-
PDF
- Split View
-
Views
-
Cite
Cite
Shikha Saxena, Jerica Gee, Sarah Klieger, Adriana Kajon, Hans Petersen, Theoklis Zaoutis, Brian Fisher, Invasive Fungal Disease in Pediatric Solid Organ Transplant Recipients, Journal of the Pediatric Infectious Diseases Society, Volume 7, Issue 3, September 2018, Pages 219–225, https://doi.org/10.1093/jpids/pix041
Close - Share Icon Share
Abstract
Solid organ transplant (SOT) recipients are at risk for invasive fungal disease (IFD). Data on IFD burden in pediatric patients are limited. We aimed to determine the incidence and outcome of IFD in a large cohort of pediatric patients who underwent SOT.
A single-center cohort of pediatric patients who underwent SOT between 2000 and 2013 was assembled retrospectively. The patients were followed for 180 days after transplant or until death to determine the presence or absence of IFD. The 2008 European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group Consensus Group criteria were used to define IFD as proven or probable. The incidence of IFD, all-cause mortality rate, and case-fatality rate at 180 days were calculated.
Among 584 pediatric patients who underwent SOT, 13 patients sustained 14 episodes of IFD (candidiasis, aspergillosis, and mucormycosis). The overall incidence was 2.2% (14.3 IFD events per 100000 patient-days). The IFD rates according to transplant type were 12.5% (1 of 8) (heart/lung), 11.4% (4 of 35) (lung), 4.7% (8 of 172) (liver), 0% (0 of 234) (kidney), and 0% (0 of 135) (heart). Three patients with IFD (2 lung and 1 heart/lung) died, and all these deaths were deemed likely attributable to the IFD; the case-fatality rate was 21.4% (3 of 14).
The overall incidence of IFD in these pediatric SOT recipients was low but varied across transplant type, with heart/lung and lung recipients having the highest IFD rate. Given the attributable case-fatality rate, the risk of death resulting from IFD is potentially high. More data on groups at higher risk, such as lung transplant recipients, are needed to guide targeted antifungal prophylaxis.
Pediatric solid organ transplant (SOT) recipients sustain immune suppression from the surgical procedure and from immunosuppressive agents administered to prevent graft rejection. This composite immunocompromised state leaves them at risk for developing opportunistic infections such as invasive fungal disease (IFD), which potentially can cause significant morbidity and death. A number of contemporary publications have documented the burden of IFD in adult SOT recipients. The Transplant-Associated Infection Surveillance Network (TRANSNET) prospectively reviewed a predominantly adult cohort of SOT recipients from 2001 to 2006 and reported a 12-month cumulative IFD incidence of 3.1%. The most common IFD was invasive candidiasis (56%), followed by invasive aspergillosis (19%) [1]. In a separate single-center adult study, Pugliese et al [2] identified 46 (16.5%) IFD events in a cohort of 278 SOT recipients. Candida albicans was the most common pathogen, followed by other Candida species and Aspergillus flavus. Infection was associated with a case-fatality rate of 35% (16 of 46) compared to a mortality rate of 3.5% (8 of 232) in the patients without IFD.
Reports that document the incidence of IFD in pediatric SOT recipients have been limited. Recent pediatric publications focused on only a specific fungal pathogen [3–5] or type of organ transplant [4–8]. Knapp et al [9], using data from TRANSNET, presented pediatric-specific IFD rates at the 50th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy in 2010. They found 49 episodes of IFD among 41 pediatric SOT recipients, which represented 3% of all SOT recipients in the TRANSNET cohort. However, a detailed report of these data has not been published. Furthermore, we found no published contemporary reports describing IFD in SOT patients using the definitions created by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) [10].
We aimed to establish the incidence and outcome of proven and probable IFD using the EORTC/MSG criteria in a cohort of pediatric SOT recipients from a large hospital in the United States. Establishing the burden of IFD in pediatric SOT recipients is important for multiple reasons. An estimation of infection risk can enhance anticipatory guidance provided to patients undergoing transplantation and their families and can inform front-line clinicians caring for SOT recipients in the posttransplant period. These data also can aid pediatric infectious diseases transplant clinicians in determining the need for fungal disease prevention initiatives.
METHODS
Study Design and Population
A single-center cohort of pediatric SOT recipients was assembled retrospectively to determine the incidence of and attributable mortality rate from proven and probable IFD after transplant. Patients younger than 19 years who underwent an SOT at the Children’s Hospital of Philadelphia between January 1, 2000, and December 31, 2013, were included in the study cohort. The study cohort was divided into 1 of 2 time epics, 2000–2006 or 2007–2013, to explore whether the incidence of IFD might have changed over time. Patients were grouped according to the type of organ transplanted. Patients who underwent a multivisceral organ transplant were categorized as follows: combined kidney/liver recipients (n = 3) were included with liver recipients, combined kidney/heart recipients (n = 1) were included with heart recipients, and combined heart/lung recipients (n = 8) were assigned to their own group. There was a separate 2-center cohort study on IFD that included liver transplant recipients from our current study [5].
In this population of pediatric SOT recipients, immunosuppressive therapies varied across transplant types but remained consistent during the study period. Lung, heart, and kidney transplant recipients typically were given antithymocyte globulin and methylprednisolone for induction followed by mycophenolate and tacrolimus for maintenance. Mycophenolate was substituted by azathioprine in a subset of lung and heart recipients, and tacrolimus was substituted by cyclosporine in a subset of heart recipients. Liver transplant recipients were given methylprednisolone for induction followed by tacrolimus alone or tacrolimus and mycophenolate for maintenance.
