-
PDF
- Split View
-
Views
-
Cite
Cite
Kristen G Valencia Deray, Lara A Danziger-Isakov, Kevin J Downes, Current and Emerging Antiviral Agents in the Prevention and Treatment of Cytomegalovirus in Pediatric Transplant Recipients, Journal of the Pediatric Infectious Diseases Society, Volume 13, Issue Supplement_1, February 2024, Pages S14–S21, https://doi.org/10.1093/jpids/piad059
Close - Share Icon Share
Abstract
Despite current prophylaxis regimens, cytomegalovirus (CMV) is common in hematopoietic cell transplantation (HCT) and solid organ transplantation (SOT) and remains a significant cause of morbidity and mortality. Newer antiviral medications are reshaping the landscape for prevention and treatment of CMV DNAemia, infection, and disease. Letermovir is approved for CMV prevention in adult HCT patients and is attractive due to the absence of marrow suppression seen with ganciclovir/valganciclovir. Letermovir should not be routinely used for CMV treatment due to its low threshold for resistance. Maribavir is approved for the treatment of refractory or resistant CMV disease in HCT and SOT recipients ≥12 years of age, though it has no current role in CMV prevention. More research is needed to fully elucidate the roles, efficacy, and safety of these newer agents in prevention and treatment of CMV in pediatric transplant recipients.
INTRODUCTION
Cytomegalovirus (CMV) continues to cause significant morbidity after solid organ transplantation (SOT) and hematopoietic cell transplantation (HCT) with recent reports showing CMV disease rates of up to 5% [1, 2] and 7% [3], respectfully. The risk of posttransplant CMV is affected by donor and recipient serostatus, organ or graft type, T-cell depletion, immunosuppressive agent, and intensity [4, 5]. Prevention strategies and newer treatment options provide effective options to diminish the impact of this virus.
As it is not possible to avoid CMV in HCT recipients (highest risk individuals are seropositive prior to transplant) and preferential use of CMV seronegative HCT and SOT donors is not routinely feasible, alternative prevention strategies are needed to mitigate risk. Further, no current antivirals eliminate latent virus, requiring ongoing monitoring and/or prevention for at-risk patients posttransplant. Several strategies have emerged and are endorsed in guidance documents including prophylaxis, monitoring with preemptive therapy, or a combination of these strategies, also referred to as surveillance after prophylaxis [4, 5]. Prophylaxis entails giving an antiviral medication, usually ganciclovir/valganciclovir at present, for a predetermined amount of time with the goal of preventing CMV DNAemia. Prophylaxis is generally targeted based on the CMV risk profile, including donor and recipient serostatus, where the highest risk exists when CMV R− SOT receive seropositive (D+) organs or CMV R+ HCT receive D− grafts. The major drawbacks of this strategy include the side effects of the antiviral medications, specifically bone marrow suppression with the use of ganciclovir/valganciclovir, and late-onset CMV disease after prophylaxis is discontinued [4, 5]. Preemptive therapy includes monitoring for CMV DNAemia at routine times posttransplant and initiating antiviral therapy if DNAemia is found at a predetermined threshold. This strategy avoids unnecessary antiviral side effects, though studies have shown that it does not prevent the indirect effects of CMV DNAemia such as graft dysfunction and all-cause mortality [4, 5]. Many experts use surveillance after prophylaxis in patients at risk for developing CMV DNAemia, such as those with a high-risk CMV profile. Secondary prophylaxis is the use of antiviral prophylaxis, after treatment for CMV DNAemia or disease, for prevention of recurrent CMV.
The optimal prevention strategy for each organ and HCT subgroup is not fully elucidated, and data support several approaches. In pediatric HCT, primary prophylaxis with valganciclovir, ganciclovir, or foscarnet may be considered in some populations; however, monitoring with preemptive therapy is also a reasonable option [6], as it may avoid the toxicities associated with the use of these agents. In the adult HCT population, letermovir has emerged as the primary prophylactic agent, but limited pediatric data have restricted its use to small cohorts to date (covered subsequently in this review). For pediatric SOT, monitoring with preemptive therapy has been successfully reported primarily in pediatric liver transplant recipients [7, 8], while the bulk of the published data support the use of universal prophylaxis in other pediatric SOT populations [1, 2, 9–11]. However, challenges exist related to optimal prophylaxis durations [12] and dosing strategies [13, 14], as well as frequent medication side effects [15, 16], which highlight the need for additional therapeutic options for both prevention and treatment.
Despite CMV prevention strategies, CMV DNAemia and disease continue to occur. Occasionally, CMV DNAemia does not respond to traditional antiviral agents either due to drug resistant or refractory disease. Refractory CMV is defined as CMV DNAemia that continues to increase despite 2 weeks of adequately dosed antiviral medication [17]. Cytomegalovirus is considered resistant if it has a genetic mutation that decreases susceptibility to one or more antiviral drugs [17]. Limited treatment strategies are available to treat resistant/refractory disease, but maribavir, a novel CMV antiviral, is now FDA approved for this indication in individuals 12 years of age and older. This article explores the potential therapeutic uses and challenges of maribavir and letermovir, two newer CMV antivirals, in addition to traditional CMV agents.
