-
PDF
- Split View
-
Views
-
Cite
Cite
Emily Grohs, Alexandra Hill-Ricciuti, Nicole Kelly, Maria Messina, Daniel A Green, Wenjing Geng, Medini K Annavajhala, Philip Zachariah, Barun Mathema, Anne-Catrin Uhlemann, Lisa Saiman, Spa Typing of Staphylococcus aureus in a Neonatal Intensive Care Unit During Routine Surveillance, Journal of the Pediatric Infectious Diseases Society, Volume 10, Issue 7, July 2021, Pages 766–773, https://doi.org/10.1093/jpids/piab014
Close - Share Icon Share
Abstract
Staphylococcus aureus protein A (spa) typing can be used to expand characterization of the epidemiology of methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) in neonatal intensive care units (NICU).
From January 2017 to June 2018, twice-monthly surveillance for S. aureus was performed in an academically affiliated NICU. Decolonization of infants colonized with S. aureus included chlorhexidine gluconate bathing and/or mupirocin for those with mupirocin-susceptible strains. Spa typing and mupirocin-resistance testing were performed. Demographic and clinical characteristics were compared between infants colonized with MSSA vs MRSA and infants with and without the most common MSSA spa type, MSSA-t279.
Overall, 14% and 2% of 1556 hospitalized infants had positive surveillance cultures for MSSA and MRSA, respectively. Thirty-six infants harbored unique MSSA spa types, 5 infants harbored unique MRSA spa types, and 30 MSSA and 6 MRSA spa types were identified in ≥2 infants. No outbreaks were identified during the study period. MSSA-t279 was isolated from 3% of infants and largely detected from infants hospitalized in one section of the NICU; 96% of t279 isolates were mupirocin resistant. Infection rates, length of hospitalization, and mortality were similar among infants initially colonized with t279 vs other MSSA spa types.
The MSSA colonization burden was 5-fold larger than that of MRSA. Numerous unique spa types were identified. The most common spa type, MSSA-t279, was not associated with increased morbidity or mortality but was mupirocin resistant and associated with clustered NICU beds. This suggests potential transmission from the environment, shared staff, and/or workflow issues requiring further study. Other decolonization strategies for S. aureus in the NICU are needed.
Staphylococcus aureus is a well-known cause of healthcare-associated infections in the neonatal intensive care unit (NICU) population [1–3]. While most of the S. aureus infections in the NICU are caused by methicillin-susceptible S. aureus (MSSA) and MSSA causes similar morbidity and mortality compared with methicillin-resistant S. aureus (MRSA), MRSA has been prioritized for infection prevention and control (IP&C) measures due to antimicrobial resistance, concerns regarding vancomycin treatment and potential toxicity, and well-described outbreaks [4–6].
Pairing molecular typing with active surveillance efforts for both MSSA and MRSA could improve our understanding of acquisition patterns and risk factors for S. aureus and guide the development of more effective, targeted IP&C strategies. Staphylococcus aureus protein A (spa) typing can rapidly identify similar strains of S. aureus and thus can identify or rule out potential transmission and avert or control outbreaks [6–9]. However, few studies have used this typing strategy in the NICU population, particularly during routine surveillance efforts and in the absence of an identified outbreak.
We sought to address these knowledge gaps by describing the epidemiology of MSSA and MRSA spa types identified during an active surveillance and decolonization program, which included mupirocin, in our NICU and hypothesized that MSSA would exhibit more genetic heterogeneity than MRSA. We compared the burden of MSSA and MRSA colonization and assessed the epidemiology and clinical impact of common spa types.
METHODS
Study Design, Setting, and Population
From January 2017 to June 2018, in recognition of emerging national efforts to reduce S. aureus infections, we implemented prospective surveillance for MSSA and MRSA in our 58-bed academically affiliated, tertiary care NICU (~1000 admissions/y) based on a previously described program [10]. In September 2017, an additional 17-bed neonatal cardiac care ICU opened on a different floor, increasing the bed count to 75 beds. Infants included in this study had S. aureus first detected by surveillance cultures. The Columbia University Irving Medical Center Institutional Review Board approved this study with a waiver of informed consent.
Surveillance Cultures, Specimen Processing, and Spa Typing
Twice-monthly surveillance for S. aureus colonization was performed for all hospitalized infants by swabbing the anterior nares and 3 skin sites (axilla, umbilical stump, and groin), which were pooled for culture. Surveillance cultures were also obtained on admission for infants transferred to the NICU at 7 days of life and older. Surveillance samples were inoculated onto both Columbia CNA (Becton-Dickinson and Co., Franklin Lakes, NJ) and chromogenic agar (Spectra MRSA; Remel, Lenexa, KS) and incubated aerobically at 37°C for 24 hours. Presumptive S. aureus colonies were confirmed by catalase and rapid latex agglutination (StaphAurex; Remel, Lenexa, KS). To distinguish MSSA and MRSA, cefoxitin disk diffusion and PBP2a Culture Colony Test (Alere, Scarborough, ME) were used. Mupirocin resistance among S. aureus isolates detected by surveillance was performed using gradient diffusion (E-test, Biomerieux, Durham, NC). The mupirocin susceptibility breakpoint was ≤4 µg/mL.
As per the standard of care, the NICU team obtained relevant clinical cultures from infants with suspected infections. In this study, infections were defined as S. aureus isolated from a sterile body site (eg, blood) or skin and soft tissue infections. Clinical specimens were processed according to culture source [11]. Full antimicrobial susceptibility testing was performed for S. aureus isolates obtained for clinical purposes by the MicroScan WalkAway 96 Plus System with Pos MIC Panel Type 34 (Beckman Coulter, Inc., Sacramento, CA), but mupirocin susceptibility testing was not performed.
