-
PDF
- Split View
-
Views
-
Cite
Cite
Chikara Ogimi, Janet A Englund, Miranda C Bradford, Xuan Qin, Michael Boeckh, Alpana Waghmare, Characteristics and Outcomes of Coronavirus Infection in Children: The Role of Viral Factors and an Immunocompromised State, Journal of the Pediatric Infectious Diseases Society, Volume 8, Issue 1, March 2019, Pages 21–28, https://doi.org/10.1093/jpids/pix093
Close - Share Icon Share
Abstract
Immunocompromised children might be predisposed to serious infections from human coronaviruses (HCoVs), including strains OC43, NL63, HKU1, and 229E; however, the virologic and clinical features of HCoV infection in immunocompromised children have not been compared to those in nonimmunocompromised children.
We retrospectively analyzed a cohort of children who presented to Seattle Children’s Hospital and in whom HCoV was detected by a multiplex respiratory polymerase chain reaction assay of a nasal sample between October 2012 and March 2016. Lower respiratory tract disease (LRTD) was defined as possible or definite infiltrate seen in chest imaging, need for oxygen, or abnormal lung examination in conjunction with a physician diagnosis of LRTD. We used logistic regression modeling to evaluate risk factors for LRTD and LRTD that necessitated oxygen use (severe LRTD), including an immunocompromised state, in children with HCoV infection.
The median ages of 85 immunocompromised and 1152 nonimmunocompromised children with HCoV infection were 6.3 and 1.6 years, respectively. The prevalence of LRTD and of severe LRTD did not differ greatly between the immunocompromised and nonimmunocompromised patients (22% vs 26% [LRTD] and 15% vs 11% [severe LRTD], respectively); however, in a multivariable model, an immunocompromised state was associated with an increased likelihood of severe LRTD (adjusted odds ratio, 2.5 [95% confidence interval, 1.2–4.9]; P = .01). Younger age, having an underlying pulmonary disorder, and the presence of respiratory syncytial virus were also associated with LRTD or severe LRTD in multivariable models. The risks of LRTD or severe LRTD did not differ among the children with different HCoV strains.
The presence of a copathogen and host factors, including an immunocompromised state, were associated with increased risk for severe LRTD. Recognizing risk factors for severe respiratory illness might assist in risk stratification.
Human coronaviruses (HCoVs), including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronaviruses, are serious respiratory pathogens [1–4]. Four other common strains of HCoV (OC43, NL63, HKU1, and 229E) are important causes of upper respiratory tract disease and lower respiratory tract disease (LRTD) [5, 6]. With the development and widespread use of new sensitive molecular diagnostic techniques, such as the polymerase chain reaction (PCR) assay [7, 8], we now have a better understanding of the human pathogenicity of these 4 strains in children [9–11]. However, comprehensive analyses of differences in clinical features among the 4 HCoV strains in children have been limited because of small sample sizes, incomplete strain evaluations, and the potential presence of respiratory copathogens. Data from a recent study suggested that HCoV-related LRTD is associated with high rates of oxygen use and death in immunocompromised hosts; however, that study included only 1 pediatric patient [12]. Immunocompromised children might be predisposed to serious HCoV infection, as seen with other respiratory viruses [13, 14]. The purpose of this study was to compare the clinical characteristics of HCoV infection and its outcomes in immunocompromised and nonimmunocompromised children. We also investigated host and virologic factors associated with LRTD.
METHODS
Study Design
We retrospectively identified all pediatric patients who presented to Seattle Children’s Hospital for acute care and in whom HCoV was detected between October 2012 and March 2016. HCoV was identified in clinical nasal samples using a multiplex respiratory PCR assay (FilmArray [BioFire Diagnostics, Salt Lake City, Utah]) that can detect 20 respiratory viral and bacterial agents, including 4 HCoV strains (OC43, NL63, HKU1, and 229E) [15]. The patients underwent respiratory viral testing as clinically indicated at the time of their evaluation at Seattle Children’s Hospital regardless of their immunocompromised status. We included only patients who presented with their first episode of HCoV infection and excluded patients whose respiratory viral tests were performed after hospitalization. Demographic and clinical data were collected from the electronic medical database and chart review. This study was approved by the Seattle Children’s Hospital Institutional Review Board.
Definitions
HCoV-related LRTD was defined as abnormal lung examination results (tachypnea, retraction, wheezing, crackles, or coarse breath sounds), new possible or definite pulmonary infiltrates found in chest imaging, or need for supplemental oxygen in conjunction with a physician diagnosis (eg, bronchiolitis, pneumonia) at the time of presentation [16]. Physical findings and oxygen use within 24 hours and chest imaging within 48 hours of presentation were included in the analyses. Severe LRTD was defined as LRTD that necessitated oxygen use. We defined an immunocompromised state as the presence of primary immunodeficiency or receipt of chemotherapy for treatment of hematologic malignancy or solid tumor, chronic immunosuppressive therapy, solid organ transplant, or hematopoietic cell transplant [13]. Nonimmunocompromised children could have other types of underlying disorders, as previously described [9, 16]. Any virus or bacteria in nasal samples detected by multiplex respiratory PCR at presentation was considered to be a respiratory copathogen. Other coinfections were determined by chart review and microbiologic results obtained within 48 hours of presentation. In immunocompromised children, the highest daily steroid doses and lowest cell counts in the 2 weeks before HCoV infection were recorded.
Statistical Analysis
Demographic and clinical characteristics of immunocompromised and nonimmunocompromised patients were summarized using counts and percentages or medians and ranges, as appropriate. The primary outcomes were LRTD and severe LRTD. To evaluate associations with virologic and host factors, we calculated 95% confidence intervals (CIs) for the percentages of patients in risk-factor categories with each primary outcome and performed bivariate logistic regression modeling for each outcome and covariate. Associations between immunocompromised status and primary outcomes were estimated using bivariate and multivariable logistic regression modeling. Logistic regression modeling was used for exploratory analyses to investigate associations between the primary outcomes and cell counts in the immunocompromised patients and to assess how adjusting for demographic and clinical factors affected the estimated association between viral strain and the primary outcomes. Covariates in the multivariable models included sex, age (<1, 1–5 years, or >5 years), presence of an underlying pulmonary disorder (yes or no), and presence of a respiratory copathogen (HCoV only, respiratory syncytial virus [RSV], or non-RSV). To investigate interactions between the HCoV strain and other respiratory copathogens, we calculated 95% CIs for the percentage of patients with LRTD in groups defined by viral strain and the presence of a non-HCoV copathogen (yes or no). All tests were 2 sided, and we used an α level of .05 without correction for multiple testing. Analyses were performed using Stata 12.1 (Stata Corp, College Station, Texas).
