-
PDF
- Split View
-
Views
-
Cite
Cite
Deborah A Ellis, April Idalski Carcone, Sylvie Naar-King, Dixy Rajkumar, Gloria Palmisano, Kathleen Moltz, Adaptation of an Evidence-Based Diabetes Management Intervention for Delivery in Community Settings: Findings From a Pilot Randomized Effectiveness Trial, Journal of Pediatric Psychology, Volume 44, Issue 1, January/February 2019, Pages 110–125, https://doi.org/10.1093/jpepsy/jsx144
Close - Share Icon Share
Abstract
To adapt an evidence-based intervention targeting diabetes management in adolescents with poorly controlled type 1 diabetes for use in a community setting by community health workers (CHWs) and to conduct pilot testing of the new intervention, REACH for Control (RFC). The study was conducted as a collaboration between university researchers and a federally qualified health center. Methods In a pilot effectiveness trial, feasibility and acceptability of RFC were evaluated based on participant enrollment, treatment dose, and consumer satisfaction. RFC effects on adolescent adherence, health outcomes, and quality of life were also assessed. The trial used a parallel group design. Families were randomized to 6 months of RFC plus standard medical care (n = 26) or standard care (SC) only (n = 24). Data were collected at baseline and 7-month posttest. A mixed-methods approach was used to analyze data.
Qualitative analyses suggested that caregivers viewed RFC and delivery of a home-based intervention by CHWs positively. Furthermore, adolescents who received RFC had statistically significant (p = .05) and clinically meaningful improvements in hemoglobin A1c (HbA1c) (0.7%) and reported significant improvements in quality of life from baseline to follow-up (p = .001). No significant changes were found for adolescents in standard medical care. However, while dose of primary intervention session delivered was acceptable, dose of follow-up sessions used for skills practice was low.
Results provide preliminary support for RFC’s acceptability and effectiveness to improve health status and quality of life when used in community settings serving high-risk, low-income families. Additional testing in a full-scale effectiveness trial appears warranted.
Type 1 diabetes (T1D) is characterized by a demanding care regimen. For children with T1D, the adolescent developmental period is marked by deterioration in both adherence and metabolic control. While biological mechanisms, including hormonal changes at puberty, directly affect metabolic control (Hamilton & Daneman, 2002; Moran et al., 1999), adolescents are also more likely to be nonadherent with almost every aspect of diabetes management (Hood, Peterson, Rohan, & Drotar, 2009; Rausch et al., 2012; Tamborlane et al., 2005). Furthermore, a subset of high-risk adolescents demonstrates much more serious health problems, as evidenced by chronically poor metabolic control (CPMC). Adolescents with T1D are expected to maintain glycosylated hemoglobin levels (hemoglobin A1c, HbA1c) <7.5% (Silverstein et al., 2005); however, many youths have persistently higher HbA1c over time, indicating CPMC. Longitudinal studies have shown that CPMC is a stable pattern that persists over time (Rohan et al., 2014; Helgeson et al., 2017). In turn, CPMC is related to both short-term diabetes complications, such as admissions for diabetic ketoacidosis (Maahs et al., 2015), and long-term diabetes complications, such as retinopathy, neuropathy, and nephropathy (Wood et al., 2013). Such complications not only negatively impact quality of life but also substantially affect medical care costs.