TRADITIONAL AGENTS
Ganciclovir was the first drug to be approved for the treatment and prevention of CMV DNAemia and disease. It is a nucleoside analog that requires phosphorylation by UL97 kinase and host kinases to inhibit viral DNA polymerase, therefore inhibiting the synthesis of CMV DNA (Figure 1) [18–20]. Valganciclovir is an oral prodrug of ganciclovir with excellent bioavailability [21]. Ganciclovir and valganciclovir are currently the drugs of choice for the prevention and treatment of CMV in HCT and SOT recipients [5, 22, 23], though their use is limited by significant toxicities including myelosuppression, predominantly neutropenia, and acute kidney injury, which both have been associated with dose modification and early cessation of therapy [15, 24, 25]. This is of particular concern with valganciclovir as optimal dosing in the pediatric population is controversial. The FDA recommended dosing for valganciclovir is based on body surface area and creatinine, but adverse effects with this dosing strategy are common [15, 16]. Therefore, in 2018, the FDA updated its dosing guidance to prevent supratherapeutic concentrations [26]. A PK study by Peled et al. showed that weight-based dosing produced more appropriate area under the curve values with continued clinical efficacy [14]. Subsequently, numerous pediatric centers have changed their dosing strategy to weight-based and achieved similar results [13].
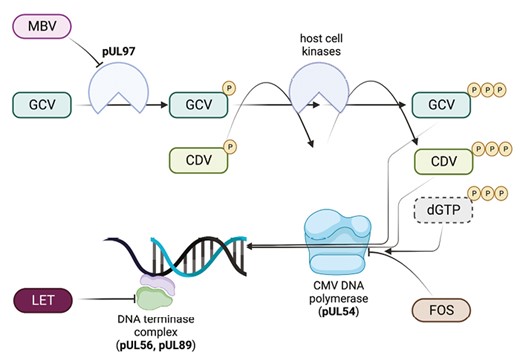
Mechanisms of activity of anti-CMV antivirals. Ganciclovir (GCV) is a nucleoside analog that requires initial phosphorylation by the viral protein kinase encoded by UL97. It is subsequently di- and tri-phosphorylated by host cellular kinases. Ganciclovir triphosphate inhibits CMV DNA polymerase (UL54) activity and also serves as substrate for the enzyme, substituting for deoxyguanosine triphosphate (dGTP) during chain elongation, slowing viral DNA synthesis. Cidofovir (CDV) is not a substrate for UL97 but is di- and tri-phosphorylated by host kinases, as with GCV monophosphate, and serves as competitive inhibitor of DNA polymerase. Foscarnet (FOS) is a noncompetitive inhibitor of CMV DNA polymerase, blocking the pyrophosphate binding site and preventing cleavage of deoxynucleotide triphosphates. Maribavir (MBV) is a potent inhibitor of the UL97 kinase, which is involved in phosphorylation of a number of viral proteins, leading to inhibition of multiple stages of CMV DNA replication, viral encapsulation, and nuclear egress. Letermovir (LET) inhibits the CMV DNA terminase complex by binding to its pUL56 subunit. The enzymatic targets are also the main sites of resistance for each drug: GCV–UL97 > UL54; CDV–UL54; FOS–UL54; MBV–UL97; LET–UL56 > UL89. Figure was made using Biorender. Abbreviations: CDV, cidofovir; CMV, cytomegalovirus; FOS foscarnet; GCV, ganciclovir; LET, letermovir; MBV, maribavir; NUC, nucleotide.
Foscarnet is a pyrophosphate analog that binds reversibly to DNA polymerase, stopping the elongation of CMV DNA [27]. Foscarnet can be used as preemptive therapy in HCT or treatment for CMV disease in HCT or SOT, in an effort to avoid the myelosuppressive effects of ganciclovir/valganciclovir [5, 22]. The most common adverse event is nephrotoxicity [27], caused by both renal tubule toxicity [28] and formation of crystal nephropathy [29]. Other toxicities include electrolyte abnormalities, seizures, genital ulcerations, anemia, and nausea [27].
Cidofovir is a nucleotide analog that inhibits CMV DNA polymerase, slowing DNA synthesis and destabilizing viral DNA [30]. Cidofovir is a second-line agent for CMV treatment and a third-line agent for CMV prevention in HCT recipients [22]. It is a third-line treatment agent in SOT recipients, but is not recommended for prophylaxis in this population [23]. Nephrotoxicity limits its use primarily to patients intolerant of other medications or with resistant or refractory infections [30]. Brincidofovir, an oral lipid prodrug of cidofovir with less nephrotoxic potential, is not currently available for treatment of CMV.
The development of CMV resistance to these agents is a major concern. The primary ganciclovir resistance mechanism is via a mutation in the UL97 phosphotransferase gene, which prevents the initial phosphorylation step required for antiviral activity [31]. A less common ganciclovir resistance mechanism, mutation in the UL54 DNA polymerase, also confers resistance to foscarnet or cidofovir [31]. Resistant and refractory CMV infection in HCT and SOT recipients remains a challenge given the limited number of traditional antiviral agents and their associated toxicities. The development of newer antiviral agents for the prevention and treatment of CMV infection and disease is paramount to improve outcomes in pediatric HCT and SOT patients. Pertinent prescribing information for antivirals discussed is located in Table 1.