MSSA and MRSA isolates were characterized by spa typing of a single colony using standard primers as previously described [12]. Spa types were assigned using the Ridom StaphType software and associated database. Spa typing of initial surveillance isolates, recurrent surveillance isolates, and isolates causing infections in colonized infants was performed on available isolates. Spa-typing results were not available in real-time or provided to clinicians.
Decolonization Protocols
Infants colonized with either MSSA or MRSA underwent decolonization based on a regimen previously described [10]. Infants eligible for decolonization were >36 weeks gestational age or >4 weeks chronologic age. Infants colonized with S. aureus received chlorhexidine gluconate (CHG) baths on days 1, 5, and 7 and those with mupirocin-susceptible strains also had mupirocin applied to the nares thrice daily for 7 days. Infants ineligible for CHG baths who had mupirocin-susceptible isolates were treated with mupirocin. Infants with mupirocin-resistant isolates were not treated with mupirocin.
Data Collection and Analysis
Demographic and selected clinical characteristics were compared between infants with MSSA vs MRSA first detected by surveillance. Those whose first S. aureus isolate was identified by clinical cultures were excluded. MSSA-positive infants with and without the most common MSSA spa type, MSSA-t279, were also compared. Clinical characteristics included comorbid conditions classified by admitting International Classification of Diseases (ICD)-10 codes [13], selected exposures that occurred within the 2 weeks prior to the first positive S. aureus culture (eg, surgical procedures and respiratory support), and outcomes (eg, S. aureus infections, in-hospital mortality, and length of stay). To explore potential acquisition patterns, the room locations of infants in the larger noncardiac NICU were mapped during the 14 days prior to the detection of MSSA-t279.
Categorical variables were compared using chi-squared or Fisher’s exact tests, when appropriate. Continuous variables were compared using the Mann-Whitney test; correlations between variables were assessed using the Pearson’s correlation coefficient. Factors associated with MSSA vs MRSA colonization or with MSSA-t279 colonization with P-value < .10 in bivariate analysis were assessed in a multivariate logistic regression using the Firth’s Penalized Likelihood Approach to account for zero cell counts. Highly correlated variables (eg, admitting cardiac disorder vs surgery exposure) were excluded from final adjusted model. All statistical analyses were performed using SAS 9.4 (Cary, NC). A P-value < .05 was considered significant.
RESULTS
Study Population
During the 18-month surveillance period, 1556 infants were admitted to the NICU and 98% (2200/2235) of eligible surveillance cultures were obtained. Overall, 16% (n = 248) of admitted infants had S. aureus first identified by surveillance cultures. Demographic and clinical characteristics, exposures, and outcomes of MSSA- (n = 211) vs. MRSA- (n = 37) colonized infants were similar (Table 1). No outbreaks were identified during the study period by the Department of Infection Prevention and Control.
| . | MSSA n = 211 . | MRSA n = 37 . | P-value . |
|---|---|---|---|
| Demographic characteristics, n (%) | |||
| Male sex | 125 (59) | 20 (54) | 0.56 |
| Gestational age, weeks, median (IQR)a | 31 (28-35) | 31 (30-34) | 0.71 |
| Birthweight, grams, median (IQR) | 1350 (960-2420) | 1350 (985-2150) | 0.68 |
| Vaginal delivery | 60 (28) | 9 (24) | 0.61 |
| In-hospital birth | 190 (90) | 36 (97) | 0.22 |
| Multiple gestation | 64 (30) | 13 (35) | 0.56 |
| Race | 0.19 | ||
| White | 120 (57) | 20 (54) | — |
| Black/African American | 45 (21) | 4 (11) | — |
| Asian | 10 (5) | 4 (11) | — |
| Unknown/Other | 36 (17) | 9 (24) | — |
| Ethnicity | 0.83 | ||
| Hispanic | 59 (28) | 11 (30) | — |
| Non-Hispanic | 91 (43) | 14 (38) | — |
| Unknown | 61 (29) | 12 (32) | — |
| Age at first positive surveillance culture, days, median (IQR) | 24 (13-40) | 18 (9-32) | 0.06 |
| Clinical characteristics, n (%) | |||
| Admitting diagnosis | |||
| Prematurity | 148 (70) | 26 (70) | 0.99 |
| Congenital disorder | 50 (24) | 8 (22) | — |
| Cardiovascular | 31 (15) | 5 (14) | 0.85 |
| Other congenital disorderb | 19 (9) | 3 (8) | 0.72 |
| Respiratory | 6 (3) | 2 (5) | 0.34 |
| Other diagnosisc | 5 (2) | 1 (3) | 1.00 |
| Procedures, devices, and antibiotic therapy within 2 wk prior to positive culture, n (%) | |||
| Surgical procedure | 19 (9) | 4 (11) | 0.76 |
| Mechanical ventilation | |||
| Invasive | 46 (22) | 6 (16) | 0.44 |
| Noninvasive | 154 (73) | 28 (76) | 0.73 |
| Central line | 88 (42) | 15 (41) | 0.89 |