High-risk adolescents with CPMC are embedded within multiple systems that contribute to poor adherence; many risk and protective factors have been identified at the level of the child, family, school, and health-care system that influence regimen adherence (see Hilliard, Harris, & Weissberg-Benchell, 2012; Naranjo, Mulvaney, McGrath, Garnero, & Hood, 2014 for recent reviews). Individual child risk factors that have been linked to poor adherence include higher rates of behavioral/emotional problems (Northam, Lin, Finch, Werther, & Cameron, 2010) and poorer coping skills (Jaser et al., 2012). Family risk factors include diabetes-related conflict (Hood, Butler, Anderson, & Laffel, 2007; Ingerski, Anderson, Dolan, & Hood, 2010) low support for diabetes care from caregivers (Palmer et al., 2011; Wysocki et al., 2009), and poor parental supervision and monitoring (Ellis, Podolski, et al., 2007; Horton, Berg, Butner, & Wiebe, 2009). Extrafamilial barriers to adequate regimen adherence among high-risk adolescents include poor school attendance (Cooper et al., 2016; Glaab, Brown, & Daneman, 2005), as school personnel are unlikely to adequately manage the diabetes care of children who are not present. Interface between the family and the medical care system also plays a crucial role, as better adherence is associated with positive relationships with medical care providers and regular clinic attendance (Urbach et al., 2005; Valenzuela et al., 2014;). Examination of community context also shows that high-risk adolescents are disproportionately likely to be from disadvantaged groups such as those of lower socioeconomic status (SES), single-parent-headed households, minorities, and those living in disadvantaged neighborhoods (Frey, Templin, Ellis, Gutai, & Podolski, 2007; Swift, Chen, Hershberger, & Holmes, 2006; Queen et al., 2017). In summary, descriptive studies of high-risk youth with CPMC show that risk factors within the child, family, school, and community systems contribute to the development of severe adherence problems. Comprehensive interventions that can address the multiple determinants of poor adherence and CPMC (adolescent, family, school, health-care system) are needed.
Adequately powered, randomized controlled trials testing behavioral interventions to improve regimen adherence in high-risk youth with poorly controlled diabetes are limited and have largely been unsuccessful in improving metabolic control (Christie et al., 2016; Nunn, King, Smart & Anderson, 2006). In addition, these interventions have largely targeted the individual youth, despite the extensive evidence for critical factors within other systems within which the adolescent is embedded that influence regimen adherence and metabolic control. One of the few empirically supported interventions to have successfully improved metabolic control in youth with poorly controlled diabetes is Behavioral Family Systems Therapy for Diabetes (BFST-D; Wysocki et al., 2007; Wysocki et al., 2008). The BFST-D intervention uses predominantly family therapy interventions, such as communication training and problem-solving to improve regimen adherence and health outcomes.
Multisystemic therapy (MST) is an intensive, home- and community-based, family intervention originally developed and validated for the treatment of adolescents with serious behavioral difficulties (Henggeler, Schoenwald, & Borduin, 2009). The MST treatment approach is an excellent fit for intervening with youth with severe adherence problems because MST interventions address problems within the individual adolescent, the family system, and the broader community systems within which the family operates (e.g., school, health-care system). MST’s home-based approach to service provision also allows barriers to accessing clinic-based services often seen among families of adolescents with chronic poor metabolic control to be minimized. Our research group has adapted and tested the use of MST for the treatment of CPMC [MST-Health Care or MST-HC; Ellis et al., 2008; Ellis et al., 2004]. For example, in a randomized controlled trial comparing MST-HC with an attention control condition for the treatment of youth with poorly controlled type 1 diabetes, we showed that adolescents with CPMC who received in MST-HC had statistically significant and clinically meaningful decreases in HbA1c as compared with adolescents in an attention control condition (Ellis et al., 2012).