Each patient was followed from the day of transplant (day 0) until 180 days after the transplant, he or she was lost to follow-up, or he or she died, whichever date came first. The transplants undergone by patients who had received more than 1 SOT during the study period were counted as separate incidents for each transplant, as long as the subsequent transplant was 180 days or more from the previous transplant. Patients who died within 24 hours of transplant were excluded, because they likely died secondary to complications of the transplant procedure and thus could not contribute at-risk time to the denominator. Patients with incomplete laboratory data were also excluded.
Outcome and Definitions
The outcome of the study was proven or probable IFD determined by using the published EORTC/MSG criteria [10]. Briefly, proven IFD is defined as recovery of a mold or yeast by culture or histopathology from a tissue specimen obtained in sterile fashion and associated with a clinical or radiologic abnormality consistent with an infectious disease process; a blood culture that yielded a mold or yeast in the context of a compatible infectious disease process; or discovery of a cryptococcal antigen in cerebrospinal fluid on serologic analysis.
Designation of probable IFD requires clinical and mycological factors in an immunocompromised host. Clinical criteria include evidence of a fungal infection on radiographic examination (such as chest computed tomography findings of a dense well-circumscribed lesion with or without a halo sign; air crescent sign; or cavity) or evidence of infection by direct clinical visualization (such as nasal ulcer with black eschar). Mycological criteria include direct evidence of a fungal pathogen from a sterile site (via cytology, direct microscopy, or culture) or indirect evidence of a pathogen (such as a positive galactomannan antigen detected in plasma, serum, bronchoalveolar lavage fluid, or cerebrospinal fluid or β-d-glucan detected in serum). Galactomannan antigen and β-d-glucan testing was available to clinicians during the study period, but rather than being used for surveillance testing, they were ordered by physicians in the setting of clinical suspicion for an IFD. The outcome of possible IFD was not included in the calculation of IFD incidence for this study, because many of the radiologic criteria are nonspecific and can be present in this patient population with other comorbid conditions.
Patients with multiple positive blood cultures for the same fungal pathogen were considered to have 1 IFD episode if the cultures revealed the same organism within 14 days based on the 14-day repeat-infection time frame set forth by the Centers for Disease Control and Prevention [11]. If the same organism was identified after 14 days, or if a different organism was identified within 14 days, the patient was considered to have had a distinct IFD event.
Antifungal Prophylaxis
Administration of antifungal prophylaxis was not routine for patients in any of the organ groups during the study period; however, some clinicians elect to administer prophylaxis. To determine intention to provide prophylaxis, antifungal therapy administration during the first 5 days after the transplant was reviewed for each patient. Primary antifungal prophylaxis is defined as initiation of an antifungal medication in this time period without clinical, laboratory, or radiographic evidence to support IFD. Secondary prophylaxis is defined as administration of an antifungal medication in a patient with previous infection or colonization with a fungal pathogen (including receipt of a colonized donor organ) but without current active infection. Previous infection or colonization was determined by reviewing laboratory results and physician notes from the 30 days before transplant to the date of the transplant.
Data Abstraction
Using a structured case-report form, demographic data, including sex, date of birth, race, SOT type, transplant date, and date of last known status, were collected for all patients in the cohort. Patients were followed for 180 days from transplant to determine the presence or absence of an IFD. In applying the definitions of proven and probable IFD, results of all cultures, pathology, and indirect mycology testing were reviewed for each SOT recipient during the study time frame. Autopsy reports, if available, were reviewed also to assess for the presence of an IFD. The chart of patients with positive mycology testing results were further reviewed for radiographic and clinical evidence in support of an IFD. Clinical data were gathered from physician progress notes, and the medication-administration record was reviewed to determine antifungal exposure.
Statistical Analysis
The incidences of IFD are reported as the rate of IFDs per patient and the rate of IFDs per patient-days follow-up. The rate of IFDs per patient was calculated by dividing the number of patients with proven or probable IFD by the total number of patients in the cohort, and the rate of IFDs per patient-days follow-up was calculated by indexing the number of IFD episodes by the cumulative number of follow-up days for the study cohort and standardizing to 100000 follow-up days. IFD rates are reported along with rates of primary and secondary antifungal prophylaxis. All-cause mortality and case-fatality rates at 180 days were calculated and are reported as a rate of death per patient. Each death of a patient with IFD was determined to be unlikely, possibly, or likely attributable to the IFD. These rates were calculated for the entire patient cohort, for each SOT subgroup, for the entire study duration, and for each time epic (2000–2006 and 2007–2013).
RESULTS
Characteristics of the Study Subjects
In this retrospective cohort, 584 subjects met the eligibility criteria for study inclusion. Demographic and clinical characteristics for the assembled cohort are presented in Table 1. The median age at transplant was 9.79 years (range, 0.04–18.92 years), 56% of the patients were male, and the majority race was white (61%). The kidney was the most common organ transplanted (n = 234) followed by the liver, heart, lung, and combined heart/lung. Of the 35 lung transplant recipients, 13 underwent a transplant as a result of cystic fibrosis (37%).
