-
PDF
- Split View
-
Views
-
Cite
Cite
Olivia Hughes, Katherine H Shelton, Helen Penny, Andrew R Thompson, Living With Physical Health Conditions: A Systematic Review of Mindfulness-Based Interventions for Children, Adolescents, and Their Parents, Journal of Pediatric Psychology, Volume 48, Issue 4, April 2023, Pages 396–413, https://doi.org/10.1093/jpepsy/jsad003
Close - Share Icon Share
Abstract
This systematic review aimed to identify and appraise studies investigating the efficacy of mindfulness-based interventions (MBIs) for improving depression, anxiety and parental stress in families affected by childhood physical illnesses, as well as feasibility and acceptability.
Embase, PsycINFO, Scopus, Medline, and PubMed were searched between February 2 and 17, 2021, and updated on August 5, 2022. Studies investigating MBIs with children and adolescents (<18 years) with physical health conditions were included, and results are presented with narrative synthesis.
Eighteen studies met eligibility criteria. Studies included children and adolescents with chronic pain, headaches, cancer, heart conditions, esophageal atresia, inflammatory bowel disease, and polycystic ovary syndrome. Most studies reported mindfulness was feasible and acceptable, although findings for different health conditions were mixed. Some studies encountered difficulties with attrition, resulting in findings being underpowered.
MBIs show promise for improving anxiety and depression in children with physical health conditions, but there is limited support for reducing stress in the family unit. A potential direction for future research might be the inclusion of parents. However, because of the heterogeneity of studies included in this review, findings must be cautiously interpreted.
Introduction
Physical health conditions require ongoing management with medication and other therapies, over a period of years or decades, and often cannot be cured (National Health Service, 2021). This applies to a range of health conditions including non-communicable diseases (e.g., cancer and cardiovascular disease), communicable diseases (e.g., human immunodeficiency virus [HIV] and acquired immunodeficiency syndrome [AIDS]), or impairments in structure (e.g., joint conditions). Many physical health conditions are long-term, such as diabetes, cardiovascular conditions, chronic respiratory conditions (e.g., asthma), neurological conditions (e.g., multiple sclerosis), chronic pain (e.g., arthritis), and inflammatory bowel disease (IBD) (National Health Service, 2021). During childhood, non-communicable diseases are globally responsible for over half of disability-affected life years (United Nations International Children’s Emergency Fund (UNICEF), 2019). Some of the most common physical health conditions affecting children include skin diseases (e.g., atopic dermatitis), migraine, and congenital abnormalities (United Nations International Children’s Emergency Fund (UNICEF), 2019). In the United Kingdom, asthma is the most prevalent childhood illness in 1.1 million children, epilepsy is diagnosed in 112,000 children, diabetes affects 36,000 children, and 1 in 5 children develop eczema (Great Ormond Street Hospital for Children: NHS Foundation Trust, 2020; Royal College of Paediatrics and Child Health, 2020).
Physical health conditions are classified by the World Health Organization (WHO) International Classification of Functioning, Disability and Health (ICF) as a dynamic interaction between a person’s health condition, environment, and personal factors (WHO, 2001), suggesting that physical health conditions could have an impact across several domains of life (McDougall et al., 2008). Indeed, children diagnosed with physical illnesses during childhood have been found to have a disproportionate level of mental health conditions by the age of 10 (Brady et al., 2020). Children with physical illnesses may experience adverse educational outcomes from poor school attendance (Emerson et al., 2016), attending medical appointments and undergoing treatment, or from deliberate avoidance (Sentenac et al., 2013). Although the psychological burden may depend on age (Ablett & Thompson, 2016), feeling ‘different’ could alter identity during an important time for development. Childhood illnesses also have the potential to dramatically affect family functioning, with parents experiencing stress (Cohn et al., 2020) as a result of adapting daily life and assuming the dual role of parent/caregiver without formal training (Ong et al., 2021). Currently, there is a need for more psychological support for children living with physical health conditions, and their families. Over recent years, mindfulness has been investigated as an approach to develop positive ways of healthily managing emotion and regulating affect (Zoogman et al., 2015).
Examples of mindfulness-based interventions (MBIs) include mindfulness-based stress reduction (MBSR) which aims to reduce behaviors associated with stress, and mindfulness-based cognitive therapy (MBCT) incorporating mindfulness exercises with cognitive behavioral therapy (CBT) techniques. Mindfulness-based interventions are based on the central concept described by Jon Kabat-Zinn (1994, p. 4) as developing a sense of “awareness that arises through paying attention on purpose, in the present moment, non-judgementally”. Mindfulness-based interventions have shown promise with parents of children with mental health conditions, including obsessive compulsive disorder (Belschner et al., 2020), attention deficit hyperactivity disorder (Behbahani et al., 2018), and autism (Dykens et al., 2014). Similarly, MBIs involving the child and their parents have been investigated with children with mental health conditions and developmental disorders (Bögels et al., 2008; Ridderinkhof et al., 2018).
Mindfulness has been investigated for children with obesity (Cotter et al., 2020; Emmanouil et al., 2018) and their families (Jastreboff et al., 2018) and has shown promising health outcomes for weight and blood pressure. Thus, mindfulness could improve quality of life by increasing effective management of symptoms and promote healthy regulation in other areas of life (e.g., lifestyle) that have a secondary impact on illness. For families, mindfulness could decrease stress in parents of children with chronic pain (Anclair et al., 2018; Seidman et al., 2019), reduce depression in parents of children with cancer (Mehranfar et al., 2012), and improve quality of life in parents of children with cerebral palsy (Mak et al., 2019). Similarly, mindful parenting has been found to increase parental responsiveness to children with cerebral palsy (Dieleman et al., 2021) and improve stress in caregivers of children with psoriasis and eczema (Heapy et al., 2022) perhaps from shifting focus from future worries, to accepting problems as they arise. Acceptance could therefore mediate parental distress (López et al., 2021), as experiential avoidance and cognitive defusion predicts negative outcomes in parents of children with chronic health conditions (e.g., burnout, stress, anxiety, depression) (Sairanen et al., 2018). Indeed, mindfulness concepts promoting present moment awareness and psychological flexibility could equip families to cope adaptively with childhood illness (Cousineau et al., 2019). There could be relevance in implementing MBIs with families affected by physical health conditions, as an alternative to more traditional CBT approaches. Specifically, practicing mindfulness could promote acceptance and healthy coping with the permanency of body symptoms or intense emotions related to long-term illness, instead of endeavoring to change maladaptive patterns of thinking.
Importantly, the use of MBIs with children and their parents is underexplored, and there is a need for research to examine the impact of physical illness on families in more depth. There have been calls for research to adopt a dyadic focus with wider investigations into the family unit to focus on the impact of being an informal caregiver (Moons et al., 2020). Although there have been studies investigating mindfulness with caregivers of children diagnosed with physical health conditions (Ruskin et al., 2021), study samples are often treated as separate groups, instead of acknowledging the shared burden. Therefore, there are several gaps in the literature which the current systematic review aims to address. This review will examine the involvement of parents/relevance of the inclusion of families in MBIs, which has not been previously investigated (Abujaradeh et al., 2018; Ahola Kohut et al., 2017). Previous systematic reviews by Abujaradeh et al. (2018) and Ahola Kohut et al. (2017) investigated MBIs in clinical samples of children and adolescents, whereas the current review will examine all samples (clinical and non-clinical). Previous reviews have examined samples of children (from 12 years of age) with chronic illnesses including mental health conditions (Abujaradeh et al., 2018), while the present review has a focus on MBIs for physical health conditions only, in samples <18 years. Since previous investigations (Ahola Kohut et al., 2017), the use of online delivery for psychological interventions has advanced, which could have implications for feasibility and acceptability of mindfulness.
Therefore, the objectives of this systematic review are twofold: (1) to employ a narrative synthesis to address a gap in knowledge in an understudied area and investigate the feasibility, acceptability and efficacy of MBIs for improving depression, anxiety and parental stress in families affected by childhood illness, and (2) to synthesize the most recent literature, since previous systematic reviews have been published (Abujaradeh et al., 2018; Ahola Kohut et al., 2017). This review will appraise existing evidence for MBIs with focus on psychological outcomes in children, and parental stress in their caregivers, to determine how well including parents in MBIs has been received, or if it might be practical to involve the family unit. Indeed, Ahola Kohut et al. (2017) suggests there is a need for further studies to focus on measures of emotional distress (e.g., anxiety and depression). This is an important area to investigate, as a synthesis of findings related to the potential role of mindfulness for promoting the psychological wellbeing of children, adolescents and families affected by childhood illnesses, could have value for the development of dyadic interventions.