The transport of effective, evidence-based interventions, such as MST-HC from research settings to real-world, community settings difficult to accomplish (Berwick, 2003; Rotheram-Borus & Duan, 2003; Schoenwald & Hoagwood, 2001). Consistent with other areas of health-care research, the pediatric diabetes literature is lacking in implementation science studies that test the effectiveness of evidence-based behavioral interventions when used in community settings, rather than in university-based efficacy trials. Moreover, the current health-care system creates many barriers to the implementation of effective behavioral interventions in real-world treatment settings where children and adolescents with chronic health conditions are seen (Strickland et al., 2015). As a result, community-based behavioral interventions for youth with T1D are essentially nonexistent; existing interventions such as the NICH program (Harris et al., 2014; Wagner, Barry, Stoeckel, Teplitsky, & Harris, 2017) have only been evaluated by case reports or program evaluation methodology, and hence, their effectiveness is unclear. Costs of behavioral interventions are an additional barrier to the translation of evidence-based interventions to community setting, given limited health-care funds (Kazak et al., 2010). The MST-HC intervention was delivered by mental health professionals with a master’s degree and the cost per adolescents was relatively high (Ellis et al., 2008). Offering behavioral interventions through paraprofessionals may be one way to reduce service costs. Community health workers (CHWs) are lay health workers who have been used to provide health-care services to underserved and/or minority communities. They have also been widely used to provide diabetes management interventions to adults with type 2 diabetes (Davis, O'Toole, Brownson, Llanos, & Fisher, 2007; Norris et al., 2006). Both the American Association of Diabetes Educators (2003) and the Centers for Disease Control (CDC) Division of Diabetes Translation (2003) endorse the use of CHWs to deliver diabetes management interventions to adults. However, their effectiveness for children with T1D in general, or adolescents with poorly controlled T1D in particular, has not been evaluated.
The current study used an effectiveness–implementation hybrid design (Curran, Bauer, Mittman, Pyne, & Stetler 2012), which allows testing of the effectiveness of an evidence-based intervention in a real-world setting while gathering information about the context for implementation and barriers/facilitators to implementation. The study was conducted as a collaboration between university researchers and a federally qualified health center providing health care to underserved, predominantly minority families. The purpose of the current study was to adapt the MST-HC intervention for use in a community setting for delivery by CHWs, and to evaluate the feasibility and acceptability of the new intervention, called REACH for Control (RFC) in a pilot clinical trial based on participant enrollment, treatment dose, and consumer satisfaction. Preliminary efficacy of the RFC intervention’s effect on adolescent adherence, health outcomes, and quality of life was also assessed. A mixed-methods approach was used to evaluate feasibility, acceptability, and preliminary efficacy of the RFC intervention. We hypothesized that adolescents receiving RFC would have improved regimen adherence, glycemic control, and quality of life as compared with adolescents receiving standard medical care who were expected to have no change.
Method
Intervention Adaptation
The CDC have provided guidance on the process of adapting and testing evidence-based behavioral interventions for use in new settings (McKleroy et al., 2006). These guidelines define adaptation as modifying important but nonessential attributes of intervention activities and delivery methods while maintaining fidelity to core intervention elements, intervention theory, and the internal logic of the intervention. The adaptation process involves (1) an assessment phase, involving assessing agencies, and agency capacity to implement a particular evidence-based intervention (goodness of fit) (2) a preparation phase, including determining what adaptations are needed with stakeholder input and pretesting materials, and (3) an implementation stage, where the intervention is pilot tested.
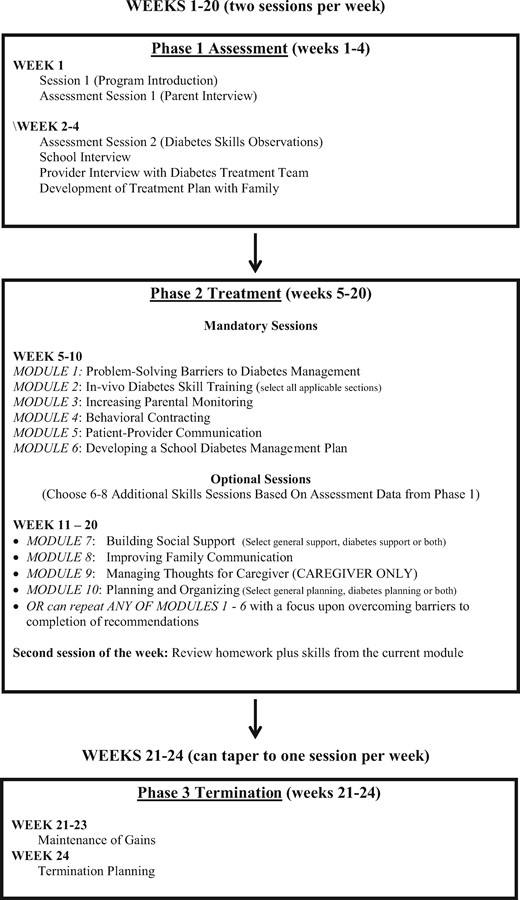