Antiviral Drugs for the Prevention and Treatment of Cytomegalovirus in Pediatric Hematopoietic Stem Cell and Solid Organ Transplant Recipients
| Agent . | Indications in Transplant Recipients . | Dosing for CMV Prevention in Pediatric Transplant . | Dosing for CMV Treatment in Pediatric Transplant . | Comments/Challenges to Use . |
|---|---|---|---|---|
| Valganciclovir | First line for treatment and prevention of CMV DNAemia and disease | 7 × BSA × creatinine clearance with upper limit of creatinine clearance of 150 mL/min PO daily (max dose 900 mg PO daily) or 16 mg/kg PO daily (max dose 900 mg PO daily) | 7 × BSA × creatinine clearance with upper limit of creatinine clearance of 150 mL/min PO BID (max dose 900 mg PO BID) or 16 mg/kg PO BID (max dose 900 mg PO BID) | Causes myelosuppression, predominately leukopenia Requires dose adjustment for renal dysfunction Oral formulations available as a tablet and a suspension |
| Ganciclovir | First line for treatment and prevention of CMV DNAemia and disease | 5 mg/kg IV once daily | 5 mg/kg IV every 12 h | Causes myelosuppression, predominately leukopenia Requires dose adjustment for renal dysfunction Intravenous administration |
| Foscarnet | Second-line agent for therapy in SOT recipients First-line agent for HCT recipients who can’t tolerate marrow suppression from ganciclovir/valganciclovir | Currently not indicated for prevention in SOT recipients 90 mg/kg IV every 24 h for prevention in HCT | 90 mg/kg IV every 12 h | Highly nephrotoxic Requires dose adjustment for renal dysfunction Intravenous administration Used for UL97-mutant ganciclovir-resistant CMV DNAemia or disease |
| Cidofovir | Third-line agent for treatment of CMV DNAemia and disease in SOT recipients Second-line agent for prevention and treatment of CMV DNAemia and disease in HCT recipients | Currently not indicated for prevention in SOT recipients 5 mg/kg/dose IV once weekly for 2 consecutive weeks followed by 5 mg/kg/dose IV once every 2 weeks, in combination with probenecid in HCT [32] | 5 mg/kg/dose IV once weekly for 2 consecutive weeks followed by 5 mg/kg/dose IV once every 2 weeks, in combination with probenecid | Highly nephrotoxic Requires dose adjustment for renal dysfunction Intravenous administration Second-line agent for UL97-mutant ganciclovir-resistant CMV DNAemia or disease |
| Maribavir | Refractory CMV DNAemia or disease (with or without resistance to traditional agents) age 12 and older | Currently not indicated for CMV prevention | 400 mg PO twice daily for patients ≥12 years of age and ≥35 kg [33] | Well-tolerated (GI side effects including taste disturbance are most common) Primarily metabolized by CYP3A4 and can increase the concentration of common immunosuppressant. Therefore, monitor immunosuppression levels closely Recommend reviewing contaminant medications as it has numerous other drug–drug interactions Oral tablet formulation only. Can be crushed and placed through orogastric or nasogastric tubes No dose adjustment is needed for impaired renal or mild to moderate hepatic function Do not coadminister with ganciclovir/valganciclovir due to concerns for antagonism No CNS penetration |
| Letermovir | Prophylaxis of CMV infection and disease in adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant | 480 mg PO or IV administered once daily (over 1 h) through 100 days posttransplant; dose should be reduced to 240 mg if coadministered with cyclosporine | Currently not indicated for treatment | Generally well-tolerated (GI side effects most common) Oral formulation only available as a tablet that must be swallowed whole CYP3A4 inhibitor and has notable drug interactions (cyclosporine, tacrolimus, and statins) that require dose adjustments and/or close monitoring when coadministered |
| Agent . | Indications in Transplant Recipients . | Dosing for CMV Prevention in Pediatric Transplant . | Dosing for CMV Treatment in Pediatric Transplant . | Comments/Challenges to Use . |
|---|---|---|---|---|
| Valganciclovir | First line for treatment and prevention of CMV DNAemia and disease | 7 × BSA × creatinine clearance with upper limit of creatinine clearance of 150 mL/min PO daily (max dose 900 mg PO daily) or 16 mg/kg PO daily (max dose 900 mg PO daily) | 7 × BSA × creatinine clearance with upper limit of creatinine clearance of 150 mL/min PO BID (max dose 900 mg PO BID) or 16 mg/kg PO BID (max dose 900 mg PO BID) | Causes myelosuppression, predominately leukopenia Requires dose adjustment for renal dysfunction Oral formulations available as a tablet and a suspension |
| Ganciclovir | First line for treatment and prevention of CMV DNAemia and disease | 5 mg/kg IV once daily | 5 mg/kg IV every 12 h | Causes myelosuppression, predominately leukopenia Requires dose adjustment for renal dysfunction Intravenous administration |
| Foscarnet | Second-line agent for therapy in SOT recipients First-line agent for HCT recipients who can’t tolerate marrow suppression from ganciclovir/valganciclovir | Currently not indicated for prevention in SOT recipients 90 mg/kg IV every 24 h for prevention in HCT | 90 mg/kg IV every 12 h | Highly nephrotoxic Requires dose adjustment for renal dysfunction Intravenous administration Used for UL97-mutant ganciclovir-resistant CMV DNAemia or disease |