Demographic and Clinical Characteristics for Single-Center Transplant Cohort (2000–2013)
| Characteristic . | Entire Cohort . | Kidney Recipients . | Liver Recipients . | Heart Recipients . | Lung Recipients . | Heart/Lung Recipients . |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| No. of patients | 584 | 234 | 172a | 135b | 35 | 8 |
| Age (median [range]) (y) | 9.79 (0.04–18.92) | 12.99 (1.11–18.92) | 2.81 (0.24–17.84) | 7.20 (0.04–18.29) | 11.32 (0.52–18.51) | 6.10 (1.11–15.39) |
| Male sex (n [%]) | 328 (56) | 144 (62) | 97 (56) | 72 (53) | 10 (29) | 5 (63) |
| Race (n [%]) | ||||||
| White | 356 (61) | 149 (64) | 107 (62) | 75 (56) | 21 (60) | 4 (50) |
| Black or African American | 127 (22) | 54 (23) | 31 (18) | 36 (27) | 3 (9) | 3 (38) |
| Asian | 20 (3) | 8 (3) | 9 (5) | 2 (1) | 1 (3) | 0 (0) |
| Unknown | 79 (14) | 23 (10) | 24 (14) | 21 (16) | 10 (29) | 1 (13) |
| >1 | 2 (<1) | 0 (0) | 1 (0.01) | 1 (0.01) | 0 (0) | 0 (0) |
| Cumulative patient-days (n) | 97801 | 40517 | 29098 | 21402 | 5777 | 1008 |
| Incidence of IFD | ||||||
| Patients with IFD (n/N [%]) | 13/584 (2.2) | 0 (0) | 8/172 (4.7) | 0 (0) | 4/35 (11.4) | 1/8 (12.5) |
| IFD events per 100000 patient-days | 14.3 | 0 (0) | 27.5 | 0 (0) | 86.6 | 99.2 |
| Characteristic . | Entire Cohort . | Kidney Recipients . | Liver Recipients . | Heart Recipients . | Lung Recipients . | Heart/Lung Recipients . |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| No. of patients | 584 | 234 | 172a | 135b | 35 | 8 |
| Age (median [range]) (y) | 9.79 (0.04–18.92) | 12.99 (1.11–18.92) | 2.81 (0.24–17.84) | 7.20 (0.04–18.29) | 11.32 (0.52–18.51) | 6.10 (1.11–15.39) |
| Male sex (n [%]) | 328 (56) | 144 (62) | 97 (56) | 72 (53) | 10 (29) | 5 (63) |
| Race (n [%]) | ||||||
| White | 356 (61) | 149 (64) | 107 (62) | 75 (56) | 21 (60) | 4 (50) |
| Black or African American | 127 (22) | 54 (23) | 31 (18) | 36 (27) | 3 (9) | 3 (38) |
| Asian | 20 (3) | 8 (3) | 9 (5) | 2 (1) | 1 (3) | 0 (0) |
| Unknown | 79 (14) | 23 (10) | 24 (14) | 21 (16) | 10 (29) | 1 (13) |
| >1 | 2 (<1) | 0 (0) | 1 (0.01) | 1 (0.01) | 0 (0) | 0 (0) |
| Cumulative patient-days (n) | 97801 | 40517 | 29098 | 21402 | 5777 | 1008 |
| Incidence of IFD | ||||||
| Patients with IFD (n/N [%]) | 13/584 (2.2) | 0 (0) | 8/172 (4.7) | 0 (0) | 4/35 (11.4) | 1/8 (12.5) |
| IFD events per 100000 patient-days | 14.3 | 0 (0) | 27.5 | 0 (0) | 86.6 | 99.2 |
Abbreviation: IFD, invasive fungal disease.
aIncludes 3 kidney/liver recipients.
bIncludes 1 kidney/heart recipient.
Demographic and Clinical Characteristics for Single-Center Transplant Cohort (2000–2013)
| Characteristic . | Entire Cohort . | Kidney Recipients . | Liver Recipients . | Heart Recipients . | Lung Recipients . | Heart/Lung Recipients . |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| No. of patients | 584 | 234 | 172a | 135b | 35 | 8 |
| Age (median [range]) (y) | 9.79 (0.04–18.92) | 12.99 (1.11–18.92) | 2.81 (0.24–17.84) | 7.20 (0.04–18.29) | 11.32 (0.52–18.51) | 6.10 (1.11–15.39) |
| Male sex (n [%]) | 328 (56) | 144 (62) | 97 (56) | 72 (53) | 10 (29) | 5 (63) |
| Race (n [%]) | ||||||
| White | 356 (61) | 149 (64) | 107 (62) | 75 (56) | 21 (60) | 4 (50) |
| Black or African American | 127 (22) | 54 (23) | 31 (18) | 36 (27) | 3 (9) | 3 (38) |
| Asian | 20 (3) | 8 (3) | 9 (5) | 2 (1) | 1 (3) | 0 (0) |
| Unknown | 79 (14) | 23 (10) | 24 (14) | 21 (16) | 10 (29) | 1 (13) |
| >1 | 2 (<1) | 0 (0) | 1 (0.01) | 1 (0.01) | 0 (0) | 0 (0) |
| Cumulative patient-days (n) | 97801 | 40517 | 29098 | 21402 | 5777 | 1008 |
| Incidence of IFD | ||||||
| Patients with IFD (n/N [%]) | 13/584 (2.2) | 0 (0) | 8/172 (4.7) | 0 (0) | 4/35 (11.4) | 1/8 (12.5) |
| IFD events per 100000 patient-days | 14.3 | 0 (0) | 27.5 | 0 (0) | 86.6 | 99.2 |
| Characteristic . | Entire Cohort . | Kidney Recipients . | Liver Recipients . | Heart Recipients . | Lung Recipients . | Heart/Lung Recipients . |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| No. of patients | 584 | 234 | 172a | 135b | 35 | 8 |
| Age (median [range]) (y) | 9.79 (0.04–18.92) | 12.99 (1.11–18.92) | 2.81 (0.24–17.84) | 7.20 (0.04–18.29) | 11.32 (0.52–18.51) | 6.10 (1.11–15.39) |
| Male sex (n [%]) | 328 (56) | 144 (62) | 97 (56) | 72 (53) | 10 (29) | 5 (63) |
| Race (n [%]) | ||||||
| White | 356 (61) | 149 (64) | 107 (62) | 75 (56) | 21 (60) | 4 (50) |
| Black or African American | 127 (22) | 54 (23) | 31 (18) | 36 (27) | 3 (9) | 3 (38) |
| Asian | 20 (3) | 8 (3) | 9 (5) | 2 (1) | 1 (3) | 0 (0) |
| Unknown | 79 (14) | 23 (10) | 24 (14) | 21 (16) | 10 (29) | 1 (13) |
| >1 | 2 (<1) | 0 (0) | 1 (0.01) | 1 (0.01) | 0 (0) | 0 (0) |
| Cumulative patient-days (n) | 97801 | 40517 | 29098 | 21402 | 5777 | 1008 |
| Incidence of IFD | ||||||
| Patients with IFD (n/N [%]) | 13/584 (2.2) | 0 (0) | 8/172 (4.7) | 0 (0) | 4/35 (11.4) | 1/8 (12.5) |
| IFD events per 100000 patient-days | 14.3 | 0 (0) | 27.5 | 0 (0) | 86.6 | 99.2 |
Abbreviation: IFD, invasive fungal disease.