| Nasogastric tube | 120 (57) | 20 (54) | 0.75 |
| Antibiotic therapy | 95 (45) | 23 (62) | 0.05 |
| Retinopathy of prematurity exam | 35 (17) | 4 (11) | 0.37 |
| Outcomes | |||
| Mortality | 4 (2) | 1 (3) | 0.56 |
| Developed Staphylococcus aureus infection | 12 (6) | 4 (11) | 0.27 |
| Length of stay, days, median (IQR) | 57 (31-88) | 58 (35-107) | 0.43 |
| . | MSSA n = 211 . | MRSA n = 37 . | P-value . |
|---|---|---|---|
| Demographic characteristics, n (%) | |||
| Male sex | 125 (59) | 20 (54) | 0.56 |
| Gestational age, weeks, median (IQR)a | 31 (28-35) | 31 (30-34) | 0.71 |
| Birthweight, grams, median (IQR) | 1350 (960-2420) | 1350 (985-2150) | 0.68 |
| Vaginal delivery | 60 (28) | 9 (24) | 0.61 |
| In-hospital birth | 190 (90) | 36 (97) | 0.22 |
| Multiple gestation | 64 (30) | 13 (35) | 0.56 |
| Race | 0.19 | ||
| White | 120 (57) | 20 (54) | — |
| Black/African American | 45 (21) | 4 (11) | — |
| Asian | 10 (5) | 4 (11) | — |
| Unknown/Other | 36 (17) | 9 (24) | — |
| Ethnicity | 0.83 | ||
| Hispanic | 59 (28) | 11 (30) | — |
| Non-Hispanic | 91 (43) | 14 (38) | — |
| Unknown | 61 (29) | 12 (32) | — |
| Age at first positive surveillance culture, days, median (IQR) | 24 (13-40) | 18 (9-32) | 0.06 |
| Clinical characteristics, n (%) | |||
| Admitting diagnosis | |||
| Prematurity | 148 (70) | 26 (70) | 0.99 |
| Congenital disorder | 50 (24) | 8 (22) | — |
| Cardiovascular | 31 (15) | 5 (14) | 0.85 |
| Other congenital disorderb | 19 (9) | 3 (8) | 0.72 |
| Respiratory | 6 (3) | 2 (5) | 0.34 |
| Other diagnosisc | 5 (2) | 1 (3) | 1.00 |
| Procedures, devices, and antibiotic therapy within 2 wk prior to positive culture, n (%) | |||
| Surgical procedure | 19 (9) | 4 (11) | 0.76 |
| Mechanical ventilation | |||
| Invasive | 46 (22) | 6 (16) | 0.44 |
| Noninvasive | 154 (73) | 28 (76) | 0.73 |
| Central line | 88 (42) | 15 (41) | 0.89 |
| Nasogastric tube | 120 (57) | 20 (54) | 0.75 |
| Antibiotic therapy | 95 (45) | 23 (62) | 0.05 |
| Retinopathy of prematurity exam | 35 (17) | 4 (11) | 0.37 |
| Outcomes | |||
| Mortality | 4 (2) | 1 (3) | 0.56 |
| Developed Staphylococcus aureus infection | 12 (6) | 4 (11) | 0.27 |
| Length of stay, days, median (IQR) | 57 (31-88) | 58 (35-107) | 0.43 |
Abbreviations: MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; IQR, interquartile range.
aOne infant had missing gestational age information.
bOther congenital disorders included gastrointestinal disorders, neurologic disorders, congenital diaphragmatic hernia, congenital tongue anomaly, craniofacial bone abnormalities, gastroschisis, overgrowth syndrome, Pierre-Robin Sequence, and Trisomy 21.
cOther disorders included cyanosis, hypotonia, persistent vomiting, and neonatal abstinence syndrome.
| . | MSSA n = 211 . | MRSA n = 37 . | P-value . |
|---|---|---|---|
| Demographic characteristics, n (%) | |||
| Male sex | 125 (59) | 20 (54) | 0.56 |
| Gestational age, weeks, median (IQR)a | 31 (28-35) | 31 (30-34) | 0.71 |
| Birthweight, grams, median (IQR) | 1350 (960-2420) | 1350 (985-2150) | 0.68 |
| Vaginal delivery | 60 (28) | 9 (24) | 0.61 |
| In-hospital birth | 190 (90) | 36 (97) | 0.22 |
| Multiple gestation | 64 (30) | 13 (35) | 0.56 |
| Race | 0.19 | ||
| White | 120 (57) | 20 (54) | — |
| Black/African American | 45 (21) | 4 (11) | — |
| Asian | 10 (5) | 4 (11) | — |
| Unknown/Other | 36 (17) | 9 (24) | — |
| Ethnicity | 0.83 | ||
| Hispanic | 59 (28) | 11 (30) | — |
| Non-Hispanic | 91 (43) | 14 (38) | — |
| Unknown | 61 (29) | 12 (32) | — |
| Age at first positive surveillance culture, days, median (IQR) | 24 (13-40) | 18 (9-32) | 0.06 |
| Clinical characteristics, n (%) | |||
| Admitting diagnosis | |||
| Prematurity | 148 (70) | 26 (70) | 0.99 |
| Congenital disorder | 50 (24) | 8 (22) | — |
| Cardiovascular | 31 (15) | 5 (14) | 0.85 |
| Other congenital disorderb | 19 (9) | 3 (8) | 0.72 |
| Respiratory | 6 (3) | 2 (5) | 0.34 |
| Other diagnosisc | 5 (2) | 1 (3) | 1.00 |
| Procedures, devices, and antibiotic therapy within 2 wk prior to positive culture, n (%) | |||
| Surgical procedure | 19 (9) | 4 (11) | 0.76 |
| Mechanical ventilation | |||
| Invasive | 46 (22) | 6 (16) | 0.44 |
| Noninvasive | 154 (73) | 28 (76) | 0.73 |
| Central line | 88 (42) | 15 (41) | 0.89 |
| Nasogastric tube | 120 (57) | 20 (54) | 0.75 |
| Antibiotic therapy | 95 (45) | 23 (62) | 0.05 |
| Retinopathy of prematurity exam | 35 (17) | 4 (11) | 0.37 |
| Outcomes | |||
| Mortality | 4 (2) | 1 (3) | 0.56 |
| Developed Staphylococcus aureus infection | 12 (6) | 4 (11) | 0.27 |
| Length of stay, days, median (IQR) | 57 (31-88) | 58 (35-107) | 0.43 |
| . | MSSA n = 211 . | MRSA n = 37 . | P-value . |
|---|---|---|---|
| Demographic characteristics, n (%) | |||
| Male sex | 125 (59) | 20 (54) | 0.56 |