Method
Eligibility Criteria
Studies were eligible for inclusion if they: (1) included a sample of children and adolescents with a physical health condition (e.g., diabetes, heart conditions, or cancer); (2) included an entire sample (<18 years old) of children or adolescents as the target population, and their parents or caregivers (e.g., involved in the MBI, provided support/assessment outcomes, or received MBI in parallel); (3) included an intervention based on a structured mindfulness program (e.g., MBSR or MBCT), and had been peer-reviewed and reported in English. Studies were excluded if they investigated single components of mindfulness (e.g., meditation, mindful eating, yoga, transcendental meditation) and trait/dispositional mindfulness in the absence of intervention. Qualitative studies, case studies and single cases were excluded unless they included an experimental design enabling assessment of efficacy. Children ‘at risk’ (not clinically diagnosed) of physical disease were excluded. Additionally, developmental and mental health conditions, and obesity were not included (Cotter et al., 2020; Emmanouil et al., 2018), and have similarly been excluded from previous systematic reviews as there exists a substantial literature base elsewhere (Abujaradeh et al., 2018). Dissertations and grey literature, protocols, conference abstracts, review, theory, and commentary papers were excluded.
Information Sources and Search Strategy
The searching and screening process was led by O.H between February 2 and 17, 2021, O.H searched five databases including Embase, PsycINFO, Scopus, Medline, and PubMed. On February 17, 2021 O.H also carried out a snowball search to identify additional eligible articles, with Google Scholar and a citation search by hand by O.H on relevant papers (references cited in articles included in this review, and references cited in systematic reviews on similar topics). Finally, the search was updated by O.H on August 5, 2022, using the same search method, but the results were filtered from 2021 onwards. Full details of each digital search strategy can be found in the Supplementary Files.
Selection Process
The search results from each database were exported into Microsoft Excel and duplicates were removed. O.H first screened studies for eligibility by title, followed by abstract. All studies conducting a MBI with the target population were subjected to full-text scrutiny by O.H, and studies not meeting eligibility criteria were excluded with reasons noted. The screened studies were independently corroborated by K.H.S and A.R.T to ensure there was agreement between researchers on included articles, and reasons for exclusion were discussed.
Data Collection Process and Data Items
The data collection process followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) statement (Page et al., 2021) (final checklist is available as Supplementary File 1), and focused on extracting information for tabulation on several outcome domains of interest including presenting a summary of psychological measures of quality of life, depression, anxiety, or parental stress, and feasibility and acceptability of mindfulness across the timeframe of intervention. Data were extracted from each report by O.H, including information on authors, geographical location, study sample (e.g., age, gender, race/ethnicity), involvement of parents, health condition and recruitment, study design, type of MBI/delivery, dropouts, control groups, outcome measures/frequency, and effect sizes/95% confidence intervals (where reported). Information on the structure of MBI was also extracted, including session content, facilitator, homework requirements, and fidelity checks (Supplementary Files). Feasibility was operationalized as being investigated by reporting information on recruitment and retention (Eldridge et al., 2016) and acceptability was determined from the perspective of the participant (e.g., from intervention feedback).
Certainty of Evidence Assessment
The certainty of evidence of each of the included articles was assessed with either the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for cohort, case–control, and cross-sectional studies (combined) (Von Elm et al., 2007), the Consolidated Standards of Reporting Trials (CONSORT) checklist for parallel group randomized trials (Schulz et al., 2010), or the CONSORT extension for randomized pilot and feasibility trials (Eldridge et al., 2016). These checklists were used to determine the quality of studies included in this review, and to assess study designs.
Study Risk of Bias Assessment
Based on the PRISMA (2020; Page et al., 2021) guideline, risk of bias assessments was examined for each individual study included in this review by O.H. To maintain rigor and corroborate risk of bias assessments, discrepancy checks were carried out independently by A.R.T, and differences in scores were resolved with discussion. The Cochrane Risk of Bias—second edition (RoB 2) was used to assess risk of bias for randomized studies (Higgins et al., 2011; Sterne et al., 2019), whereas the Risk of Bias in Nonrandomized Studies-of Interventions (ROBINS-I) Assessment Tool was used to assess risk of bias for all nonrandomized studies included in this review (Sterne et al., 2016).
Results
Study Selection
A flowchart in accordance with the PRISMA (2020) statement (Page et al., 2021) was developed to document the study selection process (Figure 1).
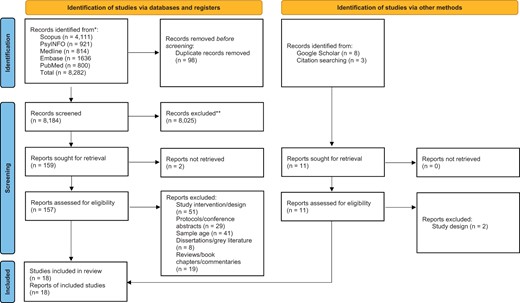
PRISMA (2020) flow diagram (Page et al., 2021) illustrating selection of studies.
Study Characteristics
Eighteen studies were included in the review (Table I). Studies were conducted between 2013 and 2022 in the United States (k = 8), Canada (k = 8), France (k = 1), and Iran (k = 1). Sample sizes ranged from 6 to 62 (mean = 22.94, SD = 15.37). The ages of children included in MBI groups ranged from 8 to 18 years (mean=14.69, SD = 1.59). Recruitment was almost exclusively carried out via university-affiliated hospitals, and disease specific-clinics and centers (k = 17), in some cases, combined with social media (Lovas et al., 2017; Young et al., 2022), and k = 1 study recruited pupils enrolled at a specialized school for children with chronic pain conditions (Lagor et al., 2013). Demographic information was collected, such as race and ethnicity (k = 8 studies), and study samples included participants who were White (n = 114), Asian (n = 20), African American and Black (n = 17), multiracial (n = 2), Hispanic Latino/Latina (n = 4), American Indian or Alaskan Native (n = 2), and ‘unknown’ or ‘other’ (n = 17). However, k = 10 studies did not report on race or ethnicity. All studies provided some information on gender of participants. Studies included children living with conditions such as: mixed chronic pain and chronic conditions (n = 133, k = 9), cancer (n = 54, k = 2), heart conditions (n = 56, k = 2), headaches (n = 20, k = 1), esophageal atresia (n = 19, k = 1), IBD (n = 80, k = 2), and polycystic ovary syndrome (n = 51, k = 1).
