-
PDF
- Split View
-
Views
-
Cite
Cite
Zainab M Al-Shammaa, Mohammed I Aladul, Narmin S Essa, Trends in anti-neuropathic medications turnover in Iraq, Journal of Pharmaceutical Health Services Research, Volume 14, Issue 4, November 2023, Pages 407–414, https://doi.org/10.1093/jphsr/rmad042
Close - Share Icon Share
Abstract
Neuropathic pain (NP) is a type of chronic pain. Numerous diseases and/or lesions are associated with the development of NP, and diabetes mellitus is the most prevalent cause. Several classes of medications were recommended and/or approved as anti-neuropathic medications. This study aimed to examine the trends in the turnover of anti-neuropathic medications in Iraq.
This was a retrospective analysis of the turnover of selected anti-neuropathic medications in the Iraqi market derived from Advanced Marketing Statistics between 2017 and 2021. The sales units were converted to defined daily doses. Regression analysis and correlation analysis were used to compare the turnover of the medications.
The overall volume of turnover of anti-neuropathic medications increased between 2017 and 2021. The anti-neuropathic market domination was shifted from carbamazepine to pregabalin, in which the average increase in pregabalin turnover of 60% [95% CI (confidence interval) 37.6–82.5] quarterly to achieve 42% of the market share (highest sales) by 2021. The overall expenditure on anti-neuropathic medications increased steadily over the study period.
The market of anti-neuropathic medications’ domination was shifted from the older antiepileptic drug (AED) (carbamazepine) to the newer AED (pregabalin). The availability and affordability of less expensive generic versions of these agents, together with the better safety profiles of the newer agents were the main driver for this shift. This indeed allowed Iraqi physicians to adhere to the latest international guidelines.
Introduction
According to the International Association for the Study of Pain, neuropathic pain (NP) is defined as “the pain that is evoked by a lesion or a disease of the peripheral or central somatosensory nervous system.” A wide range of diseases and/or lesions are associated with the development of NP, which can be broadly classified into central and peripheral [1]. The most common causes of central NP are stroke, spinal cord lesion, and multiple sclerosis while those of peripheral NP, diabetes mellitus is the most prevalent cause that leads to a condition known as diabetic neuropathy (DN) as well as other less prevalent causes like those caused by chemotherapy, post-viral infection (post-herpetic neuralgia), or trigeminal neuralgia. In general, all NP conditions share a similar management plan that focuses on providing symptomatic relief, as there are no disease-modifying medications available [2].
Globally, the management of NP is still challenging for healthcare professionals, this could be attributed to many causes including inadequate response to the available medications and the difficulty of dose escalation due to the high incidence rate of side effects [3]. Several classes of medications (antiepileptics, tricyclic antidepressants, selective norepinephrine serotonin reuptake inhibitors, and opioids) were recommended and/or approved as anti-neuropathic medications [4].
In Iraq, to date, there are no national or regional guidelines for NP management, this fact adds further challenges to the already existing challenges of NP management. The Iraqi healthcare system consists of public (free service for all Iraqi citizens) and private sectors (fully paid (out-of-pocket) services). The public sector provides services through nearly 300 public hospitals and 3000 primary health centers [5]. Medication procurement for the public sector is the responsibility of KIMADIA, a company owned by the Iraqi government. The procurement of medications by KIMADIA is governed by the National Committee of Drug Selection that produces a periodically updated list of essential medicine list and comprehensive medicine list. The essential medicine list includes medications and vaccines that should be accessible in the public sector, while the comprehensive medicine list includes additional medications that could be available in the private sector. The medications in the essential medicine list are further stratified into three levels according to their priority (need to be available) [6]. This means that level 1 medications are crucial and should be secured by KIMADIA, while level 2 and 3 medications are allowed to be available in the public sector. However, they could be secured by the peripheral healthcare facilities accordingly. The private sector is less regulated and offers a wide range of medications including both branded and generic products from national and international sources [7]. However, it is important to note that counterfeit pharmaceutical medications are also available in the private sector [8]. The absence of national guidelines and the disparity in medication availability between the public and private sectors will result in diverse trends in prescribing these medications. Being the strongest determinant of prescription medication turnover, a physician’s prescribing behavior is a complex process influenced by numerous factors. These factors include personal attributes, medication cost, patient preferences, medication efficacy and safety profile, medication accessibility, and attributes of pharmaceutical companies [9]. The aim of this study is to examine the trends in the turnover of anti-neuropathic medications in the Iraqi private sector over the last 5 years.