For the present study, the assessment phase involved developing a partnership between the university-based developers of the MST-HC intervention and a not-for-profit federally qualified health clinic (FQHC). The FQHC was approached as a partner because of its extensive experience working with minority families in the local community and using CHWs to provide health-care services. The agency had previously participated in a CDC-funded initiative that used community-based, participatory research principles to develop, implement and sustain a CHW-delivered intervention to improve health outcomes among minority adults with type 2 diabetes (Spencer et al., 2011). During a 6-month preparation phase, FQHC staff and university researchers collaborated to adapt the MST-HC intervention for use in the agency, including modifying the intervention content and the CHW training materials. The new RFC intervention maintained core components of the MST-HC intervention and treatment theory, which included (1) the home- and community-based delivery model, (2) the family-focused treatment approach that emphasized parenting interventions, (3) the flexible and tailored treatment approach that allowed different potential causes of chronic poor adherence to be addressed, (4) the inclusion of case management strategies to address nondiabetes-specific needs of high-risk families. Intervention content that could not reasonably be implemented by a CHW (e.g., individual adolescent treatment for depression, marital counseling, etc.) was eliminated and/or modified (i.e., provision of referrals/linkage to care). An overview of the RFC intervention is shown in Figure 1. Interventionist training was modified and expanded from the original MST-HC training to include additional focus on relationship-building skills, cognitive-behavioral treatment skills, and training in how to work with other professionals, such as school staff and medical care providers. In addition to the FQHC staff, seven CHWs providing health-care services to children and families were recruited from a statewide CHW professional organization to review the RFC intervention and training materials before its use and provide feedback before finalization. The preparation phase also included meetings with medical staff within the local tertiary care medical facility where adolescents with T1D were seen for medical care to develop procedures for regular communication between CHWs, CHW supervisors, and clinic diabetes care providers regarding each adolescent’s medical needs and diabetes-specific issues that arose during the course of RFC treatment. Finally, in the implementation stage, the new RFC intervention was pilot tested in a randomized effectiveness trial.
Pilot Clinical Trial
The clinical trial was designed as a pragmatic randomized effectiveness trial, as the goal was to obtain preliminary information regarding how an adapted evidence-based intervention such as RFC would perform under real-world conditions (i.e., implemented by a community treatment agency, minimal exclusion criteria). Therefore, an attention control condition was not included as part of the design; rather, the control condition was standard medical care. A parallel group design was used.
To be eligible, participants had to be between 10 and 18 years of age, have a diagnosis of T1D for at least 1 year, have CPMC as defined by the research team as a current HbA1c of ≥9%, and a mean HbA1c of ≥9% during the year before study entry, and be residing in a home setting (e.g., not in residential psychiatric treatment). No child psychiatric diagnoses were exclusionary with the exception of moderate or severe mental retardation and mood disorders/psychoses, such as bipolar disorder or schizophrenia. These were assessed by caregiver report, as the medical chart was inconsistent in reporting this information. Families were also excluded if they were not English speaking or could not complete study measures in English.
Adolescents with CPMC and their families were recruited by the university research partner from a pediatric endocrinology clinic within a tertiary care children’s hospital located in a major Midwestern metropolitan area between 2014 and 2016. Potentially eligible participants were first identified based on medical chart review through the electronic medical record of all youth seen in the diabetes clinic. In total, 652 youth seen at the clinic were eligible based on age and diagnosis with type 1 diabetes. Additional medical chart review was conducted to identify youth with elevated HbA1c (≥9% currently and in the year before study entry); 430 were ineligible based on HbA1c criteria. A letter describing the study was sent to home of all families that were eligible based on age, diagnosis, and HbA1c inclusion criteria. This was followed up by phone contacts from study research staff to confirm eligibility, and subsequently with a home-based consent visit if families indicated an interest in participating. Potential participants were recruited until the planned sample was enrolled. The research was approved by the institutional review board of the university affiliated with the hospital, where the adolescents were seen for medical care. All parents provided informed consent and all adolescents provided assent to participate. The trial was registered in ClinicalTrials.gov under registry number NCT02243072.