RESULTS
Patient and Viral Characteristics
We identified 1308 patients with a first episode of HCoV infection. After excluding 71 patients whose respiratory viral tests were performed after hospitalization, 1237 patients were included in the final analyses. Of these, 85 were immunocompromised and 1152 were nonimmunocompromised. Demographic characteristics and clinical presentation of these patients are listed in Table 1. Demographic characteristics were similar between the immunocompromised and nonimmunocompromised groups with the exception of median age, which was higher among the immunocompromised children (6.3 vs 1.6 years). The proportions of underlying disorders (other than an immunocompromised state) and site of initial presentation were similar. Patients with a hematologic malignancy or solid tumor made up the largest proportion of immunocompromised children (51%), followed by solid organ transplant recipients (19%).
Demographics and Clinical Presentation of Patients With Human Coronavirus Infection
| Characteristics . | Immunocompromised Children (N = 85) (n [%] or Median [Range]) . | Nonimmunocompromised Children (N = 1152) (n [%] or Median [Range]) . |
|---|---|---|
| Demographic | ||
| Female | 39 (46) | 515 (45) |
| Age (y) | 6 (1–21) | 2 (0–20) |
| Age categories | ||
| <1 y | 3 (4) | 429 (37) |
| 1–5 y | 30 (35) | 502 (44) |
| >5 y | 52 (61) | 221 (19) |
| Ethnicity | ||
| Non-Hispanic | 62 (73) | 907 (79) |
| Hispanic | 21 (25) | 203 (18) |
| Unknown | 2 (2) | 42 (4) |
| Underlying disorder(s) | 32 (38) | 438 (38) |
| Pulmonary | 13 (15) | 226 (20) |
| Renal/gastrointestinal | 13 (15) | 154 (13) |
| Neuromuscular | 8 (9) | 113 (10) |
| Metabolic/genetic | 6 (7) | 84 (7) |
| Cardiovascular | 2 (2) | 56 (5) |
| Prematuritya | 1 (1) | 38 (3) |
| Hematology | 0 (0) | 18 (2) |
| Reason for immunocompromised state | ||
| Solid organ transplant | 16 (19) | — |
| Hematopoietic cell transplant | 10 (12) | — |
| Hematologic malignancy/ solid tumor | 43 (51) | — |
| Chronic use of immunosuppressive agents | 10 (12) | — |
| Primary immunodeficiency | 6 (7) | — |
| Initial treatment location | ||
| Emergency department | 70 (82) | 798 (69) |
| Urgent care center | 1 (1) | 200 (17) |
| Clinic | 5 (6) | 43 (4) |
| Outside hospital | 9 (11) | 111 (10) |
| Clinical presentation | ||
| Fever | 63 (74) | 852 (74) |
| Cough | 53 (62) | 864 (75) |
| Sore throat or rhinorrhea | 61 (72) | 871 (76) |
| No respiratory symptoms | 15 (18) | 125 (11) |
| Wheeze | 8 (9) | 144 (13) |
| Abnormal respiratory signs or examination other than wheezeb | 17 (20) | 384 (33) |
| Abnormal findings in chest imaging | 12/31 (39) | 101/361 (28) |
| Bacteremia or candidemia | 4 (5) | 10 (1) |
| Human coronavirus strain(s)c | ||
| OC43 | 33 (39) | 562 (49) |
| NL63 | 23 (27) | 311 (27) |
| HKU1 | 23 (27) | 256 (22) |
| 229E | 10 (12) | 54 (5) |
| Respiratory copathogen(s) | ||
| Human coronavirus onlyd | 66 (78) | 797 (69) |
| RSV present | 5 (6) | 151 (13) |
| Copathogens other than RSV | 14 (16) | 204 (18) |
| White blood cell count > 1000 × 106 cells/Le | 53/77 (69) | — |
| Absolute neutrophil count > 500 × 106 cells/Le | 51/74 (69) | — |
| Absolute lymphocyte count > 300 × 106 cells/Le | 52/74 (70) | — |
| Absolute monocyte count > 300 × 106 cells/Le | 34/74 (46) | — |
| Characteristics . | Immunocompromised Children (N = 85) (n [%] or Median [Range]) . | Nonimmunocompromised Children (N = 1152) (n [%] or Median [Range]) . |
|---|---|---|
| Demographic | ||
| Female | 39 (46) | 515 (45) |
| Age (y) | 6 (1–21) | 2 (0–20) |
| Age categories | ||
| <1 y | 3 (4) | 429 (37) |
| 1–5 y | 30 (35) | 502 (44) |
| >5 y | 52 (61) | 221 (19) |
| Ethnicity | ||
| Non-Hispanic | 62 (73) | 907 (79) |
| Hispanic | 21 (25) | 203 (18) |
| Unknown | 2 (2) | 42 (4) |
| Underlying disorder(s) | 32 (38) | 438 (38) |
| Pulmonary | 13 (15) | 226 (20) |
| Renal/gastrointestinal | 13 (15) | 154 (13) |
| Neuromuscular | 8 (9) | 113 (10) |
| Metabolic/genetic | 6 (7) | 84 (7) |
| Cardiovascular | 2 (2) | 56 (5) |
| Prematuritya | 1 (1) | 38 (3) |
| Hematology | 0 (0) | 18 (2) |
| Reason for immunocompromised state | ||
| Solid organ transplant | 16 (19) | — |
| Hematopoietic cell transplant | 10 (12) | — |
| Hematologic malignancy/ solid tumor | 43 (51) | — |
| Chronic use of immunosuppressive agents | 10 (12) | — |
| Primary immunodeficiency | 6 (7) | — |
| Initial treatment location | ||
| Emergency department | 70 (82) | 798 (69) |
| Urgent care center | 1 (1) | 200 (17) |
| Clinic | 5 (6) | 43 (4) |
| Outside hospital | 9 (11) | 111 (10) |
| Clinical presentation | ||
| Fever | 63 (74) | 852 (74) |
| Cough | 53 (62) | 864 (75) |
| Sore throat or rhinorrhea | 61 (72) | 871 (76) |
| No respiratory symptoms | 15 (18) | 125 (11) |
| Wheeze | 8 (9) | 144 (13) |
| Abnormal respiratory signs or examination other than wheezeb | 17 (20) | 384 (33) |
| Abnormal findings in chest imaging | 12/31 (39) | 101/361 (28) |
| Bacteremia or candidemia | 4 (5) | 10 (1) |
| Human coronavirus strain(s)c | ||
| OC43 | 33 (39) | 562 (49) |
| NL63 | 23 (27) | 311 (27) |
| HKU1 | 23 (27) | 256 (22) |
| 229E | 10 (12) | 54 (5) |
| Respiratory copathogen(s) | ||
| Human coronavirus onlyd | 66 (78) | 797 (69) |
| RSV present | 5 (6) | 151 (13) |
| Copathogens other than RSV | 14 (16) | 204 (18) |
| White blood cell count > 1000 × 106 cells/Le | 53/77 (69) | — |
| Absolute neutrophil count > 500 × 106 cells/Le | 51/74 (69) | — |
| Absolute lymphocyte count > 300 × 106 cells/Le | 52/74 (70) | — |
| Absolute monocyte count > 300 × 106 cells/Le | 34/74 (46) | — |
Abbreviation: RSV, respiratory syncytial virus.