Methods
This study was conducted as a retrospective analysis of the turnover of selected anti-neuropathic medications in the Iraqi market over the period from January 2017 to December 2021. Despite the fact that amitriptyline, carbamazepine, duloxetine, gabapentin, and pregabalin are approved for the treatment of diseases other than NP such as depression, epilepsy, and anxiety, international guidelines have approved these medications as anti-neuropathic agents [10–14]. Studies have found that medications such as duloxetine, gabapentin, and pregabalin are primarily prescribed for NP [15–18]. A previous study conducted among Iraqi physicians specialized in managing NP has found that amitriptyline, carbamazepine, duloxetine, gabapentin, and pregabalin were the most commonly prescribed anti-neuropathic agents in Iraq [19].
The private sector sales data of those medications (amitriptyline, carbamazepine, duloxetine, gabapentin, and pregabalin) for the last 5 years was requested from Advanced Marketing Statistics (AMS), which is a Jordanian-based pharmaceutical market research company [20]. The AMS data cover the private pharmaceutical markets in Iraq. The provided data were at a gross national level, not at institutional or patient level, and are presented as molecule name, trade name(s), dosage form(s), cost (in US Dollars) per unit, the amount of the active ingredient(s), the expenditure, and the number of packs sold quarterly for each preparation containing the above-mentioned agents between January 2017 and December 2021.
The quarterly data for amitriptyline, carbamazepine, duloxetine, gabapentin, and pregabalin were downloaded from the database. The turnover volume for each molecule, measured in milligrams, is calculated by multiplying the number of packs sold by the amount of medicine in each unit pack. This result is then multiplied by the number of units (tablets or capsules) in each pack for each pharmaceutical product. Then the total number of milligrams for each molecule was calculated by summing the values for all products. The following formula was used in the calculation of turnover in terms of milligrams:
To compare the volume of turnover of different medications, the volume of turnover is converted to defined daily doses (DDDs). The defined daily dose is a technical measurement established by the World Health Organization (WHO) to make it possible to compare the turnover of different medicines of different strengths. The WHO defined DDD as “the assumed average maintenance dose per day for a drug used for its main indication in adults” [21]. For each medicine classified by the Anatomical Therapeutic Chemical classification, a DDD index is given. The DDD index for amitriptyline 75 mg, carbamazepine 1000 mg, duloxetine 60 mg, gabapentin 1800 mg, and pregabalin 300 mg [21]. The turnover of medicine (in DDD units) is calculated by dividing the volume of turnover in milligrams by the DDD index for that medicine. The following formula was used in the calculation [22]:
Statistical analysis
Data were analyzed using IBM® SPSS® Statistics version 24 software and Microsoft Excel® 2019. The turnover volume data in Table 1 are presented as the number of DDDs and percentages for each medication, as well as the percentage of change during the study period. Regression analysis was used to explore the nature of the relationship between the dependent variable (turnover in DDDs) and the independent variable (time in year quarter term). The percentage of change for each quarter was calculated by dividing the regression coefficient by the baseline turnover, which refers to the turnover in the first quarter of 2017. On the other hand, the expenditure on anti-neuropathic medications was calculated annually by multiplying the cost of each product sold by the number of items sold during the calendar year. The results were presented in both US Dollars and as a percentage of change during the study period (Table 2). The Defined Daily Dose Cost (DDDc) is a standardized measure of the cost for each DDD of a medication. It is calculated by dividing the median cost of medication by the turnover volume (in DDDs) for each medication. Furthermore, a correlation analysis was conducted using SPSS® software to examine the correlation between the turnover volume and the DDDc of each anti-neuropathic medication during the study period.