Procedures
Data collection occurred at baseline and 7 months postbaseline (corresponding to treatment termination). To minimize bias, data collection was conducted by research assistants hired by the university research partner rather than the CHW interventionists. Participants were randomized in a 1:1 ratio to RFC plus standard medical care or standard medical care alone. Randomization occurred immediately after baseline data collection using a permuted block algorithm with blocks of varying size to ensure equivalence across treatment condition and was conducted by the project coinvestigator using a computerized software package (http://randomization.com); treatment assignment was then provided to the research assistant collecting the data who informed the family of their status. Randomization was stratified by baseline HbA1c [≥10.5% (n = 40) vs. <10.5% (n = 10) based on the median sample HbA1c in our prior MST-HC treatment trials drawn from the same recruitment location]. Adolescents randomized to RFC received ∼6 months of home-based family treatment delivered by two CHWs (one CHW treated 14 families and the other CHW treated 9). Owing to the feasibility nature of the study, families were not randomly assigned to one of the two CHWs. All measures were collected in the participants’ homes. The research assistant was not blind to treatment assignment because of the need to complete exit interviews to assess treatment satisfaction with treatment families. Both the adolescent and the primary caregiver completed questionnaires. Families were provided $25 to compensate them for participating in each data collection session. The primary outcomes were glycemic control, regimen adherence, and quality of life.
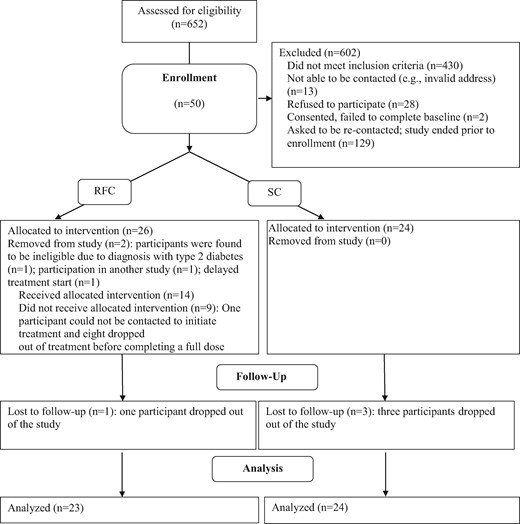
A Consort diagram for the clinical trial is shown in Figure 2. Of the 652 families screened for participation, 430 were ineligible and 13 could not be contacted. Of the remaining 209, 28 refused to participate (12%), citing lack of interest or time and the remainder (181 or 88%) expressed an interest in participating. Fifty were enrolled before closure of enrollment (as the study was a pilot, the planned sample was 25 per arm). In total, 26 were assigned to RFC and 24 were assigned to SC. Of those assigned to RFC, two were subsequently found to be ineligible and removed from the study (one was reclassified with type 2 diabetes rather than type 1 after baseline data collection, and one was found to be participating in another adherence intervention research study), and a third was removed because of a delay in initiating treatment, leaving a final n of 23. One of the RFC families and three of the SC families dropped out of the study and did not complete follow-up data collection. The overall study retention rate was 91%.
Standard Medical Care
All participants in the trial received standard medical care in the pediatric diabetes clinic from which they were recruited. Families received standard medical care in accordance with American Diabetes Association guidelines (Silverstein et al., 2005), which included visits to the clinic every 3–4 months. Participants were seen at clinic visits by a pediatric endocrinologist, nurse educator, and a dietician. Visits included monitoring of glycemic control (HbA1c) review of blood glucose records, identification of any illness management problems, and setting target goals for diabetes care. Diabetes education was provided by certified diabetes educators. The linear growth and weight gain of the adolescent was also assessed and changes in insulin dosage made as necessary. Between visits, assistance with problem-solving and blood glucose management was provided by clinic diabetes educators by phone. Additional diabetes education sessions could also be scheduled with clinic diabetes educators on request outside of regular clinic appointments.