aThis child had a gestational age of ≤34 weeks.
bDyspnea, tachypnea, retraction, stridor, crackles, or coarse breath sound.
cThe number of patients (1237) did not equal the sum of detections for each human coronavirus strain (1272) because of the detection of multiple strains in some subjects. At the time of presentation, 2 strains were detected in 31 patients, and 3 strains were detected in 2 patients.
dPatients with multiple human coronavirus strains were included in this category.
eLowest counts in the 2 weeks before the first human coronavirus detection.
Demographics and Clinical Presentation of Patients With Human Coronavirus Infection
| Characteristics . | Immunocompromised Children (N = 85) (n [%] or Median [Range]) . | Nonimmunocompromised Children (N = 1152) (n [%] or Median [Range]) . |
|---|---|---|
| Demographic | ||
| Female | 39 (46) | 515 (45) |
| Age (y) | 6 (1–21) | 2 (0–20) |
| Age categories | ||
| <1 y | 3 (4) | 429 (37) |
| 1–5 y | 30 (35) | 502 (44) |
| >5 y | 52 (61) | 221 (19) |
| Ethnicity | ||
| Non-Hispanic | 62 (73) | 907 (79) |
| Hispanic | 21 (25) | 203 (18) |
| Unknown | 2 (2) | 42 (4) |
| Underlying disorder(s) | 32 (38) | 438 (38) |
| Pulmonary | 13 (15) | 226 (20) |
| Renal/gastrointestinal | 13 (15) | 154 (13) |
| Neuromuscular | 8 (9) | 113 (10) |
| Metabolic/genetic | 6 (7) | 84 (7) |
| Cardiovascular | 2 (2) | 56 (5) |
| Prematuritya | 1 (1) | 38 (3) |
| Hematology | 0 (0) | 18 (2) |
| Reason for immunocompromised state | ||
| Solid organ transplant | 16 (19) | — |
| Hematopoietic cell transplant | 10 (12) | — |
| Hematologic malignancy/ solid tumor | 43 (51) | — |
| Chronic use of immunosuppressive agents | 10 (12) | — |
| Primary immunodeficiency | 6 (7) | — |
| Initial treatment location | ||
| Emergency department | 70 (82) | 798 (69) |
| Urgent care center | 1 (1) | 200 (17) |
| Clinic | 5 (6) | 43 (4) |
| Outside hospital | 9 (11) | 111 (10) |
| Clinical presentation | ||
| Fever | 63 (74) | 852 (74) |
| Cough | 53 (62) | 864 (75) |
| Sore throat or rhinorrhea | 61 (72) | 871 (76) |
| No respiratory symptoms | 15 (18) | 125 (11) |
| Wheeze | 8 (9) | 144 (13) |
| Abnormal respiratory signs or examination other than wheezeb | 17 (20) | 384 (33) |
| Abnormal findings in chest imaging | 12/31 (39) | 101/361 (28) |
| Bacteremia or candidemia | 4 (5) | 10 (1) |
| Human coronavirus strain(s)c | ||
| OC43 | 33 (39) | 562 (49) |
| NL63 | 23 (27) | 311 (27) |
| HKU1 | 23 (27) | 256 (22) |
| 229E | 10 (12) | 54 (5) |
| Respiratory copathogen(s) | ||
| Human coronavirus onlyd | 66 (78) | 797 (69) |
| RSV present | 5 (6) | 151 (13) |
| Copathogens other than RSV | 14 (16) | 204 (18) |
| White blood cell count > 1000 × 106 cells/Le | 53/77 (69) | — |
| Absolute neutrophil count > 500 × 106 cells/Le | 51/74 (69) | — |
| Absolute lymphocyte count > 300 × 106 cells/Le | 52/74 (70) | — |
| Absolute monocyte count > 300 × 106 cells/Le | 34/74 (46) | — |
| Characteristics . | Immunocompromised Children (N = 85) (n [%] or Median [Range]) . | Nonimmunocompromised Children (N = 1152) (n [%] or Median [Range]) . |
|---|---|---|
| Demographic | ||
| Female | 39 (46) | 515 (45) |
| Age (y) | 6 (1–21) | 2 (0–20) |
| Age categories | ||
| <1 y | 3 (4) | 429 (37) |
| 1–5 y | 30 (35) | 502 (44) |
| >5 y | 52 (61) | 221 (19) |
| Ethnicity | ||
| Non-Hispanic | 62 (73) | 907 (79) |
| Hispanic | 21 (25) | 203 (18) |
| Unknown | 2 (2) | 42 (4) |
| Underlying disorder(s) | 32 (38) | 438 (38) |
| Pulmonary | 13 (15) | 226 (20) |
| Renal/gastrointestinal | 13 (15) | 154 (13) |
| Neuromuscular | 8 (9) | 113 (10) |
| Metabolic/genetic | 6 (7) | 84 (7) |
| Cardiovascular | 2 (2) | 56 (5) |
| Prematuritya | 1 (1) | 38 (3) |
| Hematology | 0 (0) | 18 (2) |
| Reason for immunocompromised state | ||
| Solid organ transplant | 16 (19) | — |
| Hematopoietic cell transplant | 10 (12) | — |
| Hematologic malignancy/ solid tumor | 43 (51) | — |
| Chronic use of immunosuppressive agents | 10 (12) | — |
| Primary immunodeficiency | 6 (7) | — |
| Initial treatment location | ||
| Emergency department | 70 (82) | 798 (69) |
| Urgent care center | 1 (1) | 200 (17) |
| Clinic | 5 (6) | 43 (4) |
| Outside hospital | 9 (11) | 111 (10) |
| Clinical presentation | ||
| Fever | 63 (74) | 852 (74) |
| Cough | 53 (62) | 864 (75) |
| Sore throat or rhinorrhea | 61 (72) | 871 (76) |
| No respiratory symptoms | 15 (18) | 125 (11) |
| Wheeze | 8 (9) | 144 (13) |
| Abnormal respiratory signs or examination other than wheezeb | 17 (20) | 384 (33) |
| Abnormal findings in chest imaging | 12/31 (39) | 101/361 (28) |
| Bacteremia or candidemia | 4 (5) | 10 (1) |
| Human coronavirus strain(s)c | ||
| OC43 | 33 (39) | 562 (49) |
| NL63 | 23 (27) | 311 (27) |
| HKU1 | 23 (27) | 256 (22) |
| 229E | 10 (12) | 54 (5) |
| Respiratory copathogen(s) | ||
| Human coronavirus onlyd | 66 (78) | 797 (69) |
| RSV present | 5 (6) | 151 (13) |
| Copathogens other than RSV | 14 (16) | 204 (18) |
| White blood cell count > 1000 × 106 cells/Le | 53/77 (69) | — |
| Absolute neutrophil count > 500 × 106 cells/Le | 51/74 (69) | — |
| Absolute lymphocyte count > 300 × 106 cells/Le | 52/74 (70) | — |
| Absolute monocyte count > 300 × 106 cells/Le | 34/74 (46) | — |
Abbreviation: RSV, respiratory syncytial virus.