| . | 2017 . | 2018 . | 2019 . | 2020 . | 2021 . | Percentage of change in turnover quarterly . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|---|---|---|
| Amitriptyline | 3738726 | 3220893 | 4781556 | 3429571 | 6282162 | +23.6% | 1.4 to 45.9 | .038 |
| Carbamazepine | 10506218 | 13770590 | 6574148 | 7655807 | 10301243 | –7.67% | –19.6 to 4.2 | .091 |
| Duloxetine | 2852140 | 1009245 | 1935630 | 1248585 | 1647359 | –26.8% | –66.9 to 13.2 | .176 |
| Gabapentin | 5473065 | 4293454 | 4347819 | 3614615 | 4111952 | –8% | –22.65 to 5.44 | .215 |
| Pregabalin | 5505199 | 8489312 | 11541655 | 12302878 | 15856253 | +60% | 37.6 to 82.5 | .0001 |
| overall turnover | 28075349 | 30783495 | 29180808 | 28251458 | 38198969 | + 8.40% | –1.7 to 18.5 | .098 |
| . | 2017 . | 2018 . | 2019 . | 2020 . | 2021 . | Percentage of change in turnover quarterly . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|---|---|---|
| Amitriptyline | 3738726 | 3220893 | 4781556 | 3429571 | 6282162 | +23.6% | 1.4 to 45.9 | .038 |
| Carbamazepine | 10506218 | 13770590 | 6574148 | 7655807 | 10301243 | –7.67% | –19.6 to 4.2 | .091 |
| Duloxetine | 2852140 | 1009245 | 1935630 | 1248585 | 1647359 | –26.8% | –66.9 to 13.2 | .176 |
| Gabapentin | 5473065 | 4293454 | 4347819 | 3614615 | 4111952 | –8% | –22.65 to 5.44 | .215 |
| Pregabalin | 5505199 | 8489312 | 11541655 | 12302878 | 15856253 | +60% | 37.6 to 82.5 | .0001 |
| overall turnover | 28075349 | 30783495 | 29180808 | 28251458 | 38198969 | + 8.40% | –1.7 to 18.5 | .098 |
| . | 2017 . | 2018 . | 2019 . | 2020 . | 2021 . | Percentage of change in turnover quarterly . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|---|---|---|
| Amitriptyline | 3738726 | 3220893 | 4781556 | 3429571 | 6282162 | +23.6% | 1.4 to 45.9 | .038 |
| Carbamazepine | 10506218 | 13770590 | 6574148 | 7655807 | 10301243 | –7.67% | –19.6 to 4.2 | .091 |
| Duloxetine | 2852140 | 1009245 | 1935630 | 1248585 | 1647359 | –26.8% | –66.9 to 13.2 | .176 |
| Gabapentin | 5473065 | 4293454 | 4347819 | 3614615 | 4111952 | –8% | –22.65 to 5.44 | .215 |
| Pregabalin | 5505199 | 8489312 | 11541655 | 12302878 | 15856253 | +60% | 37.6 to 82.5 | .0001 |
| overall turnover | 28075349 | 30783495 | 29180808 | 28251458 | 38198969 | + 8.40% | –1.7 to 18.5 | .098 |
| . | 2017 . | 2018 . | 2019 . | 2020 . | 2021 . | Percentage of change in turnover quarterly . | 95% confidence interval . | P value . |
|---|---|---|---|---|---|---|---|---|
| Amitriptyline | 3738726 | 3220893 | 4781556 | 3429571 | 6282162 | +23.6% | 1.4 to 45.9 | .038 |
| Carbamazepine | 10506218 | 13770590 | 6574148 | 7655807 | 10301243 | –7.67% | –19.6 to 4.2 | .091 |
| Duloxetine | 2852140 | 1009245 | 1935630 | 1248585 | 1647359 | –26.8% | –66.9 to 13.2 | .176 |
| Gabapentin | 5473065 | 4293454 | 4347819 | 3614615 | 4111952 | –8% | –22.65 to 5.44 | .215 |
| Pregabalin | 5505199 | 8489312 | 11541655 | 12302878 | 15856253 | +60% | 37.6 to 82.5 | .0001 |