| Gestational age, weeks, median (IQR)a | 31 (28-35) | 31 (30-34) | 0.71 |
| Birthweight, grams, median (IQR) | 1350 (960-2420) | 1350 (985-2150) | 0.68 |
| Vaginal delivery | 60 (28) | 9 (24) | 0.61 |
| In-hospital birth | 190 (90) | 36 (97) | 0.22 |
| Multiple gestation | 64 (30) | 13 (35) | 0.56 |
| Race | 0.19 | ||
| White | 120 (57) | 20 (54) | — |
| Black/African American | 45 (21) | 4 (11) | — |
| Asian | 10 (5) | 4 (11) | — |
| Unknown/Other | 36 (17) | 9 (24) | — |
| Ethnicity | 0.83 | ||
| Hispanic | 59 (28) | 11 (30) | — |
| Non-Hispanic | 91 (43) | 14 (38) | — |
| Unknown | 61 (29) | 12 (32) | — |
| Age at first positive surveillance culture, days, median (IQR) | 24 (13-40) | 18 (9-32) | 0.06 |
| Clinical characteristics, n (%) | |||
| Admitting diagnosis | |||
| Prematurity | 148 (70) | 26 (70) | 0.99 |
| Congenital disorder | 50 (24) | 8 (22) | — |
| Cardiovascular | 31 (15) | 5 (14) | 0.85 |
| Other congenital disorderb | 19 (9) | 3 (8) | 0.72 |
| Respiratory | 6 (3) | 2 (5) | 0.34 |
| Other diagnosisc | 5 (2) | 1 (3) | 1.00 |
| Procedures, devices, and antibiotic therapy within 2 wk prior to positive culture, n (%) | |||
| Surgical procedure | 19 (9) | 4 (11) | 0.76 |
| Mechanical ventilation | |||
| Invasive | 46 (22) | 6 (16) | 0.44 |
| Noninvasive | 154 (73) | 28 (76) | 0.73 |
| Central line | 88 (42) | 15 (41) | 0.89 |
| Nasogastric tube | 120 (57) | 20 (54) | 0.75 |
| Antibiotic therapy | 95 (45) | 23 (62) | 0.05 |
| Retinopathy of prematurity exam | 35 (17) | 4 (11) | 0.37 |
| Outcomes | |||
| Mortality | 4 (2) | 1 (3) | 0.56 |
| Developed Staphylococcus aureus infection | 12 (6) | 4 (11) | 0.27 |
| Length of stay, days, median (IQR) | 57 (31-88) | 58 (35-107) | 0.43 |
Abbreviations: MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; IQR, interquartile range.
aOne infant had missing gestational age information.
bOther congenital disorders included gastrointestinal disorders, neurologic disorders, congenital diaphragmatic hernia, congenital tongue anomaly, craniofacial bone abnormalities, gastroschisis, overgrowth syndrome, Pierre-Robin Sequence, and Trisomy 21.
cOther disorders included cyanosis, hypotonia, persistent vomiting, and neonatal abstinence syndrome.
Spa typing of S. aureus Isolates
Most (79%, 486/615) of the S. aureus isolates were available for spa typing (404 MSSA and 82 MRSA); 91% of these isolates were identified by surveillance and 9% were from clinical cultures. Among the surveillance isolates available for spa typing, 61 MSSA spa types, 6 MRSA spa types, and 5 spa types shared by MSSA and MRSA were identified. Twelve MSSA isolates and zero MRSA isolates had unknown or untypeable spa types.
Thirty-six (18%) of the 195 infants colonized with MSSA with surveillance isolates available for spa typing harbored unique MSSA spa types not detected in other infants; 5 (14%) of the 37 infants colonized with MRSA harbored unique MRSA spa types. Thirty MSSA and 6 MRSA spa types were identified in 2 or more infants (Figure 1A and Figure 1B). Among 21 sets of twins and triplets, 17 (81%) shared the same spa type as their sibling(s).
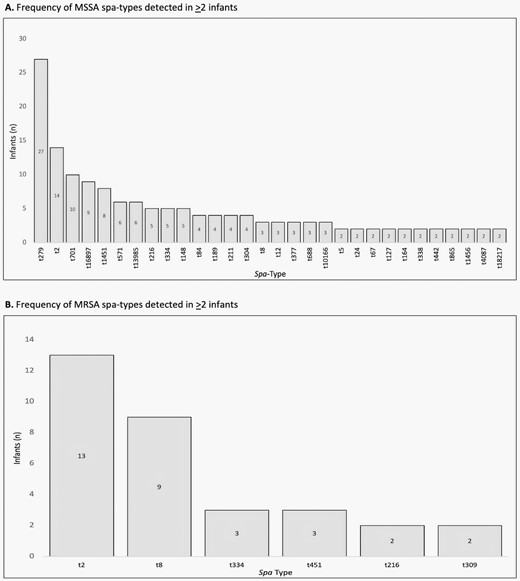
(A) Shared methicillin-sensitive Staphylococcus aureus (MSSA) spa types identified from infants’ first spa-typed S. aureus surveillance culture MSSA spa types representing infants’ first available surveillance isolate that was detected in ≥2 infants are shown. Spa-t279 was the most common, isolated in 27 infants whose first available isolate was from surveillance, followed by spa-t2 (n = 14) and t701 (n = 10). (B) Shared methicillin-resistant Staphylococcus aureus (MRSA) spa types identified from infants’ first spa-typed S. aureus surveillance culture MRSA spa types representing infants’ first available surveillance isolate detected in ≥2 infants are shown. Spa-t2 was the most common, isolated in 13 infants whose first available isolate was from surveillance, followed by t8 (n = 9).
Concordant Spa Types Among Surveillance Isolates and Infections
During the study period, 2% (34/1556) of admitted infants had S. aureus infections; 1.7% had infections with MSSA and 0.5% had infections with MRSA. Twenty-three (88%) of the 26 infants with MSSA infections had one or more surveillance cultures performed prior to infection; 10 (38%) had positive surveillance cultures for MSSA (median 5.5 days prior to infection detection, range: 2-27 days). Five of the 6 (83%) infants with both surveillance and clinical isolates had concordant MSSA spa types. Five (63%) of the 8 infants with MRSA infections had one or more surveillance cultures performed prior to infection. Two of the 3 (67%) infants with both surveillance and clinical isolates had concordant MRSA spa types.