| Cidofovir | Third-line agent for treatment of CMV DNAemia and disease in SOT recipients Second-line agent for prevention and treatment of CMV DNAemia and disease in HCT recipients | Currently not indicated for prevention in SOT recipients 5 mg/kg/dose IV once weekly for 2 consecutive weeks followed by 5 mg/kg/dose IV once every 2 weeks, in combination with probenecid in HCT [32] | 5 mg/kg/dose IV once weekly for 2 consecutive weeks followed by 5 mg/kg/dose IV once every 2 weeks, in combination with probenecid | Highly nephrotoxic Requires dose adjustment for renal dysfunction Intravenous administration Second-line agent for UL97-mutant ganciclovir-resistant CMV DNAemia or disease |
| Maribavir | Refractory CMV DNAemia or disease (with or without resistance to traditional agents) age 12 and older | Currently not indicated for CMV prevention | 400 mg PO twice daily for patients ≥12 years of age and ≥35 kg [33] | Well-tolerated (GI side effects including taste disturbance are most common) Primarily metabolized by CYP3A4 and can increase the concentration of common immunosuppressant. Therefore, monitor immunosuppression levels closely Recommend reviewing contaminant medications as it has numerous other drug–drug interactions Oral tablet formulation only. Can be crushed and placed through orogastric or nasogastric tubes No dose adjustment is needed for impaired renal or mild to moderate hepatic function Do not coadminister with ganciclovir/valganciclovir due to concerns for antagonism No CNS penetration |
| Letermovir | Prophylaxis of CMV infection and disease in adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant | 480 mg PO or IV administered once daily (over 1 h) through 100 days posttransplant; dose should be reduced to 240 mg if coadministered with cyclosporine | Currently not indicated for treatment | Generally well-tolerated (GI side effects most common) Oral formulation only available as a tablet that must be swallowed whole CYP3A4 inhibitor and has notable drug interactions (cyclosporine, tacrolimus, and statins) that require dose adjustments and/or close monitoring when coadministered |
Abbreviations: BID, twice daily; BSA, body surface area; CMV, cytomegalovirus; HCT, hemopoietic cell transplantation; IV, intravenous; kg, kilograms; mg, milligrams; mL, milliliter; min, minute; PO, orally; SOT, solid organ transplant.
Antiviral Drugs for the Prevention and Treatment of Cytomegalovirus in Pediatric Hematopoietic Stem Cell and Solid Organ Transplant Recipients
| Agent . | Indications in Transplant Recipients . | Dosing for CMV Prevention in Pediatric Transplant . | Dosing for CMV Treatment in Pediatric Transplant . | Comments/Challenges to Use . |
|---|---|---|---|---|
| Valganciclovir | First line for treatment and prevention of CMV DNAemia and disease | 7 × BSA × creatinine clearance with upper limit of creatinine clearance of 150 mL/min PO daily (max dose 900 mg PO daily) or 16 mg/kg PO daily (max dose 900 mg PO daily) | 7 × BSA × creatinine clearance with upper limit of creatinine clearance of 150 mL/min PO BID (max dose 900 mg PO BID) or 16 mg/kg PO BID (max dose 900 mg PO BID) | Causes myelosuppression, predominately leukopenia Requires dose adjustment for renal dysfunction Oral formulations available as a tablet and a suspension |
| Ganciclovir | First line for treatment and prevention of CMV DNAemia and disease | 5 mg/kg IV once daily | 5 mg/kg IV every 12 h | Causes myelosuppression, predominately leukopenia Requires dose adjustment for renal dysfunction Intravenous administration |
| Foscarnet | Second-line agent for therapy in SOT recipients First-line agent for HCT recipients who can’t tolerate marrow suppression from ganciclovir/valganciclovir | Currently not indicated for prevention in SOT recipients 90 mg/kg IV every 24 h for prevention in HCT | 90 mg/kg IV every 12 h | Highly nephrotoxic Requires dose adjustment for renal dysfunction Intravenous administration Used for UL97-mutant ganciclovir-resistant CMV DNAemia or disease |
| Cidofovir | Third-line agent for treatment of CMV DNAemia and disease in SOT recipients Second-line agent for prevention and treatment of CMV DNAemia and disease in HCT recipients | Currently not indicated for prevention in SOT recipients 5 mg/kg/dose IV once weekly for 2 consecutive weeks followed by 5 mg/kg/dose IV once every 2 weeks, in combination with probenecid in HCT [32] | 5 mg/kg/dose IV once weekly for 2 consecutive weeks followed by 5 mg/kg/dose IV once every 2 weeks, in combination with probenecid | Highly nephrotoxic Requires dose adjustment for renal dysfunction Intravenous administration Second-line agent for UL97-mutant ganciclovir-resistant CMV DNAemia or disease |