| overall turnover | 28075349 | 30783495 | 29180808 | 28251458 | 38198969 | + 8.40% | –1.7 to 18.5 | .098 |
| . | 2017 Expenditure (percentage) . | 2018 Expenditure (percentage) . | 2019 Expenditure (percentage) . | 2020 Expenditure (percentage) . | 2021 Expenditure (percentage) . | Change in the percentage of share expenditure (2017–2021) . |
|---|---|---|---|---|---|---|
| Amitriptyline | 872413 (3.15%) | 866012 (2.75%) | 912805 (2.49%) | 647645 (1.87%) | 927709 (2.46%) | 6.3% |
| Carbamazepine | 4641959 (16.8%) | 6421546 (20.43%) | 5246349 (14.36%) | 6286411 (18.20%) | 7555296 (20.06%) | 62.7% |
| Duloxetine | 1741723 (6.30%) | 898332 (2.85%) | 1931353 (5.28%) | 1311206 (3.79%) | 1461793 (3.88%) | –16% |
| Gabapentin | 7606605 (27.53%) | 5515377 (17.55%) | 6372220 (17.44%) | 5814998 (16.83%) | 5583171 (14.82%) | –26.6% |
| Pregabalin | 12766817 (46.20%) | 17723897 (56.40%) | 22068627 (60.41%) | 20475283 (59.28%) | 22121548 (58.75%) | 73.3% |
| Overall expenditure | 27629519 | 31425165 | 36531354 | 34535544 | 37649517 | 36.3% |
| . | 2017 Expenditure (percentage) . | 2018 Expenditure (percentage) . | 2019 Expenditure (percentage) . | 2020 Expenditure (percentage) . | 2021 Expenditure (percentage) . | Change in the percentage of share expenditure (2017–2021) . |
|---|---|---|---|---|---|---|
| Amitriptyline | 872413 (3.15%) | 866012 (2.75%) | 912805 (2.49%) | 647645 (1.87%) | 927709 (2.46%) | 6.3% |
| Carbamazepine | 4641959 (16.8%) | 6421546 (20.43%) | 5246349 (14.36%) | 6286411 (18.20%) | 7555296 (20.06%) | 62.7% |
| Duloxetine | 1741723 (6.30%) | 898332 (2.85%) | 1931353 (5.28%) | 1311206 (3.79%) | 1461793 (3.88%) | –16% |
| Gabapentin | 7606605 (27.53%) | 5515377 (17.55%) | 6372220 (17.44%) | 5814998 (16.83%) | 5583171 (14.82%) | –26.6% |
| Pregabalin | 12766817 (46.20%) | 17723897 (56.40%) | 22068627 (60.41%) | 20475283 (59.28%) | 22121548 (58.75%) | 73.3% |
| Overall expenditure | 27629519 | 31425165 | 36531354 | 34535544 | 37649517 | 36.3% |
| . | 2017 Expenditure (percentage) . | 2018 Expenditure (percentage) . | 2019 Expenditure (percentage) . | 2020 Expenditure (percentage) . | 2021 Expenditure (percentage) . | Change in the percentage of share expenditure (2017–2021) . |
|---|---|---|---|---|---|---|
| Amitriptyline | 872413 (3.15%) | 866012 (2.75%) | 912805 (2.49%) | 647645 (1.87%) | 927709 (2.46%) | 6.3% |
| Carbamazepine | 4641959 (16.8%) | 6421546 (20.43%) | 5246349 (14.36%) | 6286411 (18.20%) | 7555296 (20.06%) | 62.7% |
| Duloxetine | 1741723 (6.30%) | 898332 (2.85%) | 1931353 (5.28%) | 1311206 (3.79%) | 1461793 (3.88%) | –16% |
| Gabapentin | 7606605 (27.53%) | 5515377 (17.55%) | 6372220 (17.44%) | 5814998 (16.83%) | 5583171 (14.82%) | –26.6% |
| Pregabalin | 12766817 (46.20%) | 17723897 (56.40%) | 22068627 (60.41%) | 20475283 (59.28%) | 22121548 (58.75%) | 73.3% |
| Overall expenditure | 27629519 | 31425165 | 36531354 | 34535544 | 37649517 | 36.3% |