Characteristics and Outcomes of Infants with MSSA-t279
The most common MSSA spa type was MSSA-t279, initially detected by surveillance in 27 infants and isolated in 16 of the 18 surveillance months from 1 to 4 infants each month. The most common MRSA spa type was MRSA-t2, detected by surveillance in 12 infants in 10 of the 18 surveillance months.
In multivariate logistic regression analysis, those whose first MSSA-t279 was detected by surveillance (n = 27) had significantly lower gestational age compared with infants whose initial surveillance culture was another spa type (n = 168) (Table 2). In-hospital mortality, infection rates, and length of stay were similar, as was the acquisition of another spa type.
Characteristics of Infants Colonized on Initial Surveillance With MSSA-t279 vs Other MSSA Spa Types
| . | MSSA-t279 Positive (n = 27) . | Other MSSA Spa Types (n = 168) . | Crude P-value . | ORadj (95% CI)a . | Adj. P-value . |
|---|---|---|---|---|---|
| Demographic characteristics, n (%) | |||||
| Male sex | 14 (52) | 100 (60) | 0.53 | --- | |
| Gestational age, weeks, median (IQR) | 28 (26-31) | 32 (28-37) | 0.0008 | 0.90 (0.81-1.00) | 0.046 |
| Vaginal delivery | 6 (22) | 51 (31) | 0.39 | --- | |
| In-hospital birth | 27 (100) | 148 (88) | 0.08 | --- | |
| Multiple gestation | 13 (48) | 45 (27) | 0.02 | 1.78 (0.76-4.17) | 0.19 |
| Birthweight, grams, median (IQR)a | 940 (630-1310) | 1538 (990-2853) | 0.0001 | --- | |
| Race | 0.89 | --- | |||
| White | 16 (59) | 93 (55) | --- | --- | |
| Black/African American | 7 (26) | 36 (21) | --- | --- | |
| Asian | 1 (4) | 8 (5) | --- | --- | |
| Unknown/Other | 3 (11) | 31 (18) | --- | --- | |
| Ethnicity | 0.28 | -- | |||
| Hispanic | 7 (26) | 49 (29) | --- | -- | |
| Non-Hispanic | 9 (33) | 75 (45) | --- | --- | --- |
| Unknown | 11 (41) | 44 (26) | --- | ||
| Age at first positive culture, days, median (IQR) | 28 (21-48) | 23 (13-39) | 0.02 | 1.00 (0.99-1.02) | 0.37 |
| Clinical characteristics n (%) | |||||
| Admitting diagnosis | |||||
| Prematuritya | 27 (100) | 107 (64) | 0.0002 | ||
| Congenital disordersa | 0 (0) | 49 (29) | 0.001 | --- | |
| Cardiovascular | 0 (0) | 30 (18) | 0.02 | ||
| Otherb | 0 (0) | 19 (11) | 0.05 | --- | |
| Other diagnosisc | 0 (0) | 15 (6) | 0.36 | ||
| Procedures, devices, and antibiotic therapy within 2 wk prior to positive culture, n (%) | |||||
| Surgical procedure | 0 (0) | 20 (12) | 0.08 | 0.94 (0.04-20.84) | 0.97 |
| Mechanical ventilation | |||||
| Invasive | 3 (11) | 41 (24) | 0.13 | --- | |
| Noninvasive | 20 (74) | 124 (74) | 0.98 | --- | |
| Central line | 11 (41) | 70 (42) | 1.00 | --- | |
| Nasogastric tube | 13 (48) | 103 (61) | 0.20 | --- | |
| Antibiotics | 5 (19) | 82 (49) | 0.003 | 0.40 (0.15-1.07) | 0.07 |
| Retinopathy of prematurity exam | 5 (19) | 30 (18) | 0.79 | -- | -- |
| Outcomes | |||||
| Mortality | 0 (0) | 4 (2) | 1.00 | --- | --- |
| Staphylococcus aureus infection | 1(4) | 8 (5) | 1.00 | --- | --- |
| Converted to other spa type | 2 (7) | 18 (11) | 1.00 | --- | --- |
| Length of stay, days, median (IQR) | 73 (42-108) | 57 (31-87) | 0.14 | --- | --- |
| . | MSSA-t279 Positive (n = 27) . | Other MSSA Spa Types (n = 168) . | Crude P-value . | ORadj (95% CI)a . | Adj. P-value . |
|---|---|---|---|---|---|
| Demographic characteristics, n (%) | |||||
| Male sex | 14 (52) | 100 (60) | 0.53 | --- | |
| Gestational age, weeks, median (IQR) | 28 (26-31) | 32 (28-37) | 0.0008 | 0.90 (0.81-1.00) | 0.046 |
| Vaginal delivery | 6 (22) | 51 (31) | 0.39 | --- | |
| In-hospital birth | 27 (100) | 148 (88) | 0.08 | --- | |
| Multiple gestation | 13 (48) | 45 (27) | 0.02 | 1.78 (0.76-4.17) | 0.19 |
| Birthweight, grams, median (IQR)a | 940 (630-1310) | 1538 (990-2853) | 0.0001 | --- | |
| Race | 0.89 | --- | |||
| White | 16 (59) | 93 (55) | --- | --- | |
| Black/African American | 7 (26) | 36 (21) | --- | --- | |
| Asian | 1 (4) | 8 (5) | --- | --- | |
| Unknown/Other | 3 (11) | 31 (18) | --- | --- | |
| Ethnicity | 0.28 | -- | |||
| Hispanic | 7 (26) | 49 (29) | --- | -- | |
| Non-Hispanic | 9 (33) | 75 (45) | --- | --- | --- |
| Unknown | 11 (41) | 44 (26) | --- | ||
| Age at first positive culture, days, median (IQR) | 28 (21-48) | 23 (13-39) | 0.02 | 1.00 (0.99-1.02) | 0.37 |
| Clinical characteristics n (%) | |||||
| Admitting diagnosis | |||||
| Prematuritya | 27 (100) | 107 (64) | 0.0002 | ||