| Maribavir | Refractory CMV DNAemia or disease (with or without resistance to traditional agents) age 12 and older | Currently not indicated for CMV prevention | 400 mg PO twice daily for patients ≥12 years of age and ≥35 kg [33] | Well-tolerated (GI side effects including taste disturbance are most common) Primarily metabolized by CYP3A4 and can increase the concentration of common immunosuppressant. Therefore, monitor immunosuppression levels closely Recommend reviewing contaminant medications as it has numerous other drug–drug interactions Oral tablet formulation only. Can be crushed and placed through orogastric or nasogastric tubes No dose adjustment is needed for impaired renal or mild to moderate hepatic function Do not coadminister with ganciclovir/valganciclovir due to concerns for antagonism No CNS penetration |
| Letermovir | Prophylaxis of CMV infection and disease in adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant | 480 mg PO or IV administered once daily (over 1 h) through 100 days posttransplant; dose should be reduced to 240 mg if coadministered with cyclosporine | Currently not indicated for treatment | Generally well-tolerated (GI side effects most common) Oral formulation only available as a tablet that must be swallowed whole CYP3A4 inhibitor and has notable drug interactions (cyclosporine, tacrolimus, and statins) that require dose adjustments and/or close monitoring when coadministered |
| Agent . | Indications in Transplant Recipients . | Dosing for CMV Prevention in Pediatric Transplant . | Dosing for CMV Treatment in Pediatric Transplant . | Comments/Challenges to Use . |
|---|---|---|---|---|
| Valganciclovir | First line for treatment and prevention of CMV DNAemia and disease | 7 × BSA × creatinine clearance with upper limit of creatinine clearance of 150 mL/min PO daily (max dose 900 mg PO daily) or 16 mg/kg PO daily (max dose 900 mg PO daily) | 7 × BSA × creatinine clearance with upper limit of creatinine clearance of 150 mL/min PO BID (max dose 900 mg PO BID) or 16 mg/kg PO BID (max dose 900 mg PO BID) | Causes myelosuppression, predominately leukopenia Requires dose adjustment for renal dysfunction Oral formulations available as a tablet and a suspension |
| Ganciclovir | First line for treatment and prevention of CMV DNAemia and disease | 5 mg/kg IV once daily | 5 mg/kg IV every 12 h | Causes myelosuppression, predominately leukopenia Requires dose adjustment for renal dysfunction Intravenous administration |
| Foscarnet | Second-line agent for therapy in SOT recipients First-line agent for HCT recipients who can’t tolerate marrow suppression from ganciclovir/valganciclovir | Currently not indicated for prevention in SOT recipients 90 mg/kg IV every 24 h for prevention in HCT | 90 mg/kg IV every 12 h | Highly nephrotoxic Requires dose adjustment for renal dysfunction Intravenous administration Used for UL97-mutant ganciclovir-resistant CMV DNAemia or disease |
| Cidofovir | Third-line agent for treatment of CMV DNAemia and disease in SOT recipients Second-line agent for prevention and treatment of CMV DNAemia and disease in HCT recipients | Currently not indicated for prevention in SOT recipients 5 mg/kg/dose IV once weekly for 2 consecutive weeks followed by 5 mg/kg/dose IV once every 2 weeks, in combination with probenecid in HCT [32] | 5 mg/kg/dose IV once weekly for 2 consecutive weeks followed by 5 mg/kg/dose IV once every 2 weeks, in combination with probenecid | Highly nephrotoxic Requires dose adjustment for renal dysfunction Intravenous administration Second-line agent for UL97-mutant ganciclovir-resistant CMV DNAemia or disease |
| Maribavir | Refractory CMV DNAemia or disease (with or without resistance to traditional agents) age 12 and older | Currently not indicated for CMV prevention | 400 mg PO twice daily for patients ≥12 years of age and ≥35 kg [33] | Well-tolerated (GI side effects including taste disturbance are most common) Primarily metabolized by CYP3A4 and can increase the concentration of common immunosuppressant. Therefore, monitor immunosuppression levels closely Recommend reviewing contaminant medications as it has numerous other drug–drug interactions Oral tablet formulation only. Can be crushed and placed through orogastric or nasogastric tubes No dose adjustment is needed for impaired renal or mild to moderate hepatic function Do not coadminister with ganciclovir/valganciclovir due to concerns for antagonism No CNS penetration |
| Letermovir | Prophylaxis of CMV infection and disease in adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant | 480 mg PO or IV administered once daily (over 1 h) through 100 days posttransplant; dose should be reduced to 240 mg if coadministered with cyclosporine | Currently not indicated for treatment | Generally well-tolerated (GI side effects most common) Oral formulation only available as a tablet that must be swallowed whole CYP3A4 inhibitor and has notable drug interactions (cyclosporine, tacrolimus, and statins) that require dose adjustments and/or close monitoring when coadministered |
Abbreviations: BID, twice daily; BSA, body surface area; CMV, cytomegalovirus; HCT, hemopoietic cell transplantation; IV, intravenous; kg, kilograms; mg, milligrams; mL, milliliter; min, minute; PO, orally; SOT, solid organ transplant.