| . | 2017 Expenditure (percentage) . | 2018 Expenditure (percentage) . | 2019 Expenditure (percentage) . | 2020 Expenditure (percentage) . | 2021 Expenditure (percentage) . | Change in the percentage of share expenditure (2017–2021) . |
|---|---|---|---|---|---|---|
| Amitriptyline | 872413 (3.15%) | 866012 (2.75%) | 912805 (2.49%) | 647645 (1.87%) | 927709 (2.46%) | 6.3% |
| Carbamazepine | 4641959 (16.8%) | 6421546 (20.43%) | 5246349 (14.36%) | 6286411 (18.20%) | 7555296 (20.06%) | 62.7% |
| Duloxetine | 1741723 (6.30%) | 898332 (2.85%) | 1931353 (5.28%) | 1311206 (3.79%) | 1461793 (3.88%) | –16% |
| Gabapentin | 7606605 (27.53%) | 5515377 (17.55%) | 6372220 (17.44%) | 5814998 (16.83%) | 5583171 (14.82%) | –26.6% |
| Pregabalin | 12766817 (46.20%) | 17723897 (56.40%) | 22068627 (60.41%) | 20475283 (59.28%) | 22121548 (58.75%) | 73.3% |
| Overall expenditure | 27629519 | 31425165 | 36531354 | 34535544 | 37649517 | 36.3% |
Results
The overall turnover volume of anti-neuropathic medications (amitriptyline, carbamazepine, duloxetine, gabapentin, and pregabalin) in terms of DDDs increased between 2017 and 2021. The regression analysis revealed that the average quarterly change in turnover volume increased by (8.4%) [95% CI (confidence interval) –1.7 to 18.5] and was statistically insignificant (P value = .098).
Between 2017 and 2019, carbamazepine dominated the anti-neuropathic market and achieved 44% of the market share. In contrast, the market share of pregabalin doubled during the same period and achieved 42% by 2021, with a corresponding decrease in the turnover of carbamazepine of an average (7.67%) quarterly (95% CI –19.6 to 4.2). Regression analysis indicated that the increase in the turnover of pregabalin is statistically significant (P value = .0001) with an average increase in the turnover of 60% (95% CI 37.6–82.5) quarterly (Fig. 1).

Furthermore, the market share of amitriptyline increased slightly between 2019 and 2021 for which regression analysis revealed a statistically significant (P value of .038) increase in the turnover on an average of 23.6% (95% CI 1.4–45.9) quarterly. In contrast, there was a decrease in the turnover of gabapentin and duloxetine in which the average decrease was 8% (95% CI –22.65 to 5.44) and 26.8% (95% CI –66.9 to 13.2) respectively, however, it was statistically insignificant (P values were .215 and .176, respectively) (Table 1 and Figs. 1 and 2).

The annual trends in the turnover of anti-neuropathic medications.
The overall expenditure on anti-neuropathic medications increased steadily by 36.3% over the study period from 2017 to 2021 is from $27,629,519 to $37,649,517. This expenditure was driven mainly by pregabalin that increased by 73.3% from $12,766,817 (46% of overall expenditure) to $22,121,548 (60% of overall expenditure). The share of expenditure on carbamazepine only accounted for 16.8%–20% of the overall expenditure during the same period. In contrast, the share of expenditure on duloxetine and gabapentin decreased by 16% and 26.6%, respectively (Table 2).
