). Several features are atypical for interstitial nephritis in the setting of our study, including the short time to nephrotoxicity and the rapid reversal in most cases after withdrawal of the drug. Interesting to note is that neither vancomycin nor TZP is a newly approved medication, and both of them have been used in combination for >20 years; however, the description of heightened toxicity with their combination has been a more recent observation, and the interaction between these drugs, including those that result from a new manufacturing process or mode of administration, needs to be considered. A recent study by Karino et al [24] found that extending the infusion of TZP did not change the incidence of AKI in those who received intermittent infusions versus extended infusions. Therefore, the mechanism through which the combination of TZP and vancomycin causes a greater incidence of nephrotoxicity than do other antibiotic combinations or either medication alone requires additional study.
This study had several limitations. The data were collected retrospectively at 1 center and might not be generalizable to other geographic regions with different patient populations. Also, because of the retrospective nature of this study, we were unable to evaluate long-term consequences of AKI attributed to the combination of vancomycin and either TZP or cefepime. All medication administration times were assumed to have been documented accurately unless specifically noted in the electronic health record. Eleven of 42 patients with AKI experienced it within 1 calendar day after the initiation of their combination of antibiotics; however, exact times of the serum creatinine measurements were not collected, so we are unable to comment on which of these patients experienced AKI within 24 hours of combination antibiotic initiation. Residual confounding between groups might exist, because the patients were matched according to their age, sex, and chronic illness but not their indication for antibiotic therapy or severity of illness. The majority of the cohort included in this study weighed ≤20 kg at antibiotic initiation, so our findings might not be generalizable to older children. The median ages at initiation of combination antibiotics were significantly different between the 2 groups, but this difference might be explained by the fact that patients were not matched exactly according to age but, rather, by a range in ages. In addition, only a small number of patients with cystic fibrosis or malignancy were included. We did not collect data on patients who were excluded; therefore, we cannot comment on the incidence of AKI in excluded patients or to determine what, if any, difference exists between patients who were included and those who were excluded. We also did not evaluate exposure to potential nephrotoxic medications before the initiation of antibiotic combination therapy, and we did not evaluate the duration of vancomycin therapy before the start of combination therapy, which might influence the development of AKI. Despite these limitations, a strength of this study is that the groups were well balanced largely because during a portion of the study time frame, the choice of β-lactam was driven by the cefepime shortage rather than by provider preference.
In conclusion, we found that vancomycin–TZP combination therapy was associated with a higher incidence of AKI than was vancomycin–cefepime combination therapy among pediatric patients at our institution. These results are consistent with those of previous recent studies conducted in adults and children. Prescribers should be aware that vancomycin–TZP combination therapy is associated with reversible renal injury, and frequent monitoring of renal function and vancomycin levels should be performed for patients who are receiving this combination.
Notes
Financial support. This work was supported by the National Institutes of Health (grant KL2 TR 000446 to S. L. V. D. and grant UL1 TR000445 for support to the Vanderbilt Institute for Clinical and Translational Research) and the Burroughs Wellcome Fund (grant IRSA 1015006 to S. L. V. D). E. J. P. receives funding through the National Institutes of Health (grants 1P50GM115305-01, 1R01AI103348-01, 1P30AI110527-01A1, 5T32AI007474-20, and 1 R13AR71267-01), National Health and Medical Research Council of Australia, and the Australian Centre for HIV and Hepatitis Virology Research.
Potential conflicts of interest. S. L. V. D. has received honoraria from Merck as an invited speaker. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.