MARIBAVIR
Maribavir is a benzimidazole riboside that competitively inhibits the protein kinase activity of CMV enzyme pUL97, which results in the inhibition of phosphorylation of proteins [34, 35] and inhibition of multiple stages of CMV DNA replication, viral encapsulation, and nuclear egress [36–41]. Maribavir differs from ganciclovir/valganciclovir in that it is a UL97 inhibitor rather than a UL97 substrate [31], thus it can antagonize the antiviral activity of ganciclovir/valganciclovir and should not be used simultaneously [42]. Foscarnet, cidofovir, and letermovir have no activity at pUL97 and therefore, can be used concurrently with maribavir. Mutations that confer resistance to ganciclovir, valganciclovir, foscarnet, and cidofovir generally do not affect maribavir activity as the UL97 mutations conferring resistance to maribavir and ganciclovir/valganciclovir have limited overlap and differential resistance levels [43], and maribavir does not have action against the UL54 CMV DNA polymerase utilized by foscarnet and cidofovir [44]. Low-level maribavir resistance has been seen with UL27 mutations [45], though these are felt to have less of an impact on susceptibility than UL97 mutations [43]. Notably, maribavir is a CMV-specific antiviral agent; other antiviral agents should be used to treat or prevent non-CMV herpesvirus infections during maribavir administration.
Early adult trials of maribavir focused on CMV DNAemia prophylaxis. A phase II, randomized, double-blind, placebo-controlled study in CMV-seropositive HCT recipients found that maribavir was more effective than placebo for reducing the incidence of CMV DNAemia (CMV DNA polymerase chain reaction [PCR]: 7%–19% vs 46%; all P < .05), and CMV disease was only seen in three patients, all in the placebo group [46]. However, separate phase III prophylaxis trials in HCT and high-risk D+/R− liver transplant recipients found maribavir was not superior to placebo and oral ganciclovir, respectively [47, 48]. Based on these results, the evaluation for use of maribavir shifted toward treatment of CMV disease.
The pivotal SOLSTICE trial, a phase III, randomized, open-label, active-controlled trial, compared the safety and efficacy of 400 mg of maribavir twice daily to investigator-assigned therapy (valganciclovir/ganciclovir, foscarnet, or cidofovir) for refractory or resistant CMV in HCT and SOT recipients [49]. Significantly more patients in the maribavir group met the primary endpoint of confirmed CMV DNAemia clearance by week 8 of therapy (55.7% vs 23.9%; P < .01) and the secondary endpoint of CMV DNAemia clearance by week 8 with maintenance of clearance and symptom control through week 16 (18.7% vs 10.3%, P =.01) [49]. Notably, significantly more patients in the maribavir group met the primary end point even when controlling for early discontinuation of investigator-assigned therapy; therefore, maribavir’s superiority is not solely the result of better tolerance and adherence. Unfortunately, despite eligibility of patients ≥12 years, no children under 18 years of age were enrolled in the trial. Subsequently, a pharmacokinetic simulation was performed and supported adult dosing in children ≥12 years of age [50]. A phase III trial with the aim of determining the safety, tolerability, and pharmacokinetics of maribavir in children 0 to <18 years of age (NCT05319353) is planned [51]. In November 2021, the U.S. FDA approved maribavir as the first drug for treatment of resistant or refractory CMV infection in HCT and SOT recipients ≥12 years of age and weighing at least 35 kg (Table 1) [52].
Maribavir is well-tolerated with the most common side effect being dysgeusia seen in 37.2% of participants in the SOLSTICE trial [49]. Participants on maribavir also had a lower rate of marrow suppression than the valganciclovir group (24.8% vs 53.5%) and less acute kidney injury than the foscarnet group (8.5% vs 21.3%) [49]. Notably, maribavir is currently offered in a 200 mg tablet form, which can make dosing a challenge for critically ill patients where intravenous formulation may be preferable. However, alternative administration via an orogastric or nasogastric tube after crushing the tablet is feasible [33]. Maribavir is primarily metabolized by CYP3A4 so drugs that are strong inducers of this pathway, specifically many anticonvulsants, can decrease the concentration of maribavir and dose adjustments may be warranted [33]. Similarly, concentrations of immunosuppressants that are CYP3A4 substrates, such as mTOR inhibitors, may increase when taken concomitantly with maribavir so immunosuppression levels should be monitored closely [33]. Importantly, maribavir does not cross the blood–brain barrier [53].