aIncludes 3 kidney/liver recipients.
bIncludes 1 kidney/heart recipient.
Proven and Probable IFD Rates
We found 14 episodes of proven or probable IFD in 13 patients, resulting in an IFD incidence of 2.2% (13 of 584), or 14.3 IFD events per 100000 patient-days. Combined heart/lung and lung-only recipients had the highest rates of IFD, and liver recipients had the second-highest rate. None of the 13 lung transplant recipients with cystic fibrosis sustained an IFD. We also found no IFD events in kidney and heart-only recipients (Table 1). Clinical details for each episode of IFD are presented in Table 2. Invasive candidiasis accounted for the majority of IFD events (11 [79%] of 14 events), followed by invasive aspergillosis (2 [14%] of 14 events) and mucormycosis (1 [7%] of 14 events). No episodes of cryptococcosis, endemic mycosis, or other mold or yeast occurred. All IFD episodes were proven except for 1, which was classified as probable.
| Transplant Type . | Pathogen . | Site of Infection . | Days From Transplant to Diagnosis . | Status at 180 Days . |
|---|---|---|---|---|
| Heart/lung | Candida parapsilosis | Blood | 95 | Died on day 101a |
| Lung | Aspergillus spp | Multiple organs on pathology and cultureb | 19 | Died on day 19a |
| Lung | Aspergillus niger | BAL fluidc | 129 | Alive |
| Lung | Candida parapsilosis | Blood | 35 | Died on day 127a |
| Blood and braind | 119 | |||
| Lung | Mucor spp | Bloode | 67 | Alive |
| Liver | Candida albicans | Blood and liver tissue | 0f | Alive |
| Liver | Candida albicans | Blood | 2 | Alive |
| Liver | Candida albicans | Blood | 6 | Alive |
| Liver | Candida albicans | Blood | 6 | Alive |
| Liver | Candida albicans | Liver tissue | 44 | Alive |
| Liver | Candida albicans | Peritoneal fluid | 18 | Alive |
| Liver | Candida albicans | Blood | 107 | Alive |
| Liver | Candida guilliermondii | Blood | 7 | Alive |
| Transplant Type . | Pathogen . | Site of Infection . | Days From Transplant to Diagnosis . | Status at 180 Days . |
|---|---|---|---|---|
| Heart/lung | Candida parapsilosis | Blood | 95 | Died on day 101a |
| Lung | Aspergillus spp | Multiple organs on pathology and cultureb | 19 | Died on day 19a |
| Lung | Aspergillus niger | BAL fluidc | 129 | Alive |
| Lung | Candida parapsilosis | Blood | 35 | Died on day 127a |
| Blood and braind | 119 | |||
| Lung | Mucor spp | Bloode | 67 | Alive |
| Liver | Candida albicans | Blood and liver tissue | 0f | Alive |
| Liver | Candida albicans | Blood | 2 | Alive |
| Liver | Candida albicans | Blood | 6 | Alive |
| Liver | Candida albicans | Blood | 6 | Alive |
| Liver | Candida albicans | Liver tissue | 44 | Alive |
| Liver | Candida albicans | Peritoneal fluid | 18 | Alive |
| Liver | Candida albicans | Blood | 107 | Alive |
| Liver | Candida guilliermondii | Blood | 7 | Alive |
Abbreviations: BAL, bronchoalveolar lavage; IFD, invasive fungal disease.
aDeath deemed likely attributable to IFD.
bDiagnosed after death once the autopsy revealed the presence of Aspergillus spp in bilaterally transplanted lungs, heart, kidneys, gastrointestinal tract, liver, brain, thyroid gland, diaphragm, skeletal muscle, and internal genitalia.
cProbable IFD confirmed in conjunction with radiographic results.
dA brain tissue biopsy specimen and pathology revealed coagulative necrosis of the cerebellum, computed tomography of the brain revealed ring-enhancing lesions in the cerebellum, and the patient experienced neurologic symptoms, including coma.
eTwo blood cultures were positive for this pathogen, so it was considered invasive rather than contaminant.
fPatient with positive blood cultures for several days before transplant and on day of the transplant.