aThis child had a gestational age of ≤34 weeks.
bDyspnea, tachypnea, retraction, stridor, crackles, or coarse breath sound.
cThe number of patients (1237) did not equal the sum of detections for each human coronavirus strain (1272) because of the detection of multiple strains in some subjects. At the time of presentation, 2 strains were detected in 31 patients, and 3 strains were detected in 2 patients.
dPatients with multiple human coronavirus strains were included in this category.
eLowest counts in the 2 weeks before the first human coronavirus detection.
At the time of presentation, no respiratory symptoms were noted in 140 (11%) children; however, the majority of these patients (107 [76%] of 140) had a history of fever. The seasonal frequencies of each HCoV strain are shown in Figure 1. All strains were present from December through March, and cocirculation of all 4 strains was seen every year. OC43 was the most common strain except in the 2015–2016 season, when HKU1 was the most common. It is notable that 2 strains were detected in 31 (2.5%) patients and 3 strains were detected in 2 (0.2%) patients (Table 1) at presentation. The distributions of strains between the immunocompromised and nonimmunocompromised children were similar (Table 1). Respiratory copathogens were detected more commonly in the nonimmunocompromised group than in the immunocompromised group (31% vs 22%, respectively), mostly because of the increased detection of respiratory syncytial virus (RSV) (13% vs 6%, respectively) (Figure 2).
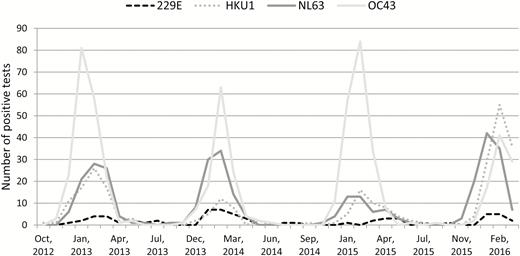
Seasonal frequencies of human coronavirus strains, Seattle Children’s Hospital, 2012–2016.
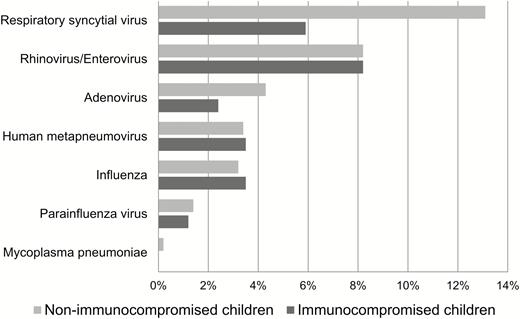
Respiratory copathogens in children with human coronavirus infection. Two respiratory copathogens were detected in 33 patients, and 3 were detected in 1 patient.
The main acute illness diagnoses for children in the immunocompromised and nonimmunocompromised groups are listed in Supplementary Table 1. Upper respiratory tract infection was the most common diagnosis in immunocompromised (36%) and nonimmunocompromised (46%) children.
Risk Factors for LRTD and Severe LRTD
Younger age, male sex, presence of an underlying pulmonary disorder, and detection of a respiratory copathogen, particularly RSV, were associated with an increased likelihood of LRTD or severe LRTD in bivariate logistic regression models (Table 2). With the exception of male sex, these predictors remained statistically significant in a multivariable logistic regression model (Table 3). Compared to the presence of HCoV only, RSV was associated with more than 5 times the risk of LRTD and severe LRTD in the multivariable model (adjusted odds ratios [aORs], 7.7 [95% CI, 5.2–11.4] and 5.9 [95% CI 3.7–9.3], respectively). The presence of an underlying pulmonary disorder was the next-strongest risk factor, with aORs of 5.9 (95% CI, 4.1–8.5) for LRTD and 4.5 (95% CI, 2.9–6.9) for severe LRTD. The prevalence of LRTD and of severe LRTD did not differ greatly between the immunocompromised and nonimmunocompromised patients (22% vs 26% and 15% vs 11%, respectively), but after adjusting for demographic and clinical covariates, an immunocompromised state was associated with an increased likelihood of severe LRTD (aOR, 2.5 [95% CI, 1.2–4.9]; P = .01). Nine children were defined as having severe LRTD on the sole basis of their need for oxygen.