The correlation analyses between the DDDc and the turnover of each anti-neuropathic medication showed a negative correlation regarding all anti-neuropathic medications. For pregabalin, the strength of the negative correlation was a strong correlation (R2 = 0.97) in which the turnover increased with the decrease in DDDc. The correlation strengths were moderate for amitriptyline and duloxetine (R2 were 0.66 and 0.57, respectively) while for gabapentin and carbamazepine, there were weak correlations (R2 were 0.18 and 0.11, respectively) (Fig. 3).
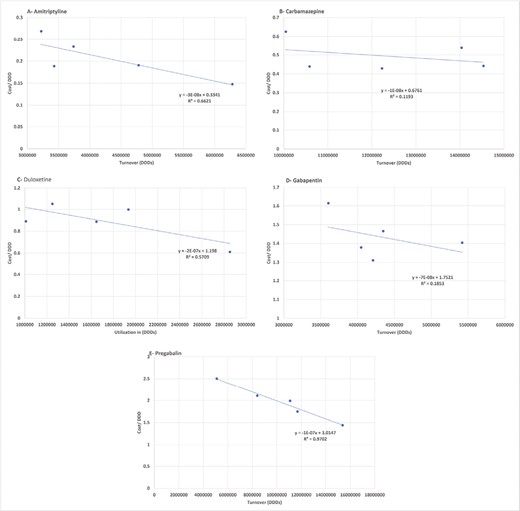
Correlation analysis between turnover and DDDc of anti-neuropathic medications.
Discussion
During the last 5 years, the market of anti-neuropathic medications has experienced steady growth, with significant changes in volume, expenditure, and market dominance. The market dominance shifted from older antiepileptic drugs (AEDs) such as carbamazepine and gabapentin to newer AED, pregabalin, which accounted for the largest share of the anti-neuropathic market by 2021. Several factors, such as the increased availability of less expensive generic versions of pregabalin and the recommendation to use this agent as a first-line treatment for NP by many international guidelines worldwide, might drive these changes in the market. However, the availability of less expensive generic versions could potentially increase the risk of abuse.
The growth in the market was largely attributed to the AEDs and specifically to the newer AED (pregabalin). This finding is consistent with many utilization studies in different countries [23–25]. In Denmark, Tsiropoulos et al. [23] study found an increase in the utilization of AEDs, which was attributed to the extension of the indications of newer AEDs to be used in neuropathic pain. Similarly, this study was consistent with the finding of Oteri et al. [24] study that was conducted in Italy in which they found an increase in the volume of utilization of AEDs and they attributed this growth to the use of newer AEDs in neuropathy. In contrast, the current study finding was inconsistent with the finding of Berman et al. [26] study, which was conducted in Israel, in which they found a plateaued level of AEDs turnover; however, they found a similar shift from old AEDs (carbamazepine) to the newer AEDs (pregabalin).
The increase in the turnover of anti-neuropathic medications in the current study could be explained by the increase in the prevalence of diabetes mellitus (DM) and its progressive nature that consequently led to the increase in complications [27]. Interestingly, the increase in the turnover of anti-neuropathic medications in Iraq was found to be remarkable between 2020 and 2021, this finding could be attributed to the outbreak of the coronavirus disease (COVID-19) pandemic infection [28]. The incidence of diabetic neuropathy and neuropathic pain was indirectly found to be associated with COVID-19 infection, furthermore, preexisting diabetic neuropathy was found to deteriorate with COVID-19 infection [29].
Although a direct comparison with other utilization studies from different countries is not appropriate due to the presence of many differences in terms of data level, the origin of the data (primary care, secondary care, or mixed data) and the study periods, however, some similarities and differences could be identified and reflect an overview of the market.