LETERMOVIR
Letermovir is a CMV DNA terminase complex (subunit pUL56) inhibitor with potent activity against human CMV [54]. Because of its site and mechanism of action, it does not inhibit CMV DNA replication and, thus, may lead to accumulation of concatemeric (ie, nonreplicative) DNA that could cause misleading interpretation of quantitative PCR results [55]. Nevertheless, it acts in a manner distinct from other CMV antiviral agents and does not exhibit cross-resistance with these other drugs. As with maribavir, it is a CMV-specific drug that has no activity against other herpesviruses; other antiviral agents should be coadministered to prevent or treat non-CMV herpesviruses, as appropriate. Letermovir is available in oral and intravenous formulations. However, there are clinically relevant drug–drug interactions between letermovir and other medications metabolized via the cytochrome P450 system that require reduced dosages of letermovir (eg, cyclosporine) [56] or necessitate the use of a reduced dose or diligent therapeutic/clinical monitoring of the coadministered medication (eg, tacrolimus [56, 57], statins [58], and voriconazole [59, 60]).
Letermovir was approved by the FDA in 2017 for the prevention of CMV infection and disease in seropositive (R+) adult allogeneic HCT recipients [61]. Approval came following a phase III, double-blind, placebo-controlled trial in which participants with undetectable CMV DNA at randomization who received 14 weeks of letermovir had fewer clinically significant CMV infections (defined as CMV disease or DNAemia requiring treatment) by week 24 after transplantation than recipients of placebo (37.5% vs 60.6%) [62]. In a secondary analysis evaluating outcomes among trial participants with detectable CMV DNA at randomization, letermovir administration was similarly associated with a lower incidence of clinically significant CMV infection compared to placebo through weeks 14 (33.1% vs 86.6%, P < .001) and 24 (64.6% vs 90.9%, P = .01) [63]. A post hoc analysis of the trial data showed that all-cause mortality at 24 weeks was significantly lower among letermovir recipients with a trend towards lower mortality at week 48 [64]. In a 2022 systematic review combining data from 48 studies and more than 7100 adult allogeneic HCT patients, Vyas and colleagues determined that letermovir prophylaxis was associated with a significant reduction in CMV reactivation, clinically significant CMV infection, CMV disease, and grade ≥2 graft-versus-host disease, as well as all-cause and nonrelapse mortality beyond day 200 posttransplant [65]. Based on the totality of data, the FDA approved an extension of prophylaxis in high-risk adult HCT patients from 100 to 200 days [66].
A recently completed phase III trial of high-risk (D+/R−) adult kidney transplant recipients determined that letermovir is noninferior to valganciclovir for the prevention of CMV disease [67]. Based on these results, the FDA has also approved letermovir for CMV prevention in high risk (D+/R−) adult kidney transplant recipients [68].
Although letermovir is only approved for primary prophylaxis, there are reports of its use as secondary prophylaxis following a CMV event [69], as well as for treatment of CMV disease in the setting of valganciclovir intolerance or failure [69, 70]. However, treatment of CMV disease does not appear to be the ideal clinical situation for its use. This stems largely from concerns about a low threshold for resistance development, particularly in the setting of high viral loads and/or poor immunologic control [71]. Letermovir’s unique mechanism of action and lack of cross-resistance make it a possible option for treatment of refractory or resistant CMV infection or disease [72, 73]; however, clinical failures and development of resistance have been reported [74–76]. As a result, no industry-sponsored CMV treatment trials are active for this drug. Additionally, dedicated studies evaluating letermovir’s safety and efficacy as secondary prophylaxis are warranted before its routine clinical adoption for this indication.
Off-label use with letermovir has been reported in SOT recipients with valganciclovir intolerance [57, 77]. Off-label use of letermovir in pediatric HCT patients has also been reported, although published data remain limited to small case series [78–81]. In general, outcomes have been favorable. However, despite these reports, the effectiveness of letermovir for primary prophylaxis in pediatric HCT recipients is unknown. Formal investigation is warranted given the differences between pediatric and adult HCT recipients themselves, as well as in outcomes associated with CMV infection in these patient populations. Further, pediatric dosing of letermovir has also not been established. A trial to evaluate this in pediatric allogeneic HCT patients is ongoing (NCT03940586). Preliminary data from adolescent participants of this trial reported at IDWeek 2022 (Washington, DC) suggest that adult dosages (Table 1) are appropriate in the adolescent population (12 to <18) [82], although final dosing recommendations for pediatric patients, particularly younger ones, have yet to be determined.
Letermovir is very well-tolerated with far fewer significant adverse effects than older drugs. In the phase III trial, the rates of hematologic toxicities and acute kidney injury events were similar between letermovir and placebo recipients, as were other adverse events [62]. Meanwhile, in the recently completed phase 3 trial in adult kidney transplant recipients [67], fewer recipients of letermovir had hematological toxicities (either leukopenia or neutropenia; 26% vs 64%, P < .0001). And, in a recent cost effectiveness analysis, with clinical parameters informed mostly from the phase 3 trial data, letermovir prophylaxis in adults HCT was found to be cost effective compared to preemptive treatment alone in a US healthcare setting [83]. With its favorable side effect profile and data supporting its efficacy and cost effectiveness, letermovir is becoming standard of care for CMV prevention in high-risk adult HCT patients.