Bivariate (Unadjusted) Associations Between Primary Outcomes and Demographic and Clinical Covariates (N = 1237)
| Covariates . | Children With LRTD . | Children With Severe LRTD . | ||||
|---|---|---|---|---|---|---|
| % of LRTD (95% CI) . | OR (95% CI) . | P . | % of severe LRTD (95% CI) . | OR (95% CI) . | P . | |
| Age | .01 | .55 | ||||
| <1 y (reference) | 29 (25–33) | 10 (8–13) | ||||
| 1–5 y | 28 (24–32) | 1.0 (0.7–1.3) | .70 | 12 (10–15) | 1.2 (0.8–1.8) | |
| >5 y | 19 (15–24) | 0.6 (0.4–0.8) | <.01 | 10 (7–14) | 1.0 (0.6–1.6) | |
| Sex | ||||||
| Male (reference) | 29 (26–33) | 11 (8–13) | ||||
| Female | 23 (19–26) | 0.7 (0.6–0.9) | .01 | 11 (9–14) | 1.1 (0.8–1.6) | .64 |
| Underlying pulmonary disorder(s) | ||||||
| No (reference) | 21 (19–24) | 8 (7–10) | ||||
| Yes | 48 (41–54) | 3.4 (2.6–4.6) | <.01 | 22 (17–28) | 3.2 (2.2–4.7) | <.01 |
| Immunocompromised state | ||||||
| No (reference) | 26 (24–29) | 11 (9–13) | ||||
| Yes | 22 (15–32) | 0.8 (0.5–1.4) | .41 | 15 (9–24) | 1.5 (0.8–2.8) | .18 |
| HCoV strain(s) | .30 | .18 | ||||
| OC43 only (reference) | 25 (22–29) | 10 (8–13) | ||||
| NL63 only | 26 (21–31) | 1.0 (0.8–1.4) | 10 (7–13) | 1.0 (0.6–1.5) | ||
| HKU1 only | 30 (25–36) | 1.3 (0.9–1.8) | 13 (9–18) | 1.3 (0.8–2.1) | ||
| 229E only | 29 (19–41) | 1.2 (0.7–2.1) | 19 (11–30) | 2.1 (1.1–4.3) | ||
| Multiple | 15 (7–31) | 0.5 (0.2–1.4) | 12 (5–27) | 1.3 (0.4–3.7) | ||
| Respiratory copathogen(s)a | <.01 | <.01 | ||||
| HCoV only (reference) | 19 (16–22) | 8 (6–10) | ||||
| RSV present | 62 (54–69) | 6.9 (4.8–9.9) | <.01 | 29 (22–36) | 4.8 (3.1–7.4) | <.01 |
| Copathogens other than RSV | 30 (24–36) | 1.8 (1.3–2.6) | <.01 | 11 (7–15) | 1.4 (0.9–2.3) | .19 |
| Covariates . | Children With LRTD . | Children With Severe LRTD . | ||||
|---|---|---|---|---|---|---|
| % of LRTD (95% CI) . | OR (95% CI) . | P . | % of severe LRTD (95% CI) . | OR (95% CI) . | P . | |
| Age | .01 | .55 | ||||
| <1 y (reference) | 29 (25–33) | 10 (8–13) | ||||
| 1–5 y | 28 (24–32) | 1.0 (0.7–1.3) | .70 | 12 (10–15) | 1.2 (0.8–1.8) | |
| >5 y | 19 (15–24) | 0.6 (0.4–0.8) | <.01 | 10 (7–14) | 1.0 (0.6–1.6) | |
| Sex | ||||||
| Male (reference) | 29 (26–33) | 11 (8–13) | ||||
| Female | 23 (19–26) | 0.7 (0.6–0.9) | .01 | 11 (9–14) | 1.1 (0.8–1.6) | .64 |
| Underlying pulmonary disorder(s) | ||||||
| No (reference) | 21 (19–24) | 8 (7–10) | ||||
| Yes | 48 (41–54) | 3.4 (2.6–4.6) | <.01 | 22 (17–28) | 3.2 (2.2–4.7) | <.01 |
| Immunocompromised state | ||||||
| No (reference) | 26 (24–29) | 11 (9–13) | ||||
| Yes | 22 (15–32) | 0.8 (0.5–1.4) | .41 | 15 (9–24) | 1.5 (0.8–2.8) | .18 |
| HCoV strain(s) | .30 | .18 | ||||
| OC43 only (reference) | 25 (22–29) | 10 (8–13) | ||||
| NL63 only | 26 (21–31) | 1.0 (0.8–1.4) | 10 (7–13) | 1.0 (0.6–1.5) | ||
| HKU1 only | 30 (25–36) | 1.3 (0.9–1.8) | 13 (9–18) | 1.3 (0.8–2.1) | ||
| 229E only | 29 (19–41) | 1.2 (0.7–2.1) | 19 (11–30) | 2.1 (1.1–4.3) | ||
| Multiple | 15 (7–31) | 0.5 (0.2–1.4) | 12 (5–27) | 1.3 (0.4–3.7) | ||
| Respiratory copathogen(s)a | <.01 | <.01 | ||||
| HCoV only (reference) | 19 (16–22) | 8 (6–10) | ||||
| RSV present | 62 (54–69) | 6.9 (4.8–9.9) | <.01 | 29 (22–36) | 4.8 (3.1–7.4) | <.01 |
| Copathogens other than RSV | 30 (24–36) | 1.8 (1.3–2.6) | <.01 | 11 (7–15) | 1.4 (0.9–2.3) | .19 |
Abbreviations: CI, confidence interval; OR, odds ratio; HCoV, human coronavirus; LRTD, lower respiratory tract disease; RSV, respiratory syncytial virus.
aPatients with multiple HCoV strains are included in this category.