| Authors/location . | Sample . | Parents involved? . | Health condition/recruitment . | Study design . | MBI/delivery . | Dropouts/attrition . | Control group . | Outcome measures . | Frequency of measures . | Effect sizes and confidence intervals (95% CI; where reported) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Abedini et al. (2021): Iran |
| Completed parent-proxy measures | Cancer: pediatric cancer hospital | Randomized clinical trial |
| n = 1 dropouts | TAU |
| Pre-intervention/baseline, post-intervention, 2-month follow-up |
|
| Ahola Kohut et al. (2019): Canada |
| One time 2-hr workshop | Inflammatory bowel disease: tertiary paediatric hospital | Prospective, mixed-methods, uncontrolled |
| n = 2 attended one session/withdrew | None |
| Baseline, post-intervention, 3 month follow-up | NR |
| Ali et al. (2017): United States |
| n = 15 completed parent-proxy measures, attended concurrent group | Chronic pain: university/community clinician referral | Pilot feasibility |
| n = 2 dropouts, 83% completion | None |
| Baseline, post-intervention, 12-week follow-up | NR |
| Andreotti et al. (2017): France |
| Children accompanied by parent, n = 18 completed parent-proxy measures |
| Randomized, two-group |
| n = 9 lost to follow-up (MBI) | Wait list |
| Pre-intervention, follow-up phone call every 1–2 weeks, post-intervention |
|
| Chadi et al. (2016): Canada |
| No |
| Pilot randomized trial |
| n = 4 dropouts, 17% attrition rate | Wait list |
| Baseline, and weeks 1, 8, 11,18 | QoL: η2 = 0.01, depression: η2 = 0.13, anxiety: η2 = 0.05, pain perception: η2 = 0.01, psychological distress: η2 = 0.09, salivary cortisol level: η2 = 0.77 |
| Chadi et al. (2019): Canada |
| No | Chronic illness: tertiary pediatric hospital | Mixed method, randomized controlled trial |
| n = 4 dropouts after randomization | eHealth platform |
| Baseline, pre-and post-intervention, 2-month follow-up |
|
| Freedenberg et al. (2015): United States |
| No | Heart diseases: cardiology clinic | Pilot, descriptive, prospective, one-group |
| 0% dropouts, 100% completion | None |
| Baseline, post-intervention | Anxiety: η2=0.59, primary engagement control coping: η2=0.07, secondary engagement control coping: η2=0.01 |
| Freedenberg et al. (2017): United States |
| No | Cardiac disease: cardiology clinic | Randomized, two-group prospective |
| 2% dropouts | Video online support group |
| Pre-intervention, post-intervention | NR |
| Hesse et al. (2015): United States |
| Completed parent-proxy measures | Headache: academic neurology clinic | Pilot nonrandomized clinical trial |
| 25% dropouts | None |
| Pre-intervention, post-intervention | NR |
| Jastrowski Mano et al. (2013): United States |
| One time concurrent session, completed parent-proxy measures | Chronic pain: pediatric pain clinic | Randomized, controlled pilot |
| 62% attendance, 18% dropouts | Psycho-education |
| Pre-intervention, post-intervention, 4 week follow-up, 12 week follow-up | NR |
| Lagor et al. (2013): Canada |
| No | Chronic illnesses: specialized school for children with chronic illnesses | Feasibility, pre- post-test, uncontrolled |
| 0% dropouts | None |
| Pre-intervention, post-intervention | NR |
| Lovas et al. (2017): Canada |
| No | Chronic pain: pediatric clinics/social media | Single-arm, nonrandomized pilot |
| 0% dropouts | None | Pain: retrospective diary somatic symptoms: CSI functional disability: FD depression/. anxiety: RCADS | Pre-intervention, post-intervention, 3 month follow-up |
|
| Malboeuf-Hurtubise et al. (2016): Canada |
| No | Cancer: university-affiliated hospital | Prospective quasi-experimental pre-test–post-test, two groups |
|
| No treatment |
| Pre-intervention, post-intervention, 6 month follow-up |
|
| Ruskin et al. (2015): Canada | n = 16, mean age = 15.75 Race NR, 100% Female | Parenting component | Chronic pain: university-affiliated hospital | Feasibility, one-group, pre-test/post-test |
| 19% dropouts | None | Pain: pain characteristics questionnaire Mindfulness: CAMM/MAAS Psychological inflexibility: AFQ-Y Acceptance of pain: CPAQ Psychological inflexibility in pain: PIPS Qualitative evaluation | Baseline, post-intervention | NR |
| Ruskin et al. (2017): Canada |
| Parenting component | Chronic pain: Pediatric tertiary care clinic | Prospective pre-post |
| 0% dropouts | None |
| Baseline, post-intervention, 3 month follow-up |
|
| Waelde et al. (2017): United States |
| Completed parent-proxy measures | Chronic pain: tertiary pain clinic | Pilot nonrandomized clinical trial |
| n = 4 attrition, n = 2 lost to follow-up | None |
| Baseline, post-intervention |
|
| Wren et al., (2021): United States |
| No | Inflammatory bowel disease: children’s IBD center | Pilot feasibility and acceptability study | Mindfulness-based Virtual Reality (MBVR) one-off session, 6 min. | Attrition 80%, completion rate 75% (60% participation rate) | None |
| Baseline, post-intervention | NR |
| Young et al. (2022): United States |
| No | Polycystic ovary syndrome: adolescent medicine clinic/social media | Pilot randomized controlled trial |
| n = 15 dropouts | Wait list |
| Baseline, post-intervention |
|
| Authors/location . | Sample . | Parents involved? . | Health condition/recruitment . | Study design . | MBI/delivery . | Dropouts/attrition . | Control group . | Outcome measures . | Frequency of measures . | Effect sizes and confidence intervals (95% CI; where reported) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Abedini et al. (2021): Iran |
| Completed parent-proxy measures | Cancer: pediatric cancer hospital | Randomized clinical trial |
| n = 1 dropouts | TAU |
| Pre-intervention/baseline, post-intervention, 2-month follow-up |
|
| Ahola Kohut et al. (2019): Canada |
| One time 2-hr workshop | Inflammatory bowel disease: tertiary paediatric hospital | Prospective, mixed-methods, uncontrolled |
| n = 2 attended one session/withdrew | None |
| Baseline, post-intervention, 3 month follow-up | NR |
| Ali et al. (2017): United States |
| n = 15 completed parent-proxy measures, attended concurrent group | Chronic pain: university/community clinician referral | Pilot feasibility |
| n = 2 dropouts, 83% completion | None |
| Baseline, post-intervention, 12-week follow-up | NR |
| Andreotti et al. (2017): France |
| Children accompanied by parent, n = 18 completed parent-proxy measures |
| Randomized, two-group |
| n = 9 lost to follow-up (MBI) | Wait list |
| Pre-intervention, follow-up phone call every 1–2 weeks, post-intervention |
|
| Chadi et al. (2016): Canada |
| No |
| Pilot randomized trial |
| n = 4 dropouts, 17% attrition rate | Wait list |
| Baseline, and weeks 1, 8, 11,18 | QoL: η2 = 0.01, depression: η2 = 0.13, anxiety: η2 = 0.05, pain perception: η2 = 0.01, psychological distress: η2 = 0.09, salivary cortisol level: η2 = 0.77 |
| Chadi et al. (2019): Canada |
| No | Chronic illness: tertiary pediatric hospital | Mixed method, randomized controlled trial |
| n = 4 dropouts after randomization | eHealth platform |
| Baseline, pre-and post-intervention, 2-month follow-up |
|
| Freedenberg et al. (2015): United States |
| No | Heart diseases: cardiology clinic | Pilot, descriptive, prospective, one-group |
| 0% dropouts, 100% completion | None |
| Baseline, post-intervention | Anxiety: η2=0.59, primary engagement control coping: η2=0.07, secondary engagement control coping: η2=0.01 |
| Freedenberg et al. (2017): United States |
| No | Cardiac disease: cardiology clinic | Randomized, two-group prospective |
| 2% dropouts | Video online support group |
| Pre-intervention, post-intervention | NR |
| Hesse et al. (2015): United States |
| Completed parent-proxy measures | Headache: academic neurology clinic | Pilot nonrandomized clinical trial |
| 25% dropouts | None |