| Transplant Type . | Pathogen . | Site of Infection . | Days From Transplant to Diagnosis . | Status at 180 Days . |
|---|---|---|---|---|
| Heart/lung | Candida parapsilosis | Blood | 95 | Died on day 101a |
| Lung | Aspergillus spp | Multiple organs on pathology and cultureb | 19 | Died on day 19a |
| Lung | Aspergillus niger | BAL fluidc | 129 | Alive |
| Lung | Candida parapsilosis | Blood | 35 | Died on day 127a |
| Blood and braind | 119 | |||
| Lung | Mucor spp | Bloode | 67 | Alive |
| Liver | Candida albicans | Blood and liver tissue | 0f | Alive |
| Liver | Candida albicans | Blood | 2 | Alive |
| Liver | Candida albicans | Blood | 6 | Alive |
| Liver | Candida albicans | Blood | 6 | Alive |
| Liver | Candida albicans | Liver tissue | 44 | Alive |
| Liver | Candida albicans | Peritoneal fluid | 18 | Alive |
| Liver | Candida albicans | Blood | 107 | Alive |
| Liver | Candida guilliermondii | Blood | 7 | Alive |
| Transplant Type . | Pathogen . | Site of Infection . | Days From Transplant to Diagnosis . | Status at 180 Days . |
|---|---|---|---|---|
| Heart/lung | Candida parapsilosis | Blood | 95 | Died on day 101a |
| Lung | Aspergillus spp | Multiple organs on pathology and cultureb | 19 | Died on day 19a |
| Lung | Aspergillus niger | BAL fluidc | 129 | Alive |
| Lung | Candida parapsilosis | Blood | 35 | Died on day 127a |
| Blood and braind | 119 | |||
| Lung | Mucor spp | Bloode | 67 | Alive |
| Liver | Candida albicans | Blood and liver tissue | 0f | Alive |
| Liver | Candida albicans | Blood | 2 | Alive |
| Liver | Candida albicans | Blood | 6 | Alive |
| Liver | Candida albicans | Blood | 6 | Alive |
| Liver | Candida albicans | Liver tissue | 44 | Alive |
| Liver | Candida albicans | Peritoneal fluid | 18 | Alive |
| Liver | Candida albicans | Blood | 107 | Alive |
| Liver | Candida guilliermondii | Blood | 7 | Alive |
Abbreviations: BAL, bronchoalveolar lavage; IFD, invasive fungal disease.
aDeath deemed likely attributable to IFD.
bDiagnosed after death once the autopsy revealed the presence of Aspergillus spp in bilaterally transplanted lungs, heart, kidneys, gastrointestinal tract, liver, brain, thyroid gland, diaphragm, skeletal muscle, and internal genitalia.
cProbable IFD confirmed in conjunction with radiographic results.
dA brain tissue biopsy specimen and pathology revealed coagulative necrosis of the cerebellum, computed tomography of the brain revealed ring-enhancing lesions in the cerebellum, and the patient experienced neurologic symptoms, including coma.
eTwo blood cultures were positive for this pathogen, so it was considered invasive rather than contaminant.
fPatient with positive blood cultures for several days before transplant and on day of the transplant.
The overall median time from transplant to IFD diagnosis was 27 days (range, 0–129 days). The median time from transplant to IFD onset was shorter for invasive candidiasis (18 days [range, 0–119 days]) and longer for invasive aspergillosis (74 days [range, 19–129 days]) and invasive mucormycosis (67 days). The risk of invasive candidiasis in liver transplant recipients occurred predominantly in the first 50 days after transplant, whereas the risk of IFD among lung recipients persisted for up to 130 days. In general, however, the frequency of IFD events decreased as the time from transplant increased; no IFD events occurred between 130 and 180 days of follow-up for any patient in the cohort (Figure 1).
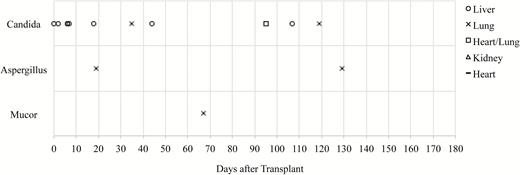
Distribution of invasive fungal disease events according to time after transplant.
A total of 52 patients received antifungal prophylaxis; 41 patients received primary prophylaxis, and 11 received secondary prophylaxis (Table 3). The group with the highest rate of antifungal prophylaxis was the lung recipients (13 [37%] of 35), followed by the heart/lung and liver recipients. Fluconazole was the most common antifungal medication given to liver, heart, and kidney recipients, whereas voriconazole was the most common agent given to lung recipients. Fluconazole and voriconazole were equally likely to be given to heart/lung recipients. Of the patients who received prophylaxis, 3.8% (2 of 52) developed an IFD, compared with the 2% (11 of 529) who did not receive prophylaxis. Of the 2 patients in whom primary prophylaxis was initiated and who subsequently developed an IFD, the antifungal agent matched the causative pathogen, but for 1 patient, the IFD occurred late in the posttransplant course (day 129).