This study found that carbamazepine dominated the market of anti-neuropathic medications from 2017 to 2019 when pregabalin became the new market dominant. This is consistent with the study of Berman et al. [26] in which they found similar domination of carbamazepine, likewise, followed by shifting by newer AEDs. Even though carbamazepine’s main use and approved indications are related to epilepsy, its contribution to the anti-neuropathic market became implicit when its turnover trend decreased gradually with the introduction of newer AEDs (e.g. pregabalin), which is used mainly for neuropathy. The use of carbamazepine in treating neuropathic pain in Iraq could be attributed to its low cost and the wide availability of different generic products, in addition to its availability in the governmental sector (in primary and secondary care institutes), as it is classified as Level 1 medications according to the latest Iraqi medical list that guaranteed its secure by the Ministry of Health. The availability in the governmental sector contributed to its use in NP in two ways, first by providing physicians in primary care centers the opportunity to prescribe medications and become more comfortable with that prescribing. As NP is a chronic condition, more common in the older age group, patients who have been prescribed the medication for the first time will most likely continue to refill the prescription and tend to be reluctant to switch to another agent [30, 31]. Furthermore, physicians are used to prescribe carbamazepine as it has been available for more than three decades and have good knowledge of its side effects.
In contrast, many studies that used different methodologies showed a decreasing trend and/or small market share of carbamazepine in comparison with newer AEDs and/or anti-neuropathic medications [32–37]. This difference in market domination of carbamazepine between the current study and other studies could be attributed to different factors including the safety concerns about carbamazepine that gave rise to the implementation of health policies that opposed some limitations on the use of carbamazepine. For instance, after the discovery of the genetic association between carbamazepine use and severe cutaneous side effects, many countries imposed a regulation that required a screening test before prescribing carbamazepine [38]. Furthermore, the requirement of close monitoring of carbamazepine by means of therapeutic drug monitoring during the initiation phase also makes carbamazepine a less attractive choice. On the other hand, the availability of health insurance systems in other countries and the reimbursements of the newly approved medications for NP by healthcare systems in developed countries, all have an impact on the market of anti-neuropathic medications [39], keeping in mind that the newer AED (pregabalin) is to date classified as Level 2 medication, which limits its availability at the public health sector in Iraq.
This study indicated a concomitant gradual increase in the turnover of pregabalin with a decrease in that of carbamazepine. This trend is found to be in line with several countries (Australia, Canada, Japan, Italy, New Zealand, Norway, Saudi Arabia, Turkey, the UK, and the USA) utilization studies [17, 33,39–49]. This trend is supported by the latest international guidelines that placed pregabalin as a first-line pharmacological agent in DN management [10, 50]. However, the higher turnover of pregabalin might also be attributed to the increasing abuse of pregabalin worldwide. This was supported by many reports about pregabalin abuse in many countries [17, 44, 51–53] that gave rise to regulation policies implemented by several healthcare systems to limit the abuse of pregabalin [49, 54, 55]. These regulations had a pronounced impact on the utilization of pregabalin [39, 49] that further supports the probability of abuse. In Iraq, pregabalin’s increasing turnover might be attributed to the increased availability of generic and locally manufactured generic pregabalin (e.g. Pregafix®) with different dosage strengths and affordable prices. This factor was also observed by Kwok et al. [39] study in which they found that the availability of generic products was associated with an increase in the utilization of pregabalin. Furthermore, the availability of medication in the private sector (community pharmacies) increases the accessibility to non-controlled prescription medications since pregabalin is not listed as a controlled drug in the official Iraqi list of controlled drugs [56], in turn, this is also attributed to the increased turnover of pregabalin and shed light to possible misuse taking place. These findings highlight the need to conduct further studies to closely examine the trends in the prescribing of these medications.