| Pre-intervention, post-intervention | NR |
| Jastrowski Mano et al. (2013): United States |
| One time concurrent session, completed parent-proxy measures | Chronic pain: pediatric pain clinic | Randomized, controlled pilot |
| 62% attendance, 18% dropouts | Psycho-education |
| Pre-intervention, post-intervention, 4 week follow-up, 12 week follow-up | NR |
| Lagor et al. (2013): Canada |
| No | Chronic illnesses: specialized school for children with chronic illnesses | Feasibility, pre- post-test, uncontrolled |
| 0% dropouts | None |
| Pre-intervention, post-intervention | NR |
| Lovas et al. (2017): Canada |
| No | Chronic pain: pediatric clinics/social media | Single-arm, nonrandomized pilot |
| 0% dropouts | None | Pain: retrospective diary somatic symptoms: CSI functional disability: FD depression/. anxiety: RCADS | Pre-intervention, post-intervention, 3 month follow-up |
|
| Malboeuf-Hurtubise et al. (2016): Canada |
| No | Cancer: university-affiliated hospital | Prospective quasi-experimental pre-test–post-test, two groups |
|
| No treatment |
| Pre-intervention, post-intervention, 6 month follow-up |
|
| Ruskin et al. (2015): Canada | n = 16, mean age = 15.75 Race NR, 100% Female | Parenting component | Chronic pain: university-affiliated hospital | Feasibility, one-group, pre-test/post-test |
| 19% dropouts | None | Pain: pain characteristics questionnaire Mindfulness: CAMM/MAAS Psychological inflexibility: AFQ-Y Acceptance of pain: CPAQ Psychological inflexibility in pain: PIPS Qualitative evaluation | Baseline, post-intervention | NR |
| Ruskin et al. (2017): Canada |
| Parenting component | Chronic pain: Pediatric tertiary care clinic | Prospective pre-post |
| 0% dropouts | None |
| Baseline, post-intervention, 3 month follow-up |
|
| Waelde et al. (2017): United States |
| Completed parent-proxy measures | Chronic pain: tertiary pain clinic | Pilot nonrandomized clinical trial |
| n = 4 attrition, n = 2 lost to follow-up | None |
| Baseline, post-intervention |
|
| Wren et al., (2021): United States |
| No | Inflammatory bowel disease: children’s IBD center | Pilot feasibility and acceptability study | Mindfulness-based Virtual Reality (MBVR) one-off session, 6 min. | Attrition 80%, completion rate 75% (60% participation rate) | None |
| Baseline, post-intervention | NR |
| Young et al. (2022): United States |
| No | Polycystic ovary syndrome: adolescent medicine clinic/social media | Pilot randomized controlled trial |
| n = 15 dropouts | Wait list |
| Baseline, post-intervention |
|
Note. NR = not reported; MBI = mindfulness based intervention; TAU = treatment as usual; MBSR = mindfulness-based stress reduction; MBCT = mindfulness-based cognitive behavioral therapy; CI = confidence interval; PCQ = Pain Characteristics Questionnaire; PCS = Pain Catastrophizing Scale; CPAQ-R = Chronic Pain Acceptance Questionnaire—Revised; MASC = Multidimensional Anxiety Scale for Children; CDS = Columbia Depression Scale; FDI = functional disability index; CAMM = child and adolescent mindfulness measure; PedsQL = pediatric quality of life inventory; DASS-21 = Depression Anxiety Stress Scales; LEIDS-R = Leiden index of depression sensitivity; RPA = responses to positive affect; ATS-R = attitudes towards self—revised; FCRI = Fear of Cancer Reoccurrence Inventory Severity Subscale; HADS = Hospital Anxiety and Depression Scale; RSQ = Responses to Stress Questionnaire; K-SADS-PL = kiddie schedule for affective disorders and schizophrenia-present and life-time version; CBCL = child behavior checklist; YSR = youth self-report; PFSD = Pain–Frequency–Duration Scale; PCS-C = Pain Catastrophizing Scale for Children; STAI-C = state-trait anxiety inventory for children; MSES = Mindfulness Self-efficacy Scale; CDS = Columbia Depression Scale; SEQ = Self-efficacy Questionnaire; PIPS = Psychological Inflexibility in Pain Scale; PROMIS = peer relationship short form; BYI-II = Beck youth inventories second edition; CSI = childhood somatization inventory; FDI = functional disability inventory; RCADS = Revised Child Anxiety and Depression Scale; MAAS = Mindful Attention and Awareness Scale; PSS = Perceived Stress Scale; CRSQ = Children’s Response Style Questionnaire; Aggression Scale, Questionnaire; HIV Quality of Life Scale; CAS = cognitive assessment system; CWS = color-word Stroop; ES = emotion Stroop; MASC2 = Multidimensional Anxiety Scale for Children Second Edition; FIQR = Fibromyalgia Impact Questionnaire Revised; SIQR = Analogue Symptom Impact Questionnaire Revised; MAAS-A = Mindful Attention Awareness Scale for Adolescents; STAI-C = Speilberger State-Trait Anxiety Inventory—Child Version; CDI = Children’s Depression Inventory; CERQ-k = Cognitive Emotion Regulation Questionnaire Kids Version; SSSS = MacArthur Scale of Subjective Socioeconomic Status; DMS = Diabetes Management Scale; BGM = blood glucose meter; CES-D = Center for Epidemiologic Studies Depression Scale; PSS-Fr = Perceived Social Support Friends Scale; PCQL-32-PF = pediatric cancer quality of life inventory; BIS = Body Image Scale; BDI-Y-II = Beck Youth Depression and Anxiety Scales second edition; IDPESQ-14 = Psychological Distress Scale; RSE = Rosenberg Self-esteem Scale; PI = Youth-Validated Illness Perception Questionnaire (brief version); PedMIDAS = pediatric migraine disability assessment; CPAQ-A = Chronic Pain Acceptance Questionnaire Adolescent Version; AFQ-Y = Avoidance and Fusion Questionnaire for Youth; CPAQ = Chronic Pain Acceptance Questionnaire; PIPS = Psychological Inflexibility in Pain Scale; NRS = Numeric Rating Scale; CDI = children’s depression inventory; SPPFI = Stanford pediatric pain functioning inventory; CHIP-AE = child health and illness profile adolescent edition; SCL-90R = symptom checklist-90 revised; BDI = Beck depression inventory; BAI = Beck anxiety inventory; PSQI = Pittsburgh sleep quality index; PANAS = positive and negative affect schedule; DIET-SE = Diet Self-Efficacy Scale; PACE = Adolescent Physical Activity Survey; VAS = Visual Analogue Scale.
| Authors/location . | Sample . | Parents involved? . | Health condition/recruitment . | Study design . | MBI/delivery . | Dropouts/attrition . | Control group . | Outcome measures . | Frequency of measures . | Effect sizes and confidence intervals (95% CI; where reported) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Abedini et al. (2021): Iran |
| Completed parent-proxy measures | Cancer: pediatric cancer hospital | Randomized clinical trial |
| n = 1 dropouts | TAU |
| Pre-intervention/baseline, post-intervention, 2-month follow-up |
|
| Ahola Kohut et al. (2019): Canada |
| One time 2-hr workshop | Inflammatory bowel disease: tertiary paediatric hospital | Prospective, mixed-methods, uncontrolled |
| n = 2 attended one session/withdrew | None |
| Baseline, post-intervention, 3 month follow-up | NR |
| Ali et al. (2017): United States |
| n = 15 completed parent-proxy measures, attended concurrent group | Chronic pain: university/community clinician referral | Pilot feasibility |
| n = 2 dropouts, 83% completion | None |
| Baseline, post-intervention, 12-week follow-up | NR |
| Andreotti et al. (2017): France |
| Children accompanied by parent, n = 18 completed parent-proxy measures |
| Randomized, two-group |
| n = 9 lost to follow-up (MBI) | Wait list |
| Pre-intervention, follow-up phone call every 1–2 weeks, post-intervention |
|
| Chadi et al. (2016): Canada |
| No |
| Pilot randomized trial |
| n = 4 dropouts, 17% attrition rate | Wait list |
| Baseline, and weeks 1, 8, 11,18 | QoL: η2 = 0.01, depression: η2 = 0.13, anxiety: η2 = 0.05, pain perception: η2 = 0.01, psychological distress: η2 = 0.09, salivary cortisol level: η2 = 0.77 |
| Chadi et al. (2019): Canada |
| No | Chronic illness: tertiary pediatric hospital | Mixed method, randomized controlled trial |