| Transplant Type . | Transplantation in 2000–2006 (Jan 1, 2000–Dec 31, 2006) . | Transplantation in 2007–2013 (Jan 1, 2007–Dec 31, 2013) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate of IFD Events per Patient (n/N [%]) . | IFD Events per 100000 Patient-Days . | IFD Case- Fatality Rate (n/N [%]) . | All-Cause Mortality Rate (n/N [%]) . | Rate of Any Antifungal Prophylaxis (%); Rate of Mold-Specific Prophylaxis (%)a . | Rate of IFD Events per Patient (n/N [%]) . | IFD Events Per 100000 Patient-Days . | IFD Case- Fatality Rate (n/N [%]) . | All-Cause Mortality Rate (n/N [%]) . | Rate of Any Antifungal Prophylaxis (%); Rate of Mold-Specific Prophylaxis (%)a . | |
| All SOTs | 12/292 (4) | 25.5 | 3/12 (25) | 15/292 (5) | 7; 2 | 2/292 (1) | 3.9 | 0 | 8/292 (3) | 11; 4 |
| Heart/lung | 1/4 (25) | 229.9 | 1/1 (100) | 2/4 (50) | 25; 0 | 0/4 (0) | 0 | 0 | 2/4 (50) | 25; 25 |
| Lung | 4/18 (22) | 147.2 | 2/4 (50) | 3/18 (16) | 28; 22b | 1/17 (6) | 32.7 | 0 | 0 (0) | 47; 47c |
| Liver | 7/63 (11) | 68.5 | 0 (0) | 0/63 | 17; 2d | 1/109 (1) | 5.3 | 0 | 4/109 (4) | 16; 1 |
| Kidney | 0/132 (0) | 0 | 0 (0) | 0/132 | 0; 0e | 0/102 (0) | 0 | 0 | 0 (0) | 2; 0 |
| Heart | 0/75 (0) | 0 | 0 (0) | 12/75 (16) | 3; 1d | 0/60 (0) | 0 | 0 | 2/60 (3) | 8; 3d |
| Transplant Type . | Transplantation in 2000–2006 (Jan 1, 2000–Dec 31, 2006) . | Transplantation in 2007–2013 (Jan 1, 2007–Dec 31, 2013) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate of IFD Events per Patient (n/N [%]) . | IFD Events per 100000 Patient-Days . | IFD Case- Fatality Rate (n/N [%]) . | All-Cause Mortality Rate (n/N [%]) . | Rate of Any Antifungal Prophylaxis (%); Rate of Mold-Specific Prophylaxis (%)a . | Rate of IFD Events per Patient (n/N [%]) . | IFD Events Per 100000 Patient-Days . | IFD Case- Fatality Rate (n/N [%]) . | All-Cause Mortality Rate (n/N [%]) . | Rate of Any Antifungal Prophylaxis (%); Rate of Mold-Specific Prophylaxis (%)a . | |
| All SOTs | 12/292 (4) | 25.5 | 3/12 (25) | 15/292 (5) | 7; 2 | 2/292 (1) | 3.9 | 0 | 8/292 (3) | 11; 4 |
| Heart/lung | 1/4 (25) | 229.9 | 1/1 (100) | 2/4 (50) | 25; 0 | 0/4 (0) | 0 | 0 | 2/4 (50) | 25; 25 |
| Lung | 4/18 (22) | 147.2 | 2/4 (50) | 3/18 (16) | 28; 22b | 1/17 (6) | 32.7 | 0 | 0 (0) | 47; 47c |
| Liver | 7/63 (11) | 68.5 | 0 (0) | 0/63 | 17; 2d | 1/109 (1) | 5.3 | 0 | 4/109 (4) | 16; 1 |
| Kidney | 0/132 (0) | 0 | 0 (0) | 0/132 | 0; 0e | 0/102 (0) | 0 | 0 | 0 (0) | 2; 0 |
| Heart | 0/75 (0) | 0 | 0 (0) | 12/75 (16) | 3; 1d | 0/60 (0) | 0 | 0 | 2/60 (3) | 8; 3d |
Abbreviations: IFD, invasive fungal disease; SOT, solid organ transplant.
aPatients who received antifungal prophylaxis in the first 5 days after transplant.
bThree patients received secondary prophylaxis.
cFive patients received secondary prophylaxis.
dOne patient received secondary prophylaxis.
eThree patients were excluded because of missing medication-administration records for first 5 days after transplant.
| Transplant Type . | Transplantation in 2000–2006 (Jan 1, 2000–Dec 31, 2006) . | Transplantation in 2007–2013 (Jan 1, 2007–Dec 31, 2013) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate of IFD Events per Patient (n/N [%]) . | IFD Events per 100000 Patient-Days . | IFD Case- Fatality Rate (n/N [%]) . | All-Cause Mortality Rate (n/N [%]) . | Rate of Any Antifungal Prophylaxis (%); Rate of Mold-Specific Prophylaxis (%)a . | Rate of IFD Events per Patient (n/N [%]) . | IFD Events Per 100000 Patient-Days . | IFD Case- Fatality Rate (n/N [%]) . | All-Cause Mortality Rate (n/N [%]) . | Rate of Any Antifungal Prophylaxis (%); Rate of Mold-Specific Prophylaxis (%)a . | |
| All SOTs | 12/292 (4) | 25.5 | 3/12 (25) | 15/292 (5) | 7; 2 | 2/292 (1) | 3.9 | 0 | 8/292 (3) | 11; 4 |
| Heart/lung | 1/4 (25) | 229.9 | 1/1 (100) | 2/4 (50) | 25; 0 | 0/4 (0) | 0 | 0 | 2/4 (50) | 25; 25 |
| Lung | 4/18 (22) | 147.2 | 2/4 (50) | 3/18 (16) | 28; 22b | 1/17 (6) | 32.7 | 0 | 0 (0) | 47; 47c |
| Liver | 7/63 (11) | 68.5 | 0 (0) | 0/63 | 17; 2d | 1/109 (1) | 5.3 | 0 | 4/109 (4) | 16; 1 |
| Kidney | 0/132 (0) | 0 | 0 (0) | 0/132 | 0; 0e | 0/102 (0) | 0 | 0 | 0 (0) | 2; 0 |
| Heart | 0/75 (0) | 0 | 0 (0) | 12/75 (16) | 3; 1d | 0/60 (0) | 0 | 0 | 2/60 (3) | 8; 3d |
| Transplant Type . | Transplantation in 2000–2006 (Jan 1, 2000–Dec 31, 2006) . | Transplantation in 2007–2013 (Jan 1, 2007–Dec 31, 2013) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate of IFD Events per Patient (n/N [%]) . | IFD Events per 100000 Patient-Days . | IFD Case- Fatality Rate (n/N [%]) . | All-Cause Mortality Rate (n/N [%]) . | Rate of Any Antifungal Prophylaxis (%); Rate of Mold-Specific Prophylaxis (%)a . | Rate of IFD Events per Patient (n/N [%]) . | IFD Events Per 100000 Patient-Days . | IFD Case- Fatality Rate (n/N [%]) . | All-Cause Mortality Rate (n/N [%]) . | Rate of Any Antifungal Prophylaxis (%); Rate of Mold-Specific Prophylaxis (%)a . | |