The growth of the Iraqi pharmaceutical market and the increased number of generic firms’ entry into the pharmaceutical market resulted in price competition between these companies and offering discounts to provide the medication at affordable prices for the patients or governmental tenders. The impact of price competition was evident in the turnover of anti-neuropathic medications, especially for pregabalin, in which the current study found a strong negative correlation between the turnover and the DDDc reflected by the increased number of rivals (generic products) available in the market. This finding was consistent with the Bilgener et al. study [46].
In contrast, this study found a decreasing trend in the turnover of gabapentin and a smaller market share in comparison with pregabalin. This finding was in line with several utilization studies conducted in different countries (Australia and Italy) [40, 44]. This finding could be attributed to the tendency of physicians to prescribe the newer gabapentinoid (pregabalin) owing to its better efficacy, potency, and improved pharmacokinetic properties [57], in addition to its cost-effectiveness [58]. The finding of the current study was inconsistent with several studies conducted in other countries (Israel, Saudi Arabia, the UK, and the USA) in which they found an increasing or stable (plateaued) trend in gabapentin utilization [17, 26, 42, 47, 49, 59]. This inconsistency might be explained by many factors including variations in healthcare systems’ policies and regulations to limit the misuse of pregabalin and data on gabapentin’s less liability to be addictive [60]. Furthermore, the patent protection and the high prices of branded pregabalin in some countries like the UK resulted in recommending therapeutic substitution of generic gabapentin with branded pregabalin for patients with diabetic neuropathy to reduce costs [61].
Similarly, our study found a decreasing trend in duloxetine turnover, this finding is consistent with the Saudi study of Althunian et al. [49]. This finding could be attributed to the high cost of branded duloxetine and the limited number of generic products that are available in the Iraqi market. The trend of duloxetine is found to be increasing or stable in two studies [59, 62], this trend is further supported by the non-inferior efficacy of duloxetine along with its better safety profile in comparison with other approved anti-neuropathic medications [63]. In contrast, this study found a slight increase in the trend of amitriptyline turnover with a minor contribution to the market of anti-neuropathic medications. This finding might be contributed to the fact that the international guidelines are in between placing amitriptyline as the first or second line [64]. Amitriptyline was also found to have the lowest safety profile in a comparative study of six anti-neuropathic medications’ safety and efficacy despite its low cost [65].
Although the unit cost of all anti-neuropathic medications including pregabalin decreased annually, there was a steady increase in the annual expenditure on anti-neuropathic medications. This could be attributed to the increase in DM prevalence and subsequent increase in its complications including DN. Pregabalin was shown to be the main driver of the annual expenditure on anti-neuropathic medications. This finding could be explained by the high unit cost of pregabalin during the study period in comparison with other anti-neuropathic medications [66].
The strength of the current study is that it is the first drug utilization study to be conducted in Iraq, due to the unavailability of trusted data before. Furthermore, no previous study was conducted to determine the trend of the utilization of anti-neuropathic agents in the Middle East area. However, this study also has some limitations, since the data were limited to the private sector since the governmental data are confidential and the healthcare system for dispensing drugs at governmental institutes is still uncomputerized and the only available data include the supplied data rather than the dispensed one. The AMS data for Iraq started in 2017; therefore, this short time frame was unsuitable to conduct more complicated statistical analysis like the interrupted time series analysis, which is the most robust quasi-experimental design for drug utilization studies.
Conclusion
This study provides an overview of the turnover of commonly prescribed anti-neuropathic medications in Iraq from 2017 to 2021. The dominance in the market of anti-neuropathic medications has shifted from the older AED (carbamazepine) to the newer AED (pregabalin) due to the availability and affordability of less expensive generic versions of these agents, which also have better safety profiles. This indeed allowed Iraqi physicians to adhere to the latest international guidelines. However, the increasing turnover of these medications could also be an alarming sign for monitoring the prescribing and dispensing processes to avoid the potential misuse of these agents.
Acknowledgment
The authors acknowledge the AMS for the data provided.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Funding
This study was not funded by any organization and the researchers are independent of any funding bodies.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.