| n = 4 dropouts after randomization | eHealth platform |
| Baseline, pre-and post-intervention, 2-month follow-up |
|
| Freedenberg et al. (2015): United States |
| No | Heart diseases: cardiology clinic | Pilot, descriptive, prospective, one-group |
| 0% dropouts, 100% completion | None |
| Baseline, post-intervention | Anxiety: η2=0.59, primary engagement control coping: η2=0.07, secondary engagement control coping: η2=0.01 |
| Freedenberg et al. (2017): United States |
| No | Cardiac disease: cardiology clinic | Randomized, two-group prospective |
| 2% dropouts | Video online support group |
| Pre-intervention, post-intervention | NR |
| Hesse et al. (2015): United States |
| Completed parent-proxy measures | Headache: academic neurology clinic | Pilot nonrandomized clinical trial |
| 25% dropouts | None |
| Pre-intervention, post-intervention | NR |
| Jastrowski Mano et al. (2013): United States |
| One time concurrent session, completed parent-proxy measures | Chronic pain: pediatric pain clinic | Randomized, controlled pilot |
| 62% attendance, 18% dropouts | Psycho-education |
| Pre-intervention, post-intervention, 4 week follow-up, 12 week follow-up | NR |
| Lagor et al. (2013): Canada |
| No | Chronic illnesses: specialized school for children with chronic illnesses | Feasibility, pre- post-test, uncontrolled |
| 0% dropouts | None |
| Pre-intervention, post-intervention | NR |
| Lovas et al. (2017): Canada |
| No | Chronic pain: pediatric clinics/social media | Single-arm, nonrandomized pilot |
| 0% dropouts | None | Pain: retrospective diary somatic symptoms: CSI functional disability: FD depression/. anxiety: RCADS | Pre-intervention, post-intervention, 3 month follow-up |
|
| Malboeuf-Hurtubise et al. (2016): Canada |
| No | Cancer: university-affiliated hospital | Prospective quasi-experimental pre-test–post-test, two groups |
|
| No treatment |
| Pre-intervention, post-intervention, 6 month follow-up |
|
| Ruskin et al. (2015): Canada | n = 16, mean age = 15.75 Race NR, 100% Female | Parenting component | Chronic pain: university-affiliated hospital | Feasibility, one-group, pre-test/post-test |
| 19% dropouts | None | Pain: pain characteristics questionnaire Mindfulness: CAMM/MAAS Psychological inflexibility: AFQ-Y Acceptance of pain: CPAQ Psychological inflexibility in pain: PIPS Qualitative evaluation | Baseline, post-intervention | NR |
| Ruskin et al. (2017): Canada |
| Parenting component | Chronic pain: Pediatric tertiary care clinic | Prospective pre-post |
| 0% dropouts | None |
| Baseline, post-intervention, 3 month follow-up |
|
| Waelde et al. (2017): United States |
| Completed parent-proxy measures | Chronic pain: tertiary pain clinic | Pilot nonrandomized clinical trial |
| n = 4 attrition, n = 2 lost to follow-up | None |
| Baseline, post-intervention |
|
| Wren et al., (2021): United States |
| No | Inflammatory bowel disease: children’s IBD center | Pilot feasibility and acceptability study | Mindfulness-based Virtual Reality (MBVR) one-off session, 6 min. | Attrition 80%, completion rate 75% (60% participation rate) | None |
| Baseline, post-intervention | NR |
| Young et al. (2022): United States |
| No | Polycystic ovary syndrome: adolescent medicine clinic/social media | Pilot randomized controlled trial |
| n = 15 dropouts | Wait list |
| Baseline, post-intervention |
|
| Authors/location . | Sample . | Parents involved? . | Health condition/recruitment . | Study design . | MBI/delivery . | Dropouts/attrition . | Control group . | Outcome measures . | Frequency of measures . | Effect sizes and confidence intervals (95% CI; where reported) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Abedini et al. (2021): Iran |
| Completed parent-proxy measures | Cancer: pediatric cancer hospital | Randomized clinical trial |
| n = 1 dropouts | TAU |
| Pre-intervention/baseline, post-intervention, 2-month follow-up |
|
| Ahola Kohut et al. (2019): Canada |
| One time 2-hr workshop | Inflammatory bowel disease: tertiary paediatric hospital | Prospective, mixed-methods, uncontrolled |
| n = 2 attended one session/withdrew | None |
| Baseline, post-intervention, 3 month follow-up | NR |
| Ali et al. (2017): United States |
| n = 15 completed parent-proxy measures, attended concurrent group | Chronic pain: university/community clinician referral | Pilot feasibility |
| n = 2 dropouts, 83% completion | None |
| Baseline, post-intervention, 12-week follow-up | NR |
| Andreotti et al. (2017): France |
| Children accompanied by parent, n = 18 completed parent-proxy measures |
| Randomized, two-group |
| n = 9 lost to follow-up (MBI) | Wait list |
| Pre-intervention, follow-up phone call every 1–2 weeks, post-intervention |
|
| Chadi et al. (2016): Canada |
| No |
| Pilot randomized trial |
| n = 4 dropouts, 17% attrition rate | Wait list |
| Baseline, and weeks 1, 8, 11,18 | QoL: η2 = 0.01, depression: η2 = 0.13, anxiety: η2 = 0.05, pain perception: η2 = 0.01, psychological distress: η2 = 0.09, salivary cortisol level: η2 = 0.77 |
| Chadi et al. (2019): Canada |
| No | Chronic illness: tertiary pediatric hospital | Mixed method, randomized controlled trial |
| n = 4 dropouts after randomization | eHealth platform |
| Baseline, pre-and post-intervention, 2-month follow-up |
|
| Freedenberg et al. (2015): United States |
| No | Heart diseases: cardiology clinic | Pilot, descriptive, prospective, one-group |
| 0% dropouts, 100% completion | None |
| Baseline, post-intervention | Anxiety: η2=0.59, primary engagement control coping: η2=0.07, secondary engagement control coping: η2=0.01 |
| Freedenberg et al. (2017): United States |
| No | Cardiac disease: cardiology clinic | Randomized, two-group prospective |
| 2% dropouts | Video online support group |
| Pre-intervention, post-intervention | NR |
| Hesse et al. (2015): United States |
| Completed parent-proxy measures | Headache: academic neurology clinic | Pilot nonrandomized clinical trial |
| 25% dropouts | None |
| Pre-intervention, post-intervention | NR |
| Jastrowski Mano et al. (2013): United States |
| One time concurrent session, completed parent-proxy measures | Chronic pain: pediatric pain clinic | Randomized, controlled pilot |
| 62% attendance, 18% dropouts | Psycho-education |
| Pre-intervention, post-intervention, 4 week follow-up, 12 week follow-up | NR |
| Lagor et al. (2013): Canada |
| No | Chronic illnesses: specialized school for children with chronic illnesses | Feasibility, pre- post-test, uncontrolled |
| 0% dropouts | None |
| Pre-intervention, post-intervention | NR |
| Lovas et al. (2017): Canada |
| No | Chronic pain: pediatric clinics/social media | Single-arm, nonrandomized pilot |
| 0% dropouts | None | Pain: retrospective diary somatic symptoms: CSI functional disability: FD depression/. anxiety: RCADS | Pre-intervention, post-intervention, 3 month follow-up |
|
| Malboeuf-Hurtubise et al. (2016): Canada |
| No | Cancer: university-affiliated hospital | Prospective quasi-experimental pre-test–post-test, two groups |
|
| No treatment |
| Pre-intervention, post-intervention, 6 month follow-up |
|
| Ruskin et al. (2015): Canada | n = 16, mean age = 15.75 Race NR, 100% Female | Parenting component | Chronic pain: university-affiliated hospital | Feasibility, one-group, pre-test/post-test |
| 19% dropouts | None | Pain: pain characteristics questionnaire Mindfulness: CAMM/MAAS Psychological inflexibility: AFQ-Y Acceptance of pain: CPAQ Psychological inflexibility in pain: PIPS Qualitative evaluation | Baseline, post-intervention | NR |
| Ruskin et al. (2017): Canada |
| Parenting component | Chronic pain: Pediatric tertiary care clinic | Prospective pre-post |
| 0% dropouts | None |
| Baseline, post-intervention, 3 month follow-up |
|