| Congenital disordersa | 0 (0) | 49 (29) | 0.001 | --- | |
| Cardiovascular | 0 (0) | 30 (18) | 0.02 | ||
| Otherb | 0 (0) | 19 (11) | 0.05 | --- | |
| Other diagnosisc | 0 (0) | 15 (6) | 0.36 | ||
| Procedures, devices, and antibiotic therapy within 2 wk prior to positive culture, n (%) | |||||
| Surgical procedure | 0 (0) | 20 (12) | 0.08 | 0.94 (0.04-20.84) | 0.97 |
| Mechanical ventilation | |||||
| Invasive | 3 (11) | 41 (24) | 0.13 | --- | |
| Noninvasive | 20 (74) | 124 (74) | 0.98 | --- | |
| Central line | 11 (41) | 70 (42) | 1.00 | --- | |
| Nasogastric tube | 13 (48) | 103 (61) | 0.20 | --- | |
| Antibiotics | 5 (19) | 82 (49) | 0.003 | 0.40 (0.15-1.07) | 0.07 |
| Retinopathy of prematurity exam | 5 (19) | 30 (18) | 0.79 | -- | -- |
| Outcomes | |||||
| Mortality | 0 (0) | 4 (2) | 1.00 | --- | --- |
| Staphylococcus aureus infection | 1(4) | 8 (5) | 1.00 | --- | --- |
| Converted to other spa type | 2 (7) | 18 (11) | 1.00 | --- | --- |
| Length of stay, days, median (IQR) | 73 (42-108) | 57 (31-87) | 0.14 | --- | --- |
Symbol “--” indicates not determined and bolded results indicate significant associations.
Abbreviations: MSSA, methicillin-sensitive Staphylococcus aureus; CI, confidence interval; OR, odds ratio; IQR, interquartile range; adj., adjusted.
aFinal adjusted model included gestational age, age at first positive culture, in-hospital birth, multiple gestation, surgery, and antibiotics. Prematurity and birthweight highly correlated with gestational age (r = 0.756, P < .001) so excluded from final model; cardiac admitting disorders highly correlated with surgery so excluded from final model.
bOther congenital disorders included: gastrointestinal or neurological disorders, growth abnormalities, and genetic conditions.
cOther diagnoses included: respiratory disorders, hypotonia, neonatal abstinence syndrome, and persistent vomiting.
Characteristics of Infants Colonized on Initial Surveillance With MSSA-t279 vs Other MSSA Spa Types
| . | MSSA-t279 Positive (n = 27) . | Other MSSA Spa Types (n = 168) . | Crude P-value . | ORadj (95% CI)a . | Adj. P-value . |
|---|---|---|---|---|---|
| Demographic characteristics, n (%) | |||||
| Male sex | 14 (52) | 100 (60) | 0.53 | --- | |
| Gestational age, weeks, median (IQR) | 28 (26-31) | 32 (28-37) | 0.0008 | 0.90 (0.81-1.00) | 0.046 |
| Vaginal delivery | 6 (22) | 51 (31) | 0.39 | --- | |
| In-hospital birth | 27 (100) | 148 (88) | 0.08 | --- | |
| Multiple gestation | 13 (48) | 45 (27) | 0.02 | 1.78 (0.76-4.17) | 0.19 |
| Birthweight, grams, median (IQR)a | 940 (630-1310) | 1538 (990-2853) | 0.0001 | --- | |
| Race | 0.89 | --- | |||
| White | 16 (59) | 93 (55) | --- | --- | |
| Black/African American | 7 (26) | 36 (21) | --- | --- | |
| Asian | 1 (4) | 8 (5) | --- | --- | |
| Unknown/Other | 3 (11) | 31 (18) | --- | --- | |
| Ethnicity | 0.28 | -- | |||
| Hispanic | 7 (26) | 49 (29) | --- | -- | |
| Non-Hispanic | 9 (33) | 75 (45) | --- | --- | --- |
| Unknown | 11 (41) | 44 (26) | --- | ||
| Age at first positive culture, days, median (IQR) | 28 (21-48) | 23 (13-39) | 0.02 | 1.00 (0.99-1.02) | 0.37 |
| Clinical characteristics n (%) | |||||
| Admitting diagnosis | |||||
| Prematuritya | 27 (100) | 107 (64) | 0.0002 | ||
| Congenital disordersa | 0 (0) | 49 (29) | 0.001 | --- | |
| Cardiovascular | 0 (0) | 30 (18) | 0.02 | ||
| Otherb | 0 (0) | 19 (11) | 0.05 | --- | |
| Other diagnosisc | 0 (0) | 15 (6) | 0.36 | ||
| Procedures, devices, and antibiotic therapy within 2 wk prior to positive culture, n (%) | |||||
| Surgical procedure | 0 (0) | 20 (12) | 0.08 | 0.94 (0.04-20.84) | 0.97 |
| Mechanical ventilation | |||||
| Invasive | 3 (11) | 41 (24) | 0.13 | --- | |
| Noninvasive | 20 (74) | 124 (74) | 0.98 | --- | |
| Central line | 11 (41) | 70 (42) | 1.00 | --- | |
| Nasogastric tube | 13 (48) | 103 (61) | 0.20 | --- | |
| Antibiotics | 5 (19) | 82 (49) | 0.003 | 0.40 (0.15-1.07) | 0.07 |
| Retinopathy of prematurity exam | 5 (19) | 30 (18) | 0.79 | -- | -- |
| Outcomes | |||||
| Mortality | 0 (0) | 4 (2) | 1.00 | --- | --- |
| Staphylococcus aureus infection | 1(4) | 8 (5) | 1.00 | --- | --- |
| Converted to other spa type | 2 (7) | 18 (11) | 1.00 | --- | --- |
| Length of stay, days, median (IQR) | 73 (42-108) | 57 (31-87) | 0.14 | --- | --- |
| . | MSSA-t279 Positive (n = 27) . | Other MSSA Spa Types (n = 168) . | Crude P-value . | ORadj (95% CI)a . | Adj. P-value . |