Bivariate (Unadjusted) Associations Between Primary Outcomes and Demographic and Clinical Covariates (N = 1237)
| Covariates . | Children With LRTD . | Children With Severe LRTD . | ||||
|---|---|---|---|---|---|---|
| % of LRTD (95% CI) . | OR (95% CI) . | P . | % of severe LRTD (95% CI) . | OR (95% CI) . | P . | |
| Age | .01 | .55 | ||||
| <1 y (reference) | 29 (25–33) | 10 (8–13) | ||||
| 1–5 y | 28 (24–32) | 1.0 (0.7–1.3) | .70 | 12 (10–15) | 1.2 (0.8–1.8) | |
| >5 y | 19 (15–24) | 0.6 (0.4–0.8) | <.01 | 10 (7–14) | 1.0 (0.6–1.6) | |
| Sex | ||||||
| Male (reference) | 29 (26–33) | 11 (8–13) | ||||
| Female | 23 (19–26) | 0.7 (0.6–0.9) | .01 | 11 (9–14) | 1.1 (0.8–1.6) | .64 |
| Underlying pulmonary disorder(s) | ||||||
| No (reference) | 21 (19–24) | 8 (7–10) | ||||
| Yes | 48 (41–54) | 3.4 (2.6–4.6) | <.01 | 22 (17–28) | 3.2 (2.2–4.7) | <.01 |
| Immunocompromised state | ||||||
| No (reference) | 26 (24–29) | 11 (9–13) | ||||
| Yes | 22 (15–32) | 0.8 (0.5–1.4) | .41 | 15 (9–24) | 1.5 (0.8–2.8) | .18 |
| HCoV strain(s) | .30 | .18 | ||||
| OC43 only (reference) | 25 (22–29) | 10 (8–13) | ||||
| NL63 only | 26 (21–31) | 1.0 (0.8–1.4) | 10 (7–13) | 1.0 (0.6–1.5) | ||
| HKU1 only | 30 (25–36) | 1.3 (0.9–1.8) | 13 (9–18) | 1.3 (0.8–2.1) | ||
| 229E only | 29 (19–41) | 1.2 (0.7–2.1) | 19 (11–30) | 2.1 (1.1–4.3) | ||
| Multiple | 15 (7–31) | 0.5 (0.2–1.4) | 12 (5–27) | 1.3 (0.4–3.7) | ||
| Respiratory copathogen(s)a | <.01 | <.01 | ||||
| HCoV only (reference) | 19 (16–22) | 8 (6–10) | ||||
| RSV present | 62 (54–69) | 6.9 (4.8–9.9) | <.01 | 29 (22–36) | 4.8 (3.1–7.4) | <.01 |
| Copathogens other than RSV | 30 (24–36) | 1.8 (1.3–2.6) | <.01 | 11 (7–15) | 1.4 (0.9–2.3) | .19 |
| Covariates . | Children With LRTD . | Children With Severe LRTD . | ||||
|---|---|---|---|---|---|---|
| % of LRTD (95% CI) . | OR (95% CI) . | P . | % of severe LRTD (95% CI) . | OR (95% CI) . | P . | |
| Age | .01 | .55 | ||||
| <1 y (reference) | 29 (25–33) | 10 (8–13) | ||||
| 1–5 y | 28 (24–32) | 1.0 (0.7–1.3) | .70 | 12 (10–15) | 1.2 (0.8–1.8) | |
| >5 y | 19 (15–24) | 0.6 (0.4–0.8) | <.01 | 10 (7–14) | 1.0 (0.6–1.6) | |
| Sex | ||||||
| Male (reference) | 29 (26–33) | 11 (8–13) | ||||
| Female | 23 (19–26) | 0.7 (0.6–0.9) | .01 | 11 (9–14) | 1.1 (0.8–1.6) | .64 |
| Underlying pulmonary disorder(s) | ||||||
| No (reference) | 21 (19–24) | 8 (7–10) | ||||
| Yes | 48 (41–54) | 3.4 (2.6–4.6) | <.01 | 22 (17–28) | 3.2 (2.2–4.7) | <.01 |
| Immunocompromised state | ||||||
| No (reference) | 26 (24–29) | 11 (9–13) | ||||
| Yes | 22 (15–32) | 0.8 (0.5–1.4) | .41 | 15 (9–24) | 1.5 (0.8–2.8) | .18 |
| HCoV strain(s) | .30 | .18 | ||||
| OC43 only (reference) | 25 (22–29) | 10 (8–13) | ||||
| NL63 only | 26 (21–31) | 1.0 (0.8–1.4) | 10 (7–13) | 1.0 (0.6–1.5) | ||
| HKU1 only | 30 (25–36) | 1.3 (0.9–1.8) | 13 (9–18) | 1.3 (0.8–2.1) | ||
| 229E only | 29 (19–41) | 1.2 (0.7–2.1) | 19 (11–30) | 2.1 (1.1–4.3) | ||
| Multiple | 15 (7–31) | 0.5 (0.2–1.4) | 12 (5–27) | 1.3 (0.4–3.7) | ||
| Respiratory copathogen(s)a | <.01 | <.01 | ||||
| HCoV only (reference) | 19 (16–22) | 8 (6–10) | ||||
| RSV present | 62 (54–69) | 6.9 (4.8–9.9) | <.01 | 29 (22–36) | 4.8 (3.1–7.4) | <.01 |
| Copathogens other than RSV | 30 (24–36) | 1.8 (1.3–2.6) | <.01 | 11 (7–15) | 1.4 (0.9–2.3) | .19 |
Abbreviations: CI, confidence interval; OR, odds ratio; HCoV, human coronavirus; LRTD, lower respiratory tract disease; RSV, respiratory syncytial virus.
aPatients with multiple HCoV strains are included in this category.
| Covariates . | LRTD . | Severe LRTD . | ||
|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Age categories (vs <1 y) | <.01 | .29 | ||
| 1–5 y | 0.6 (0.4–0.8) | 0.8 (0.5–1.2) | ||
| >5 y | 0.3 (0.2–0.5) | 0.6 (0.3–1.1) | ||
| Female | 0.8 (0.6–1.1) | .13 | 1.3 (0.9–1.9) | .15 |
| Underlying pulmonary disorder | 5.9 (4.1–8.5) | <.01 | 4.5 (2.9–6.9) | <.01 |
| Immunocompromised state | 1.5 (0.8–2.8) | .17 | 2.5 (1.2–4.9) | .01 |
| Respiratory copathogen(s) (vs HCoV onlya) | <.01 | <.01 | ||
| RSV present | 7.7 (5.2–11.4) | 5.9 (3.7–9.3) | ||
| Copathogen(s) other than RSV | 1.8 (1.3–2.6) | 1.4 (0.8–2.3) | ||
| Covariates . | LRTD . | Severe LRTD . | ||
|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Age categories (vs <1 y) | <.01 | .29 | ||
| 1–5 y | 0.6 (0.4–0.8) | 0.8 (0.5–1.2) | ||
| >5 y | 0.3 (0.2–0.5) | 0.6 (0.3–1.1) | ||
| Female | 0.8 (0.6–1.1) | .13 | 1.3 (0.9–1.9) | .15 |
| Underlying pulmonary disorder | 5.9 (4.1–8.5) | <.01 | 4.5 (2.9–6.9) | <.01 |
| Immunocompromised state | 1.5 (0.8–2.8) | .17 | 2.5 (1.2–4.9) | .01 |
| Respiratory copathogen(s) (vs HCoV onlya) | <.01 | <.01 | ||
| RSV present | 7.7 (5.2–11.4) | 5.9 (3.7–9.3) | ||
| Copathogen(s) other than RSV | 1.8 (1.3–2.6) | 1.4 (0.8–2.3) | ||
Abbreviations: CI, confidence interval; HCoV, human coronavirus; LRTD, lower respiratory tract disease; OR, odds ratio; RSV, respiratory syncytial virus.