| Waelde et al. (2017): United States |
| Completed parent-proxy measures | Chronic pain: tertiary pain clinic | Pilot nonrandomized clinical trial |
| n = 4 attrition, n = 2 lost to follow-up | None |
| Baseline, post-intervention |
|
| Wren et al., (2021): United States |
| No | Inflammatory bowel disease: children’s IBD center | Pilot feasibility and acceptability study | Mindfulness-based Virtual Reality (MBVR) one-off session, 6 min. | Attrition 80%, completion rate 75% (60% participation rate) | None |
| Baseline, post-intervention | NR |
| Young et al. (2022): United States |
| No | Polycystic ovary syndrome: adolescent medicine clinic/social media | Pilot randomized controlled trial |
| n = 15 dropouts | Wait list |
| Baseline, post-intervention |
|
Note. NR = not reported; MBI = mindfulness based intervention; TAU = treatment as usual; MBSR = mindfulness-based stress reduction; MBCT = mindfulness-based cognitive behavioral therapy; CI = confidence interval; PCQ = Pain Characteristics Questionnaire; PCS = Pain Catastrophizing Scale; CPAQ-R = Chronic Pain Acceptance Questionnaire—Revised; MASC = Multidimensional Anxiety Scale for Children; CDS = Columbia Depression Scale; FDI = functional disability index; CAMM = child and adolescent mindfulness measure; PedsQL = pediatric quality of life inventory; DASS-21 = Depression Anxiety Stress Scales; LEIDS-R = Leiden index of depression sensitivity; RPA = responses to positive affect; ATS-R = attitudes towards self—revised; FCRI = Fear of Cancer Reoccurrence Inventory Severity Subscale; HADS = Hospital Anxiety and Depression Scale; RSQ = Responses to Stress Questionnaire; K-SADS-PL = kiddie schedule for affective disorders and schizophrenia-present and life-time version; CBCL = child behavior checklist; YSR = youth self-report; PFSD = Pain–Frequency–Duration Scale; PCS-C = Pain Catastrophizing Scale for Children; STAI-C = state-trait anxiety inventory for children; MSES = Mindfulness Self-efficacy Scale; CDS = Columbia Depression Scale; SEQ = Self-efficacy Questionnaire; PIPS = Psychological Inflexibility in Pain Scale; PROMIS = peer relationship short form; BYI-II = Beck youth inventories second edition; CSI = childhood somatization inventory; FDI = functional disability inventory; RCADS = Revised Child Anxiety and Depression Scale; MAAS = Mindful Attention and Awareness Scale; PSS = Perceived Stress Scale; CRSQ = Children’s Response Style Questionnaire; Aggression Scale, Questionnaire; HIV Quality of Life Scale; CAS = cognitive assessment system; CWS = color-word Stroop; ES = emotion Stroop; MASC2 = Multidimensional Anxiety Scale for Children Second Edition; FIQR = Fibromyalgia Impact Questionnaire Revised; SIQR = Analogue Symptom Impact Questionnaire Revised; MAAS-A = Mindful Attention Awareness Scale for Adolescents; STAI-C = Speilberger State-Trait Anxiety Inventory—Child Version; CDI = Children’s Depression Inventory; CERQ-k = Cognitive Emotion Regulation Questionnaire Kids Version; SSSS = MacArthur Scale of Subjective Socioeconomic Status; DMS = Diabetes Management Scale; BGM = blood glucose meter; CES-D = Center for Epidemiologic Studies Depression Scale; PSS-Fr = Perceived Social Support Friends Scale; PCQL-32-PF = pediatric cancer quality of life inventory; BIS = Body Image Scale; BDI-Y-II = Beck Youth Depression and Anxiety Scales second edition; IDPESQ-14 = Psychological Distress Scale; RSE = Rosenberg Self-esteem Scale; PI = Youth-Validated Illness Perception Questionnaire (brief version); PedMIDAS = pediatric migraine disability assessment; CPAQ-A = Chronic Pain Acceptance Questionnaire Adolescent Version; AFQ-Y = Avoidance and Fusion Questionnaire for Youth; CPAQ = Chronic Pain Acceptance Questionnaire; PIPS = Psychological Inflexibility in Pain Scale; NRS = Numeric Rating Scale; CDI = children’s depression inventory; SPPFI = Stanford pediatric pain functioning inventory; CHIP-AE = child health and illness profile adolescent edition; SCL-90R = symptom checklist-90 revised; BDI = Beck depression inventory; BAI = Beck anxiety inventory; PSQI = Pittsburgh sleep quality index; PANAS = positive and negative affect schedule; DIET-SE = Diet Self-Efficacy Scale; PACE = Adolescent Physical Activity Survey; VAS = Visual Analogue Scale.
Certainty of Evidence in Individual Studies
All studies (n = 18) met the majority of criteria on the STROBE checklist for cohort, case–control, and cross-sectional studies (combined) (n = 11), CONSORT for parallel group randomized trials (n = 2), and the CONSORT extension for randomized pilot and feasibility trials (n = 5).
Risk of Bias in Individual Studies
Of the included studies, k = 7 randomized participants to either an MBI or control group, and k = 11 were nonrandomized designs. The RoB 2 (Higgins et al., 2011; Sterne et al., 2019) assessed randomized studies across five specific domains: (1) randomization process; (2) deviations from intended interventions; (3) missing outcome data; (4) measurement of the outcome, and (5) selection of the reported result (Higgins et al., 2011; Sterne et al., 2019). The ROBINS-I (Sterne et al., 2016) assessed nonrandomized studies across seven specific domains: (1) bias due to confounding; (2) bias due to selection of participants; (3) bias in classification of interventions; (4) bias due to deviations from intended interventions; (5) bias due to missing data; (6) bias in measurement of outcomes, and (7) bias in selection of the reported result. Graphical depictions were created by uploading the RoB 2 and ROBINS-I assessment form results generated with Microsoft excel into Robvis software to visually display the risk of bias assessment (McGuinness & Higgins, 2020). The RoB 2 results are presented in Figure 2A and B, and the ROBINS-I results are presented in Figure 3A and B. The RoB 2 provided overall judgments for k = 6 randomized studies as ‘high risk’ of bias, and k = 1 as ‘some concerns’. The ROBINS-I overall judgments suggested k = 8 studies were at ‘moderate’ risk of bias, k = 2 were at ‘high’ risk and k = 1 was ‘low’ risk. The ROBINS-I (Sterne et al., 2016) provided overall judgments for k = 11 studies, as k = 3 ‘serious’ risk, and k = 8 ‘moderate’ risk.
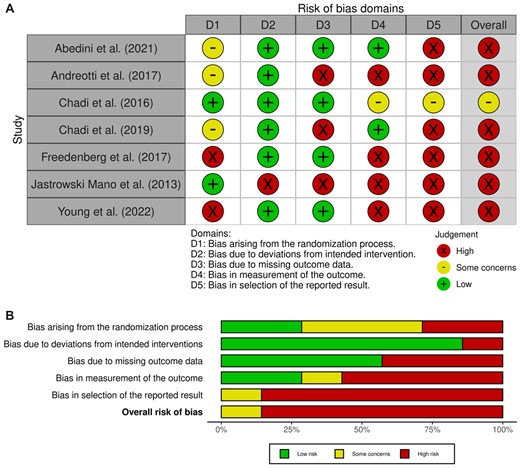
(A) Risk of bias for randomized trials (RoB 2). (B) Risk of bias for randomized trials (RoB 2) (Higgins et al., 2011; Sterne et al., 2019).
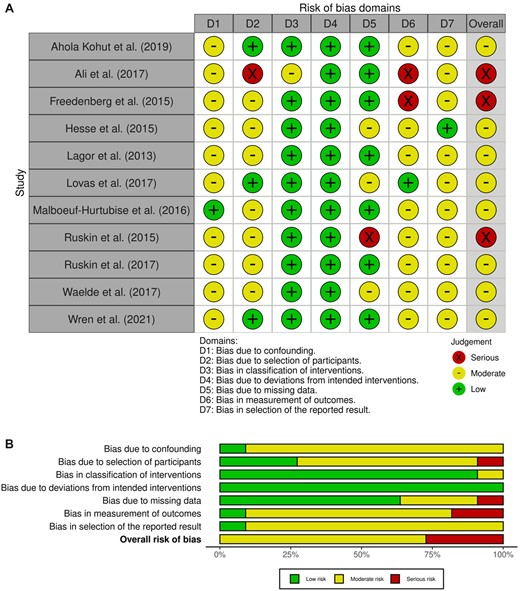
(A) Risk of Bias in Nonrandomized studies of Interventions (ROBINS-I). (B) Risk of Bias in Nonrandomized studies-of Interventions (ROBINS-I) (Sterne et al., 2016).
Mindfulness-Based Interventions
Supplementary Table 2 (Supplementary File) presents a summary of the content of MBIs. Nearly all studies involved face-to-face group delivery (k = 16). Two were digitalized or involved online components, including mindfulness-based virtual reality (MBVR; k = 1) and a dedicated website (k = 1). The majority of MBIs delivered to children and adolescents were modified (k = 13) for developmental age (e.g., MBCT-C, MARS-A) and physical health condition (e.g., PCOS Kind Mind), or MBSR (k = 4), and MBVR (k = 1). Although several studies described following standardized MBSR and MBCT protocols when adapting/designing interventions, the inclusion of fidelity checks was limited (k = 5) (Supplementary Table 2). The duration of MBI interventions ranged from a brief, one-time session (k = 1, Wren et al., 2021), to longer interventions lasting 4 weeks (k = 1), 5 weeks (k = 1), 6 weeks/42 days (k = 6), and 8 weeks (k = 9). The lengths of MBI sessions ranged from 4 min to 2 hr in length (mean = 79.16 min, SD = 34.46). In terms of numbers of sessions, they ranged from a singular one-off session to 42 days of daily practices. One study included a mindful retreat, lasting 4 hr (Ali et al., 2017).