|---|---|---|---|---|---|
| Demographic characteristics, n (%) | |||||
| Male sex | 14 (52) | 100 (60) | 0.53 | --- | |
| Gestational age, weeks, median (IQR) | 28 (26-31) | 32 (28-37) | 0.0008 | 0.90 (0.81-1.00) | 0.046 |
| Vaginal delivery | 6 (22) | 51 (31) | 0.39 | --- | |
| In-hospital birth | 27 (100) | 148 (88) | 0.08 | --- | |
| Multiple gestation | 13 (48) | 45 (27) | 0.02 | 1.78 (0.76-4.17) | 0.19 |
| Birthweight, grams, median (IQR)a | 940 (630-1310) | 1538 (990-2853) | 0.0001 | --- | |
| Race | 0.89 | --- | |||
| White | 16 (59) | 93 (55) | --- | --- | |
| Black/African American | 7 (26) | 36 (21) | --- | --- | |
| Asian | 1 (4) | 8 (5) | --- | --- | |
| Unknown/Other | 3 (11) | 31 (18) | --- | --- | |
| Ethnicity | 0.28 | -- | |||
| Hispanic | 7 (26) | 49 (29) | --- | -- | |
| Non-Hispanic | 9 (33) | 75 (45) | --- | --- | --- |
| Unknown | 11 (41) | 44 (26) | --- | ||
| Age at first positive culture, days, median (IQR) | 28 (21-48) | 23 (13-39) | 0.02 | 1.00 (0.99-1.02) | 0.37 |
| Clinical characteristics n (%) | |||||
| Admitting diagnosis | |||||
| Prematuritya | 27 (100) | 107 (64) | 0.0002 | ||
| Congenital disordersa | 0 (0) | 49 (29) | 0.001 | --- | |
| Cardiovascular | 0 (0) | 30 (18) | 0.02 | ||
| Otherb | 0 (0) | 19 (11) | 0.05 | --- | |
| Other diagnosisc | 0 (0) | 15 (6) | 0.36 | ||
| Procedures, devices, and antibiotic therapy within 2 wk prior to positive culture, n (%) | |||||
| Surgical procedure | 0 (0) | 20 (12) | 0.08 | 0.94 (0.04-20.84) | 0.97 |
| Mechanical ventilation | |||||
| Invasive | 3 (11) | 41 (24) | 0.13 | --- | |
| Noninvasive | 20 (74) | 124 (74) | 0.98 | --- | |
| Central line | 11 (41) | 70 (42) | 1.00 | --- | |
| Nasogastric tube | 13 (48) | 103 (61) | 0.20 | --- | |
| Antibiotics | 5 (19) | 82 (49) | 0.003 | 0.40 (0.15-1.07) | 0.07 |
| Retinopathy of prematurity exam | 5 (19) | 30 (18) | 0.79 | -- | -- |
| Outcomes | |||||
| Mortality | 0 (0) | 4 (2) | 1.00 | --- | --- |
| Staphylococcus aureus infection | 1(4) | 8 (5) | 1.00 | --- | --- |
| Converted to other spa type | 2 (7) | 18 (11) | 1.00 | --- | --- |
| Length of stay, days, median (IQR) | 73 (42-108) | 57 (31-87) | 0.14 | --- | --- |
Symbol “--” indicates not determined and bolded results indicate significant associations.
Abbreviations: MSSA, methicillin-sensitive Staphylococcus aureus; CI, confidence interval; OR, odds ratio; IQR, interquartile range; adj., adjusted.
aFinal adjusted model included gestational age, age at first positive culture, in-hospital birth, multiple gestation, surgery, and antibiotics. Prematurity and birthweight highly correlated with gestational age (r = 0.756, P < .001) so excluded from final model; cardiac admitting disorders highly correlated with surgery so excluded from final model.
bOther congenital disorders included: gastrointestinal or neurological disorders, growth abnormalities, and genetic conditions.
cOther diagnoses included: respiratory disorders, hypotonia, neonatal abstinence syndrome, and persistent vomiting.
Overall, MSSA-t279 was isolated from 47 (3%) of the 1556 admitted infants including the 27 infants whose first surveillance isolate was MSSA-t279 and 16 (34%) infants who were initially colonized with another spa type. Four (9%) other infants had initial detection of MSSA-t279 from a clinical culture (ie, eye discharge, surgical wound, or blood). The majority (96%, 88/92) of MSSA-t279 surveillance isolates were mupirocin resistant. The room locations of 40 infants with MSSA-t279 during the 14 days prior to the identification of this spa type are displayed in Figure 2; Rooms 3, 9, 10, 12, and 14, which were spatially clustered, housed most (70%, n = 28) of these infants.
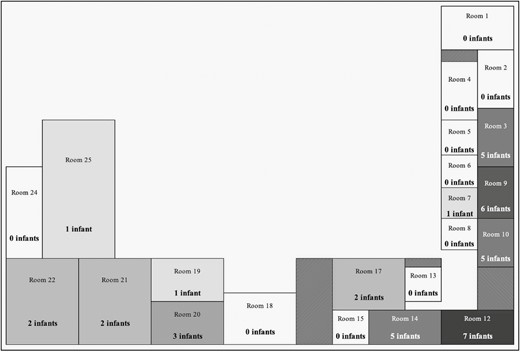
Bed locations of infants with methicillin-sensitive Staphylococcus aureus (MSSA)-t279 throughout the study period. The number of infants hospitalized in each room in the 14 days prior to their first detection of spa-type 279 (MSSA-t279) is shown. Rooms are 1-bed rooms, 2- and 3-bed pods, and 5-bed room. The shades depict the relative number of infants per room; darker shades represent a greater number of infants. Patterned boxes denote hallways and doorways. Of the 40 infants hospitalized in the larger neonatal intensive care units throughout the study period, 28 had been hospitalized in 5 rooms (3, 9, 10, 12, and 14) that clustered together.