aPatients with multiple HCoV strains are included in this category.
| Covariates . | LRTD . | Severe LRTD . | ||
|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Age categories (vs <1 y) | <.01 | .29 | ||
| 1–5 y | 0.6 (0.4–0.8) | 0.8 (0.5–1.2) | ||
| >5 y | 0.3 (0.2–0.5) | 0.6 (0.3–1.1) | ||
| Female | 0.8 (0.6–1.1) | .13 | 1.3 (0.9–1.9) | .15 |
| Underlying pulmonary disorder | 5.9 (4.1–8.5) | <.01 | 4.5 (2.9–6.9) | <.01 |
| Immunocompromised state | 1.5 (0.8–2.8) | .17 | 2.5 (1.2–4.9) | .01 |
| Respiratory copathogen(s) (vs HCoV onlya) | <.01 | <.01 | ||
| RSV present | 7.7 (5.2–11.4) | 5.9 (3.7–9.3) | ||
| Copathogen(s) other than RSV | 1.8 (1.3–2.6) | 1.4 (0.8–2.3) | ||
| Covariates . | LRTD . | Severe LRTD . | ||
|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Age categories (vs <1 y) | <.01 | .29 | ||
| 1–5 y | 0.6 (0.4–0.8) | 0.8 (0.5–1.2) | ||
| >5 y | 0.3 (0.2–0.5) | 0.6 (0.3–1.1) | ||
| Female | 0.8 (0.6–1.1) | .13 | 1.3 (0.9–1.9) | .15 |
| Underlying pulmonary disorder | 5.9 (4.1–8.5) | <.01 | 4.5 (2.9–6.9) | <.01 |
| Immunocompromised state | 1.5 (0.8–2.8) | .17 | 2.5 (1.2–4.9) | .01 |
| Respiratory copathogen(s) (vs HCoV onlya) | <.01 | <.01 | ||
| RSV present | 7.7 (5.2–11.4) | 5.9 (3.7–9.3) | ||
| Copathogen(s) other than RSV | 1.8 (1.3–2.6) | 1.4 (0.8–2.3) | ||
Abbreviations: CI, confidence interval; HCoV, human coronavirus; LRTD, lower respiratory tract disease; OR, odds ratio; RSV, respiratory syncytial virus.
aPatients with multiple HCoV strains are included in this category.
In unadjusted logistic regression models, the risks of LRTD and severe LRTD did not differ among the children with different HCoV strains (Table 2); this lack of association remained after adjusting for demographic and clinical covariates (P = .16 and .12, respectively). Among nonimmunocompromised patients, the prevalence of LRTD was consistently higher for patients coinfected with a non-HCoV respiratory pathogen regardless of HCoV strain (Figure 3). Among the immunocompromised patients, the prevalence of LRTD did not appear to be affected by the presence of a copathogen, regardless of the HCoV strain, although small sample sizes resulted in wide CIs. The prevalence of LRTD seemed somewhat higher with beta-coronaviruses (OC43 and HKU1) than with alpha-coronaviruses (NL63 and 229E); however, this result did not reach statistical significance (Supplementary Figure 1).
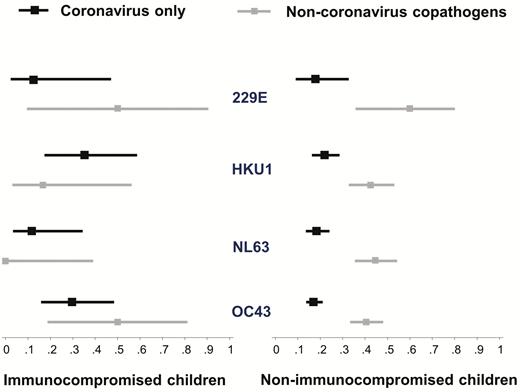
Proportions (square markers) and 95% confidence intervals (bars) of lower respiratory tract disease according to human coronavirus strain and immunocompromised status.
Of 66 immunocompromised children who did not present with LRTD, 2 developed LRTD within 4 days of presentation. Three nonimmunocompromised patients died within 28 days of presentation; 2 of those deaths might have been associated with HCoV infection. The first of these 2 deaths was of a 4-month-old boy with a history of diffuse hypotonia who was admitted for acute-on-chronic respiratory failure in the setting of severe LRTD associated with HCoV strain OC43. On day 24 of admission, care was withdrawn because of his underlying disorder and poor clinical course. The second of these deaths was of a 7-year-old boy with a history of severe asthma who suffered from cardiopulmonary arrest, likely in the setting of status asthmatics associated with HCoV strain 229E. Care was withdrawn on day 3 of admission in response to his brain death, respiratory failure, and multiple organ failure.
A white blood cell count was ordered for 77 of the immunocompromised patients in the 2 weeks before HCoV detection, and neutrophil, lymphocyte, and monocyte counts were measured for 74 of them. Cell counts were not found to be associated with LRTD or severe LRTD in the multivariable model (Supplementary Table 2).
Other Outcomes
The frequencies of outcomes other than LRTD and severe LRTD in the immunocompromised and nonimmunocompromised groups are listed in Supplementary Table 3. The rate of hospitalization in immunocompromised children was higher than that in nonimmunocompromised children (58% vs 39%, respectively); however, the rates of hospitalization that resulted from respiratory distress were similar between the 2 groups (15% vs 20%, respectively). Of the hospitalized patients, febrile illness accounted for approximately half of the indications for hospitalization in the immunocompromised children (24 [49%] of 49) but only 12% (53 of 444) in the nonimmunocompromised children. In 53 children who were hospitalized for febrile illness (including 16 patients with febrile neutropenia), no other etiology for fever other than respiratory viruses was discovered, and the majority of these patients (48 [91%] of 53) received antibiotic treatment. Fever was the only symptom in 5 immunocompromised children with febrile neutropenia and in 8 nonimmunocompromised children during their entire hospitalization.
DISCUSSION
With the widespread use of molecular diagnostic techniques, the epidemiology and clinical features of 4 common HCoV strains (OC43, NL63, HKU1, and 229E) in children have increasingly been reported [17–21]. However, few large systematic studies have addressed host and viral factors for severe respiratory illness caused by HCoV, and even fewer data describing outcomes in immunocompromised children are available. In this study, we evaluated the risk factors for LRTD and severe LRTD in a large cohort of pediatric patients, including a significant number of immunocompromised children. Younger age and presence of an underlying pulmonary disorder, a respiratory copathogen (especially RSV), and an immunocompromised state were associated with increased risk of LRTD or severe LRTD in multivariable models. Striking seasonality was found; all 4 strains were present predominantly from December through March every year, and clinical outcomes among patients infected with each of the 4 strains were similar.