Parent Caregiver Involvement in MBIs
Across the 18 studies, the involvement of parents varied in extent (k = 10). Level of involvement ranged from parents completing parent-proxy measures (Abedini et al., 2021; Ali et al., 2017; Andreotti et al., 2017; Hesse et al., 2015; Waelde et al., 2017), and two studies (Ruskin et al., 2015, 2017) incorporated a parenting component recognizing caregivers reinforce children’s coping. Three studies offered parents a concurrent group for familiarity and explanations of homework (Ahola Kohut et al., 2019; Ali et al., 2017; Jastrowski Mano et al., 2013). Only one study by Andreotti et al. (2017) involved complete parental participation in the MBI alongside their child.
Child Anxiety and Depression
Ten studies reported significant improvements in measures of anxiety, and six reported significant improvements in depression following participation in MBIs. All studies reported effect sizes, but Andreotti et al. (2017) did not specify which effect size calculation was used, and only one study (Young et al., 2022) reported individual 95% CIs. For children with chronic conditions participating in an adapted MBI, there were significant decreases in anxiety from pre-to-post intervention (p = .028), although no other significant differences for depression or quality of life were found (Lagor et al., 2013). Chadi et al.’s (2019) results suggested a significant reduction in anxiety and depression scores of adolescents with chronic illness immediately post-intervention for MARS-A via eHealth, with a large effect size (p = .048, Cohen’s d = 0.934), but this was not maintained as significant at 2-month follow-up. Improvements were recorded in 80% of children with somatic syndromes for anxiety and 60% for stress after MBSR, and anxiety scores remained statistically significant at 12-week follow-up (p = .47) (Ali et al., 2017). For IBD, there was a significant decrease in anxiety post-MBI (p < .001), and pain (p = .001) which was investigated based on age, and was significant for adolescents over, and under the age of 18 years (Wren et al., 2021). Similar findings were reported for adolescents with IBD by Ahola Kohut et al. (2019), with significant differences in emotional functioning following participation in an MBI-A group, and improvements in depression. There were significant reports of reduced rumination (effect size 0.64), and decreases in anxiety and depression in children with esophageal atresia after participation in a home-based MBI (Andreotti et al., 2017). Freedenberg et al. (2015) found a significant decrease in anxiety in adolescents with implantable cardioverter defibrillators and pacemakers, following MBSR with a large effect size (n2 = 0.59), and anxiety decreased from baseline to post-intervention with 90% of adolescents reporting lowered anxiety, but depression did not change significantly. However, this was not followed up in a later study by the same authors, and neither anxiety or depression scores were significant following MBSR or video groups (Freedenberg et al., 2017). Conversely, Hesse et al. (2015) reported no reduction in anxiety scores, but did find decreased depressive symptoms in adolescents with headaches, which could be explained by differences in intensity of home practice.
Not all studies identified significant differences. Chadi et al. (2016) measured psychological outcomes in female adolescents with chronic pain following MBI and reported no significant changes to scores of psychological distress, depression, anxiety, pain perception, or quality of life. Similar findings were reported by Ruskin et al. (2015) and no significant differences were reported for negative emotionality following MBI. Young et al. (2022) found no significant differences after MBI for depression, anxiety, stress, mindfulness, or self-esteem in adolescents with polycystic ovary syndrome. Similarly, Malboeuf-Hurtubise et al. (2016) found no significant differences between groups of adolescents with cancer for mood from pre- to post-intervention, but did report a significant different in levels of negative emotionality pre- to post-intervention in the control group (p = .04). Findings regarding follow-up were mixed, however, MBCT-C showed significant reductions in internalizing symptoms (Cohen’s d = 3.39) and attention problems in children hospitalized with cancer from baseline to follow-up (Cohen’s d = 2.52) when compared to treatment as usual from pre-intervention to follow-up with large effect sizes (Abedini et al., 2021).
Caregiver Stress and Anxiety
Waelde et al. (2017) found that parents worry in relation to their child’s pain significantly decreased over the 6-week MBI period, with a large effect size (Cohen’s d = 0.75). Parents reported the value of concurrent caregiver sessions, and felt that incorporating mindfulness into their own lives and connecting with other parents had helped to reduce stress (Ali et al., 2017). For the family, there were reports of increased bonding, and feelings of calm (Andreotti et al., 2017). This positive impact for the family was supported by qualitative evaluations conducted by Hesse et al. (2015) where out of 15 parents, 93.3% felt participation in MBI had been beneficial for their child, with 20% describing increased calmness in their children, an increased ability to cope with stress, depression, and pain, and better relations between siblings and parents in the household. However, some caregivers expressed worry about an illness-focused group potentially triggering distressing memories for their child (Chadi et al., 2016; Jastrowski Mano et al., 2013; Malboeuf-Hurtubise et al., 2016).
Feasibility and Acceptability of MBIs
In terms of feasibility of MBIs, there were several barriers to recruitment, including difficulties in getting to the location of the MBI, and living too far from the location of the intervention (Chadi et al., 2016; Malboeuf-Hurtubise et al., 2016; Ruskin et al., 2015). For example, Waelde et al. (2017) reported 4 of 20 participants not meeting attendance criteria, because of transport difficulties, and health problems. In some cases, the MBI was at the end of school-term and conflicted with events (Malboeuf-Hurtubise et al., 2016). Issues were also reported by Ahola Kohut et al. (2019) with 56.8% of approached participants declining from scheduling conflicts. Some studies encountered more difficulties than others. Jastrowski Mano et al. (2013) reported a recruitment rate of 49.2%, with 80.7% of approached participants withdrawing before the start of the study, resulting in the cancellation of a treatment wave, and data being individually plotted. Despite these challenges, Freedenberg et al. (2015) succeeded with 100% of participants completing the MBI and study measures, which was similar in further study by the same researchers with a 95% completion rate (Freedenberg et al., 2017). There were no dropouts in a study by Lovas et al. (2017). In terms of parental recruitment and retention, Andreotti et al. (2017) reported 57% of exercises were completed with one or two parents (mean parental help = 67.3%), however, only 4 of 19 children were regularly joined by parents at sessions. From parental feedback, this was explained by parents being surprised at the effort required in assisting their child in attending sessions (Andreotti et al., 2017). Similar findings were reported by Ali et al., (2017), with inconsistent parent participation, only attending sessions 66% of the time.
The acceptability of MBIs could have been influenced by delivery. When comparing in-person MBI delivery with eHealth, participants in an eHealth group had significant pre-post mindfulness reduction in salivary cortisol levels (Chadi et al., 2019). One study had to change the delivery of mindfulness from face-to-face group sessions to online via video conferencing software after difficulties were encountered with travel and participants’ time (Young et al., 2022). However, the efficacy of MBSR was compared with an online support group for cardiac disease, and no differences were found between groups as the intervention group had significantly higher baseline anxiety and depression scores, which could have been a result of inaccurate randomization (Freedenberg et al., 2017). Chadi et al. (2019) implemented measures against preferences affecting randomization, and excluded adolescents who based expressed a preference for eHealth or refused to participate in face-to-face MBI (e.g., if they thought they lived too far), supporting the suggestion that digital interventions could increase accessibly for participants living within a reasonable distance of a location offering in-person sessions. Studies that conducted intervention evaluation feedback and exit interviews reported on children’s experiences of mindfulness. Preferences for session length were mixed, with some children preferring 60-min sessions (Waelde et al., 2017), and others favoring 90-min sessions instead of 2-hr sessions (Ahola Kohut et al., 2019). This was also true for meditation, as some children did not enjoy long practices (Ahola Kohut et al., 2019). Despite this, one study reported suggestions for extending the MBI to be several weeks longer (Freedenberg et al., 2017). Other suggested improvements included having immediate exercises for dealing with flare-ups of pain (Ruskin et al., 2015). Across studies, participants appeared to value some mindfulness exercises more than others. For example, one study reported participants had mixed views on meditation and yoga exercises (Freedenberg et al., 2017), and others preferred real-life examples to metaphorical exercises (Ahola Kohut et al., 2019). ‘Wise mind’, painting (Ruskin et al., 2015), breathing exercises, meditation, and relaxation (Freedenberg et al., 2017) were also preferred by children. Group-delivery was favored for same-age peer interactions and allowing participants to speak openly about their feelings (Ahola Kohut et al., 2019; Lagor et al., 2013; Malboeuf-Hurtubise et al., 2016; Waelde et al., 2017). Ruskin et al. (2017) reported a 90.5% completion rate, with all adolescents reporting being highly satisfied and would recommend MBI-A to a friend.