CURRENT AND POTENTIAL FUTURE CLINICAL APPLICATIONS OF LETERMOVIR AND MARIBAVIR IN PEDIATRIC TRANSPLANT RECIPIENTS
Primary Prophylaxis of CMV
Currently, letermovir is only approved for primary prophylaxis of CMV infection and disease in adult CMV-seropositive allogeneic HCT [61] and high-risk adult kidney transplant recipients [68]. Based on dosing from adolescent PK studies [78], some centers have begun using this agent for primary prophylaxis more routinely among adolescent HCT patients. We anticipate that letermovir will increasingly be used off-label in pediatric HCT, including young patients once dosing is known. This is especially likely in the highest-risk patients (ie, T-cell-depleted grafts) or in patients who have developed toxicities with other antiviral agents. Despite letermovir’s appeal, close clinical monitoring for CMV DNAemia with preemptive therapy still remains a viable option in pediatric HCT recipients.
Letermovir may also be attractive for CMV prevention in pediatric SOT recipients. Unfortunately, there are significant drug–drug interactions that may limit its use. As a result, close monitoring of drug concentrations (eg, tacrolimus) or for clinical symptoms of toxicity (eg, myopathy with coadministration of statins) is imperative. We would strongly suggest that dedicated studies be performed to fully understand all clinically relevant drug interactions before letermovir is routinely used for CMV prevention in any patient populations routinely prescribed these medications.
The best way to monitor for breakthrough CMV DNAemia on letermovir prophylaxis has not yet been elucidated. This poses a unique challenge given that letermovir use can result in nonreplicative CMV DNAemia [55], creating challenges to the interpretation of viral loads. Future studies are needed to understand how best to distinguish nonreplicative CMV DNAemia from replicative virus.
Meanwhile, maribavir should not be used as primary prophylaxis in pediatric HCT or SOT. Although it has been studied for this indication in adult populations, primary prophylaxis does not appear to be its greatest utility.
Secondary Prophylaxis
Which pediatric transplant recipients require secondary prophylaxis and, when used, for how long remains controversial. While most experts would offer secondary prophylaxis to a patient who has recovered from CMV disease and is still in the early posttransplant period (ie, when primary prophylaxis would be offered), other scenarios may be more subjective. In our opinion, letermovir could be a viable option when secondary prophylaxis is deemed necessary, particularly for patients who are intolerant of traditional antiviral agents. Due to the low barrier to resistance development, resulting in mutations within the UL56 gene [75, 84], letermovir is best suited as a preventative agent in the absence of ongoing DNAemia, particularly if the patient is significantly immunosuppressed. Meanwhile, maribavir is not currently indicated for secondary prophylaxis. Although the treatment course utilized in the SOLTICE trial (400 mg twice daily for 8 weeks) may have included a period of secondary prophylaxis for individuals whose DNAemia cleared promptly [49], the optimal dosing and duration for this indication are unknown.
Treatment of CMV
Ganciclovir and valganciclovir remain the primary drugs for treatment of CMV infection and disease in children. In the absence of resistant virus or contraindications to one of these agents, they remain as first-line therapy for the majority of CMV infections in pediatric transplant recipients. Maribavir is the first drug approved for treatment of refractory or resistant CMV in patients 12 years of age and older, with an ongoing trial to establish dosing in younger children (NCT05319353). With an improved safety profile compared to foscarnet and cidofovir, maribavir may become the preferred treatment option for refractory or resistant CMV infection/disease in the future.
However, there are several caveats that may temper enthusiasm for the use of maribavir in pediatric transplant recipients. First, the drug is currently only available as 200 mg tablets, which may be challenging to dose accurately in small children (<10 kg). Second, maribavir has poor penetration into the eye and central nervous system. Alternative agents or the use of combination therapy are needed when encephalitis, meningitis, or retinitis are of concern. Lastly, maribavir may not be an ideal agent for treatment of CMV infections with high viral loads. A notable fraction of patients in the phase II trial had recurrence of DNAemia while actively receiving maribavir, some of whom developed maribavir-resistant virus [85]. Ultimately, the optimal strategy for treatment of refractory infections with persistently high viral loads remains to be established.
CONCLUSIONS
Letermovir and maribavir are newer antiviral agents with promise to significantly improve CMV prevention and treatment, respectively, in adult and pediatric HCT and SOT recipients. With their widespread use in adult transplant recipients, their specific roles in pediatric transplant will become clearer. While off-label use is likely to occur in children given the toxicities associated with traditional anti-CMV agents, dedicated pediatric trials are urgently needed.
Notes
Financial support. K.J.D. is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23HD091365).
Supplement sponsorship. This article appears as part of the supplement “Advances in Pediatric Transplant Infectious Diseases,” sponsored by Eurofins Viracor.
Potential conflicts of interest. K.V.D. is a local investigator for pediatric Pfizer SARS-CoV-2 vaccination trials. L.D.-I. is a consultant for Takeda, Merck, and Roche Diagnostics. L.D.-I. is a site investigator for AiCuris, Ansun BioPharma, Astellas, Merck, Pfizer, and Takeda. K.J.D. has no relevant conflicts of interest to report.