| All SOTs | 12/292 (4) | 25.5 | 3/12 (25) | 15/292 (5) | 7; 2 | 2/292 (1) | 3.9 | 0 | 8/292 (3) | 11; 4 |
| Heart/lung | 1/4 (25) | 229.9 | 1/1 (100) | 2/4 (50) | 25; 0 | 0/4 (0) | 0 | 0 | 2/4 (50) | 25; 25 |
| Lung | 4/18 (22) | 147.2 | 2/4 (50) | 3/18 (16) | 28; 22b | 1/17 (6) | 32.7 | 0 | 0 (0) | 47; 47c |
| Liver | 7/63 (11) | 68.5 | 0 (0) | 0/63 | 17; 2d | 1/109 (1) | 5.3 | 0 | 4/109 (4) | 16; 1 |
| Kidney | 0/132 (0) | 0 | 0 (0) | 0/132 | 0; 0e | 0/102 (0) | 0 | 0 | 0 (0) | 2; 0 |
| Heart | 0/75 (0) | 0 | 0 (0) | 12/75 (16) | 3; 1d | 0/60 (0) | 0 | 0 | 2/60 (3) | 8; 3d |
Abbreviations: IFD, invasive fungal disease; SOT, solid organ transplant.
aPatients who received antifungal prophylaxis in the first 5 days after transplant.
bThree patients received secondary prophylaxis.
cFive patients received secondary prophylaxis.
dOne patient received secondary prophylaxis.
eThree patients were excluded because of missing medication-administration records for first 5 days after transplant.
Table 3 compares the diagnoses of IFD and frequency of antifungal prophylaxis use in the first and second periods of the 14-year study (2000–2006 and 2007–2013). Although the same number of transplants occurred in each period, the overall IFD rate decreased from 4% (12 of 292 patients), or 25.5 events per 100000 patient-days, to 1% (2 of 292 patients), or 3.9 events per 100000 patient-days. The IFD rate in each SOT recipient subgroup also decreased. Meanwhile, the overall rate of antifungal prophylaxis increased from 7% (19 of 289) to 11% (33 of 292) from the first to the second period, respectively. The proportion of patients who received specifically antimold prophylaxis also increased from 2% (6 of 289) to 4% (12 of 292).
Death
The all-cause mortality rate at 180 days was 4.3% (25 of 584). This rate decreased slightly from 5% (15 of 292) to 3% (8 of 292) between the first and second periods of the study, respectively (Table 3). Death occurred in 3 patients with IFD (2 lung recipients and 1 heart/lung recipient), all in the first period of the study. On the basis of chart reviews of physician progress notes, discharge summaries, and autopsy reports, all 3 deaths were deemed likely attributable to the IFD, which results in an attributable case-fatality rate of 21.4% (3 of 14).
The first death was of a 17-year-old girl after bilateral lung transplant who developed multiple organ dysfunction and died on day 19 after transplant. The autopsy examination revealed disseminated angioinvasive aspergillosis that affected multiple organs, including the lungs, heart, kidneys, and brain; portmortem cultures were positive for aspergillosis. The second death was in a 17-month-old boy after a heart/lung transplant who had blood cultures positive for Candida parapsilosis identified 95 days after transplant. Despite treatment with liposomal amphotericin B, the blood cultures remained positive, and he died 101 days after his transplant as a result of cardiac arrest in the setting of fungal sepsis. The final death was in a 6-year-old boy after bilateral lung transplant who had blood cultures positive for C parapsilosis, which started on day 119 and persisted despite treatment with liposomal amphotericin B. He sustained acute neurologic decline associated with ring-enhancing lesions in the right cerebellum; biopsy specimen testing revealed coagulative necrosis. He eventually became comatose and died on day 127 after withdrawal of support.
DISCUSSION
The results of this large cohort study of pediatric SOT recipients provide contemporary evidence on the burden of IFD in this immunosuppressed population. The overall IFD rate for SOT recipients was low at 2.2% (14.3 IFD events per 100000 patient-days) but ranged from 0% to 12.5% (0 to 99.2 IFD events per 100000 patient-days) across SOT types; heart/lung and lung recipients had the highest rates. Most of the IFD events occurred within 90 days of follow-up, although IFD events also were identified at 130 days of follow-up. Three of the 13 patients who sustained an IFD most likely died as a result of their infection (attributable case-fatality rate, 21.4%). These results suggest that the burden of IFD is relatively low in pediatric SOT recipients, but the mortality rate in patients with IFD is high. The incidence was reported as a rate of IFD per patient to enable comparison with results in the published literature. It was reported also as a rate of IFD per patient-days to enable comparison between patient groups by accounting for variability in patient follow-up times. Although not investigated in the present study, differences in the immunosuppressive regimens between the SOT groups could account for differences in patient susceptibility to IFD.