DISCUSSION
This study demonstrated the dynamic epidemiology of S. aureus in the NICU population during a period of routine surveillance when no outbreaks were identified. As others have noted, the burden of MSSA was substantially higher than that of MRSA [14]. Overall, 14% and 2% of 1556 admitted infants had positive surveillance cultures for MSSA and MRSA, respectively, and 1.7% and 0.5% had infections with MSSA and MRSA, respectively. While MRSA has understandably been a priority for IP&C, MSSA should receive greater attention in the NICU population due to its greater burden and the potential for clusters of infections and outbreaks.
We confirmed our hypothesis that the genetic diversity of MSSA was greater than that of MRSA. By pairing our surveillance program with spa typing, we expanded our understanding of acquisition and transmission patterns of S. aureus. We identified 72 different spa types during 18 months of surveillance. Over half of the spa types (57%, n = 41/72) were isolated from only one infant or infants from multiple gestations suggesting that some infants acquired S. aureus during delivery or postnatally. We and others have demonstrated that ~30% of women have anovaginal colonization with S. aureus [15–17]. Additionally, Milstone et al. [18] recently demonstrated postnatal acquisition of MSSA from parents. However, many spa types were shared by 3–6 infants and some were shared by 8 or more infants suggesting transmission within the NICU. Confirmation of patient-to-patient transmission would require whole genome sequencing (WGS), which was unavailable in this study. We also found spa type concordance among the majority of available isolates associated with both colonization and infection, confirming the important role of colonization prior to invasive infection [19].
Though S. aureus isolated in this study exhibited genetic heterogeneity, MSSA-t279 was the most common spa type and detected in 3% of infants of whom 37% (16/43) were previously colonized by another spa type. This mupirocin-resistant strain was associated with NICU pods that clustered together, suggesting that room structure or staffing patterns provided opportunities for MSSA-t279 transmission and colonization. Goldstein et al. [20] noted similar findings when mapping MRSA colonization and infection in a NICU; neighboring bed spaces that housed MRSA-positive infants were significantly associated with increased MRSA acquisition. This further not only underscores the importance of horizontal IP&C strategies, eg, environmental cleaning and disinfection and hand hygiene, but may also suggest the need to explore a deeper analysis of room structure, workflow, and staffing patterns in NICU “hot spots” to better understand the root causes of transmission [21–24].
To our knowledge, this is the first identification of a mupirocin-resistant dominant MSSA clone in a NICU. Mupirocin resistance has been well reported in other populations, but previous decolonization programs in NICUs did not assess or found very little mupirocin resistance; additionally, previous mupirocin resistance reported in these populations reflected MRSA isolates [10, 25–28]. We have been decolonizing infants with mupirocin during MRSA outbreaks for over a decade [29]; therefore, the emergence of mupirocin resistance in MSSA in the NICU is concerning. High-level mupirocin resistance in MRSA is mediated by the mupA gene, which is carried on a plasmid [25]; future studies will assess the mechanism of mupirocin resistance in MSSA-t279. While colonization is the most significant risk factor for MRSA infections [5], no alternative approved methods currently exist to decolonize the anterior nares of infants nor to decolonize other body sites that may harbor S. aureus. Notably, MSSA-t279 was not more likely than other spa types to cause infections nor associated with increased mortality, suggesting that mupirocin resistance was not associated with more adverse clinical outcomes.
This study had limitations. Because this study was performed at a large, tertiary care NICU, these results may not be generalizable to all NICUs. As surveillance efforts were conducted every 2 weeks, neonates with shorter NICU hospitalizations may not have had surveillance cultures obtained, thus biasing the study population toward neonates with longer hospitalizations who are at increased risk of healthcare-associated infections and comorbidities. Not all clinical isolates were available for spa typing so that the frequency of spa types could be underestimated; we were also unable to assess whether certain spa types were associated with infection. Similarly, the relative proportions of other spa types may be overestimated. Spa typing single colonies could miss diversity if an infant harbored more than one spa type. The use of spa typing, rather than WGS, may overestimate the proportion of infants colonized with related strains. Mupirocin resistance testing was not performed on clinical isolates, so that the proportion of mupirocin-resistant isolates could be underestimated. The effectiveness of decolonization methods was not assessed. Due to the small number of infants initially colonized with MSSA-t279, we were underpowered to detect significant risk factors for acquisition of this vs other spa types.
In conclusion, this study enhances our understanding of the epidemiology of S. aureus colonization, particularly MSSA, in this vulnerable population. Through an active surveillance program, we found that S. aureus exhibited genetic heterogeneity with most of the infants harboring unique spa types, suggesting that visiting family members may be the reservoir for S. aureus. We also identified transmission of several spa types within the NICU, including a common mupirocin-resistant MSSA clone, the emergence of which furthers the evidence that an alternative decolonization method for S. aureus is greatly needed. We also identified the role of specific geographic areas in the NICU associated with the acquisition of the dominant spa type, supporting horizontal transmission of S. aureus through shared staff, the environment, or patient care equipment. Research directions include studies to better understand the mechanism(s) of mupirocin resistance in MSSA; the use of WGS to further elucidate the transmission patterns of S. aureus; and studies to assess the environment, workflow, and staffing patterns to identify additional IP&C strategies to reduce the S. aureus burden in the NICU.
Notes
Financial support. This work was supported by a National Institutes of Health TL1 grant (grant number: TL1 TR001875).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.