The expansion of immunocompromised populations has raised concern about whether these patients are at risk for severe HCoV respiratory tract disease [22, 23]. Recent data suggested a significant association between HCoV-related LRTD and a need for respiratory support and increased risk of death in immunocompromised adults [12]. Previous pediatric studies of HCoV infection have included small numbers of immunocompromised children [10, 24, 25]; few data from studies that focused specifically on this high-risk population exist. In our study, an immunocompromised state was associated with a higher risk of severe LRTD after we adjusted for clinical and virologic covariates. This observation is important, because HCoVs are highly prevalent in immunocompromised hosts [25, 26], and our finding might be informative for risk stratification and interventional studies in this vulnerable population.
In this study, we also found that younger age, presence of an underlying pulmonary disorder, and detection of a respiratory copathogen were associated with LRTD or severe LRTD. The age distributions in the immunocompromised and nonimmunocompromised groups differed. We assume that the broader age distribution in the immunocompromised group reflects the overall age distribution of the immunocompromised population, although these data were not available to us. A prospective longitudinal cohort study examined the effect of 3 HCoV strains on children younger than 5 years and found a substantial burden of LRTD, especially in children younger than 2 years [27]. Lee and Storch [9] characterized clinical features of hospitalized children younger than 5 years infected with HCoV strains OC43 and NL63. The presence of congenital heart disease and a chronic respiratory disorder was associated with severe disease. These reports are consistent with our findings. The overall rates of respiratory copathogens in our study (22% in immunocompromised children and 31% in nonimmunocompromised children) are lower than those of previous studies in children (35%–75%) [9, 28–30]. Conflicting data regarding the clinical implications of detecting multiple respiratory viruses exist. In children, detection of multiple viruses has been associated with less-severe disease [31], but the opposite result was reported for adults [32]. The specific combination of respiratory copathogens might be important. In this study, we found that compared to HCoV alone, the odds of LRTD or severe LRTD were much higher in children with RSV coinfection than in those with coinfection with a non-RSV copathogen. This result was likely driven by the higher pathogenicity of RSV, as indicated by previous studies [9, 28]. Higher rates of severe LRTD in patients with HCoV plus a copathogen were also found in another pediatric study; RSV accounted for half of the copathogens [19].
Although there is increased recognition of the 4 common strains of HCoV as being pathogenic, a consensus view on the clinical differences among them has not been established because of limited sample sizes, the presence of unexamined strains, and the frequent presence of respiratory copathogens in previous reports. To our knowledge, ours is the largest study to have investigated the associations between particular HCoV strains and clinical outcomes in children. Neither LRTD nor severe LRTD was associated with a particular HCoV strain after we adjusted for covariates, including presence of respiratory copathogens. We were not able to show a particular effect of strain type in the subset analysis of immunocompromised children, although our data do not suggest an effect on acquisition or disease severity. We did find marked seasonality of the HCoV strains, with higher rates of detection for all 4 strains in winter but much lower rates in the other seasons of every year. This effect was not driven by testing bias, because our institution uses multiplex PCR testing for respiratory pathogens year-round. The overall seasonality trend is consistent with that shown in other reports [10, 27, 33].
In our study, 19 immunocompromised and 34 nonimmunocompromised children admitted for febrile illness regardless of respiratory symptoms were not found to have a febrile source other than HCoV and a respiratory copathogen. The majority of these patients received antibiotics as treatment. In other pediatric studies, respiratory viruses, including HCoV, were detected by PCR frequently in febrile children without localizing signs [9, 13]. Furthermore, respiratory viral infection was implicated recently as playing a possible etiologic role in febrile neutropenia, although the detection of respiratory viruses seemed to be associated more with the presence of respiratory symptoms [34, 35]. Differentiating active infection from asymptomatic shedding is clinically challenging, especially in high risk populations. Future studies that clarify the role of respiratory viruses in patients with fever without a source or with febrile neutropenia might provide useful information for decreasing the rates of hospitalization and antibiotic usage.
The main limitations of this study are the retrospective observational nature of the analyses and the relatively small size of the immunocompromised cohort. Prolonged shedding of respiratory viruses, including HCoV, in asymptomatic immunocompromised and nonimmunocompromised children has been well described [36–38]. Two recent large prospective studies of symptomatic patients with respiratory viruses compared to asymptomatic controls suggested that most of the HCoV detections were associated with symptomatic illness [11, 39]. Nonetheless, the presence of HCoV does not necessarily indicate its etiologic role in clinical symptoms. Furthermore, testing bias by the attending physician was still possible despite the fact that we investigated only patients who visited our institution for acute care. However, most physicians would have a lower threshold for testing (ie, for less severe presentations) in a population at high risk; thus, the positive relationship between an immunocompromised state and severe LRTD would be weakened rather than accentuated. Therefore, a testing bias would be unlikely to alter our interpretation of the role of the immunocompromised state. Last, we were not able to evaluate the viral load in respiratory samples because of the nature of multiplex respiratory PCR test.
In summary, we found an association between virologic and host factors, including an immunocompromised state, and an increased risk of serious HCoV-related LRTD in children. LRTD was associated with the presence of a non-HCoV respiratory copathogen, especially RSV, but not with a particular HCoV strain. Given the universal distribution of HCoVs, recognition of risk factors for severity of respiratory illness might assist in patient risk stratification. Additional studies are warranted. Differentiating active respiratory virus infection from asymptomatic shedding in febrile patients might be useful in decision making around antibiotic use.
Notes
Acknowledgments. We thank Jennifer Phillips for database services and Katherine Carson for assistance with chart review.
Financial support. This work was supported by a Pediatric Infectious Diseases Society fellowship award funded by Horizon Pharma (to C. O.), the National Institutes of Health (grants K24HL093294 [to M. B.], K23 AI114844 [to A. W.], and T32HD00723332 [to C. O.]), and the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1 TR000423 (to M. C. B.).
Potential conflicts of interest. M. C. B. received research support and served as a consultant for Gilead Sciences, Merck, Ansun Bioscience, and Aviragen Therapeutics and served as a consultant for Humabs Biomed; and J. A. E. received research support from GlaxoSmithKline, Gilead, Pfizer, MedImmune, Novavax, and Chimerix and served as a consultant for Gilead Sciences, Inc. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. declare no relevant conflict of interest.