Analysis of the IFD trends over the 14 years of the study revealed a lower IFD rate in the second half (2007–2013) than in the first half (2000–2006) (3.9 vs 25.5 IFD events per 100000 patient-days, respectively). We also found a decrease in the all-cause mortality rate from 5% to 3% and no deaths attributable to IFD in 2007–2013. Contributors to these epidemiologic changes are likely multifactorial. We found a modest increase in the use of antifungal prophylaxis from 7% to 11%, which could account for some of the decrease in IFD rates. Another significant factor might be more widespread and effective infection-control initiatives. In fact, the Centers for Disease Control and Prevention recognized better adoption of prevention guidelines as a major contributor toward a 46% reduction in central line–associated candidemia from 2001 to 2009 [12]. In addition, it is possible that over time, clinicians had increased suspicion for IFD and thus prescribed empirical antifungal treatment before the patient actually met criteria for IFD. Finally, the evolution of surgical techniques and other supportive care measures might have translated into a reduced risk for IFD.
Compared to a large adult cohort from TRANSNET, from which a 12-month cumulative incidence for IFD of 3.1% in kidney, liver, pancreas, lung, heart, and small bowel recipients was reported, our pediatric cohort had a slightly lower 6-month incidence of 2.2%. The median times to diagnosis in the adult TRANSNET surveillance cohort (2001–2006) were longer than those in our study (103 days for invasive candidiasis, 184 days for aspergillosis, and 312 days for zygomycosis) [1]. However, that cohort was followed for a much longer time than the 180 days for our cohort, and thus direct comparison of timing is limited. Our results are similar to those reported by Knapp et al [9] from a pediatric TRANSNET cohort in which 3% of SOT recipients had IFD, and the most common organisms were Candida spp (78%), followed by Aspergillus spp (8%). Data on the timing of IFD onset in the pediatric TRANSNET cohort have not been published.
As noted already, most previous pediatric studies focused on specific SOT groups in defining IFD incidence. Danziger-Isakov et al [7] found an incidence of 10.5% for pulmonary fungal disease in pediatric lung transplant recipients. Although this rate seems similar to our rate of 11.4%, our rate also includes nonpulmonary bloodstream events. The incidence of pulmonary IFD in our lung transplant cohort was 5.7% (2 of 35), lower than the previously reported 10.5%. De Luca et al [5] determined the rate of invasive candidiasis in liver transplant recipients to be 2.5%. Although we considered other fungal pathogens in our investigation, Candida spp were the only fungal pathogens identified in our liver recipients. As already mentioned, a subset of our liver recipients overlaps with those in the De Luca et al study, which contributes to the similar rates of IFD between the 2 studies.
Overall, we found a relatively low rate of IFD in both time periods of this study. Although antifungal prophylaxis increased from 7% to 11%, approximately 90% of the patients were not administered prophylaxis, and the IFD rate remained relatively low. Thus, this evidence does not support a recommendation for routine antifungal prophylaxis to be given to all pediatric SOT recipients. Still, it is likely that certain factors, such as previous IFD, surgical complications, or high-risk transplantation (heart/lung, lung, or liver), could put some patients at a higher risk for IFD and warrant the use of targeted prophylaxis. However, these data are too limited to provide specific recommendations; additional investigations are needed to identify patients at high risk within these transplant types and to inform guidelines for targeted prophylaxis.
Notable limitations to this study exist. First, although ours was a large cohort of pediatric SOT recipients and was inclusive of most SOT types, the number of patients in each organ group was still relatively small. The small number of patients in each SOT group and even smaller number of IFD events within each group reduces the stability around the point estimates for the all-cause mortality rates, IFD incidence, and case-fatality rates.
Second, the burden of IFD might be underestimated, because events that occurred after 180 days would have been missed. The strict EORTC/MSG criteria used to define IFD might have further resulted in underestimation of clinically relevant IFD events. Third, as with all single-center studies, the results might not be generalizable to similar patient populations at other centers. Fourth, data on the duration of antifungal medication were not collected, only the date of first dose within 5 days after transplant. Thus, we cannot determine whether a certain duration of prophylaxis would be beneficial.
Last, the routine clinical care of lung transplant recipients might result in an increased likelihood for them to meet EORTC/MSG criteria for probable IFD. Lung recipients undergo bronchoscopy routinely in the posttransplant period and undergo microbiology sampling regardless of their clinical symptoms. Therefore, it is possible that the mycological criteria are met more commonly in lung recipients because of increased sampling. Alternatively, the rate of probable IFD among lung recipients could be underestimated, because the galactomannan antigen in bronchoalveolar lavage fluid was not routinely measured during this study period.
Despite these limitations, the results of this study provide contemporary incidence rates for IFD among pediatric SOT recipients. Although the incidence of IFD for most SOT recipients is low, rates vary according to organ type, and the mortality rate attributable to IFD is high. Future investigations should focus on better defining specific factors to identify patients at the highest risk who would benefit from targeted antifungal prophylaxis.
Notes
Financial support: This work was supported by NIH/NIAID contract HHSN2722011000040C.
Potential conflicts of interest: B. F. has received research support from Pfizer, Merck & Co., and Ansun Biopharma, and T. Z. has been a consultant to Nabriva Therapeutics, Merck & Co., Astellas Pharma, Pfizer, and Cubist Pharmaceuticals, has received research funding from Merck & Co. and Cubist Pharmaceuticals, and received payment for development of a Terranova CME presentation. All other authors: no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.