Discussion
Using narrative synthesis, we aimed to (1) address a knowledge gap in an understudied area, and investigate the feasibility, acceptability and efficacy of MBIs for improving depression, anxiety and parental stress in families affected by childhood illness and (2) synthesize the most recent literature for this patient demographic (Abujaradeh et al., 2018; Ahola Kohut et al., 2017). Based on the gaps identified from the existing literature, this review included the results of six new research studies that have been published since 2017. A total of 18 studies met eligibility criteria and were selected for inclusion. Mindfulness-based interventions may be effective for improving the psychological wellbeing in children and adolescents with physical health conditions, with evidence suggesting promising outcomes related to illness. Mindfulness showed promise in improving anxiety associated with chronic pain (Ali et al., 2017; Lagor et al., 2013), and cardiac disease (Freedenberg et al., 2015, 2017), improved depression in adolescents with headaches (Hesse et al., 2015), improved emotional functioning in IBD (Ahola Kohut et al., 2019; Wren et al., 2021), and alleviated distress in chronic pain (Chadi et al., 2016). However, while promising, there were mixed findings (Malboeuf-Hurtubise et al., 2016) and some studies reported a worsening of symptoms (Ruskin et al., 2017; Waelde et al., 2017). The worsening of symptoms may have been a consequence of learning mindfulness techniques as a beginner and might be expected to lessen with practice and gaining experience. The discrepancies in significance (Chadi et al., 2016) could suggest mindfulness teaches the adolescent skills to cope with and manage pain, as evidenced by reports of feeling less alone and learning to manage negative affect (Ahola Kohut et al., 2019; Ruskin et al., 2015), however, this could depend on variables such as willingness and commitment to practice. The heterogeneity of interventions and range in sample sizes makes it difficult to determine how effective mindfulness is, as some interventions involved larger groups, were developmentally tailored, and ranged in length and delivery. This could have resulted in some children gaining greater mindfulness skill, which may have been additionally influenced by the inconsistent inclusion of mindfulness retreats (Ali et al., 2017). As well as this, inconsistencies were reported in the completion of homework and home practices indicating that some children were practicing and reinforcing skills more than others (Malboeuf-Hurtubise et al., 2016).
Importantly, when considering the overall quality of studies included in this review, the risk of bias assessments indicated several studies were at moderate or high risk of bias (Higgins et al., 2011; Sterne et al., 2016, 2019). The absence of control groups, lack of clarity regarding intervention fidelity, combined with differences in facilitator qualifications could have added to the risk of bias in already underpowered study samples, and meant overall generalizations could not be determined by pooling results (Jastrowski Mano et al., 2013; Ruskin et al., 2015). However, Zoogman (2015) suggests that instructor experience, intervention design, and session length do not moderate outcomes in mindfulness, but sample origin could (e.g., clinical/non-clinical). Thus, MBIs may be more therapeutic for certain health conditions (Zoogman et al., 2015), however, the heterogeneity of study designs adds complexity to determining the efficacy of mindfulness with different health conditions.
Evidence for the value of mindfulness with families affected by physical health conditions was limited. Previous reviews into the use of MBIs with children have reported similar findings showing a lack of parental involvement in such programs, and have suggested the potential importance of including caregivers in MBI to promote the use of mindfulness in daily life (Bockmann & Yu, 2022). Involving parents in MBIs could increase completion of homework, and reinforce practice in the home environment (Bockmann & Yu, 2022). Although limited, this review indicates that including parents in interventions may be associated with reduced parental worry (Hesse et al., 2015; Waelde et al., 2017); reduced disease burden on other family members, and increased problem solving and coping behavior (Law et al., 2019; Martire & Helgeson, 2017). It was difficult to establish generalizations regarding intervention modality and duration as the findings were mixed without clear patterns. Several barriers to participation were highlighted, such as participants not feeling comfortable with a group format and travel restrictions (Ruskin et al., 2015) which could suggest the value of offering a choice of delivery. Indeed, digitally delivered MBIs were reportedly acceptable for adolescents, which could support the use of an online format for children and their families.
This systematic review addresses important gaps in the evidence base and provides an assessment of the most recent findings for the use (and delivery) of MBIs (e.g., including developments of MBVR/online platforms) (Chadi et al., 2019; Young et al., 2022) that have not been investigated in previous reviews (Abujaradeh et al., 2018; Ahola Kohut et al., 2017). This shift from traditional face-to-face MBIs could be an effective alternative, as in many cases, parent caregivers will be responsible for transporting children; and busy family schedules could hamper attendance as a result. Therefore, online delivery could be viable to enhance feasibility of an MBI from overcoming practical challenges (e.g., transport) that could affect attrition. Although, more research is needed to determine the effectiveness of digital MBIs for improving outcomes in physical health conditions (Young et al., 2022). Thus, the present systematic review makes a valuable contribution from the inclusion of recent studies. The findings from this review could provoke new ways of thinking in the field of mindfulness, and might contribute to the evidence for targeting childhood physical illnesses systemically with family-focused interventions.
Limitations
However, our findings should be cautiously interpreted because of the variation in study designs including several of the studies being pilot investigations, methods used, and intervention characteristics. There is a clear need for more rigorous scientific evidence investigating the role of mindfulness mechanisms with larger sample sizes, standardized, comparable outcome measures, and control groups. A meta-analysis was not possible due to the inconsistency in reporting of effect sizes and the heterogeneity of studies, which makes assessing the role of mindfulness for improving the quality of life of families difficult and resulted in this review being primarily a narrative synthesis with commentary and description of the available literature. The search strategy could also be replicated with a rigorous list of common childhood illnesses as disease descriptors specifically named as terms in the databases.
Implications and Future Research
Children and adolescents with physical health conditions may experience psychological distress from living with illness. Mindfulness-based interventions have shown potential for alleviating the disease-related burden, depression, anxiety, and parental stress. Our findings could have clinical implications in terms of supporting the need for the inclusion of tailored services delivering dyadic, psychological interventions for children and families affected by childhood physical illness in both clinical and non-clinical settings to improve patient outcomes. However, clinical practice should ensure MBIs are appropriate for the symptoms associated with the condition diagnosed. More research with empirically robust methods, such as homogenous intervention designs, fidelity checking, and comparable control groups is needed to understand the role mindfulness could play in assisting adaptive adjustment to physical health conditions. Future research should endeavor to focus on dyadic research with families affected by childhood physical health conditions to better capture the mechanism underlying intervention efficacy. MBIs should be collaboratively constructed (involving children and their families) to allow insight into how barriers to participation (such as mode of delivery, length of sessions, nature of exercises) can be addressed. From the risk of bias assessments, the findings from this review suggest that in order to fully assess the efficacy of MBIs for children with physical health conditions and their families, further large-scale randomized controlled trials are needed. This would allow a robust examination of the potential for clinically meaningful change after participation in an MBI, as most of the studies identified in this review relied on small, single-groups of participants.
Supplementary Data
Supplementary data can be found at: https://dbpia.nl.go.kr/jpepsy.
Funding
This study was completed in partial fulfillment of a PhD studentship funded by Cardiff University School of Psychology and supported by Health Education and Improvement Wales (HEIW). The funders had no role in this review.
Conflicts of interest: None declared.
Registration and protocol
Olivia Hughes, Andrew Thompson, Katherine Shelton, Helen Penny. A Systematic Review of Mindfulness-based Interventions for Children and Adolescents Living with Physical Health Conditions and their Families. PROSPERO 2021: CRD42021234011. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021234011. An amendment was made to the title to reflect the design of the review, comparators were updated to include studies not using control groups, and the database list was refined.
Data Availability
Data are available by request to the corresponding author.