REACH for Control
Treatment Overview
Participants randomized to RFC received the intervention in additional to standard medical care in the diabetes clinic. RFC treatment was provided by CHWs hired, trained, and supervised through the FQHC. In the current study, RFC treatment was provided by two CHWs. RFC was a home- and community-based, family intervention that lasted ∼6 months and had three phases: assessment (Phase 1), treatment (Phase 2), and relapse prevention (Phase 3) (see Figure 1). Both the adolescent and his/her primary caregiver were expected to attend all sessions. In addition, extended family members (other parents/caregivers, older siblings) were encouraged to attend as desired by the family. Treatment sessions were held twice per week. The first, “primary” weekly session was a full-length assessment or treatment session and the second, “follow-up” weekly session was a brief check-in session to assist families with follow-through on goals set during the primary session. The expected number of primary sessions across all three phases (assessment, cognitive-behavioral skill training [CBST] treatment sessions, and relapse prevention) was 20, while the expected number of follow-up sessions was 16 (assessment sessions did not have follow-up components). All sessions were scheduled at the family’s convenience. There was no scheduling requirement for time elapsed between primary and follow-up session within each week; this determination was made by the CHW based the family’s needs. Each mandatory or optional CBST module was delivered in a 60–90-min session, which was followed later in the week by a 30-min follow-up session to assess progress and conduct an additional skills practice.
Phase 1
Phase 1 (Weeks 1–4) was an assessment phase in which the CHW conducted an initial intake and a comprehensive functional analysis (FA) regarding nonadherence to diabetes care. FA was used to identify contributing factors to nonadherence as well as antecedents and consequences of nonadherent behavior across the family, school, and community settings. This FA process was the same as used in the research group’s prior MST-HC trials and consisted of detailed interviews with the adolescent, primary caregiver, and other adults (school personnel) and the medical treatment team as well as in vivo observations to identify the various factors that contributed to the adolescent’s poor adherence/poor metabolic control. Based on the FA, an individualized treatment plan was developed with the family that addressed the particular causes of nonadherence for each youth. The treatment plan consisted of all mandatory modules and specific optional modules chosen with the family based on needs (see Figure 1).
Phase 2
Phase 2 (Weeks 5–20) was a treatment phase and consisted of a combination of “mandatory” CBST modules received by each family and “optional” CBST modules chosen by the CHW and the family based on the results of the FA. “Mandatory” RFC modules were (1) tailored diabetes education and skills training (working with the family to complete diabetes care correctly in the home, school, and/or community based on youth needs in the areas of insulin administration, blood glucose testing, and/or dietary management), (2) teaching families problem-solving strategies to identify barriers to diabetes care and develop solutions, (3) increasing parental supervision and monitoring of diabetes care, (4) teaching parents to use behavioral contracting to reinforce better youth diabetes care, (5) creating a family–school diabetes management plan for use in school, and (6) improving family communication with medical providers. “Optional” modules that were individualized based on data gathered during the assessment phase data included improving parent–adolescent communication, building social support for caregivers, increasing caregiver planning/organizing skills, and resource provision to address nondiabetes-specific needs (e.g., adolescent mental health problems, marital problems, financial difficulties, transportation, etc.). Consistent with CBST, all modules in Phase 2 were delivered through a combination of didactic information provision/psychoeducation about the skill (e.g. Why a behavioral contract might be useful for improving the youth’s regimen adherence; Provision of information about what an effective behavioral contract consists of) followed by skills practice with the family (e.g., creation of a behavioral contract for the youth and practice in filling of the contract). Homework was assigned at the end of each session. During this phase, treatment success was monitored by weekly review of the adolescent’s blood glucose meter for frequency of testing and test values (i.e., number of values in or out of target range). In addition, the CHW attended any diabetes clinic appointments with the family that occurred during treatment and addressed any other family resource needs (e.g., housing; finances; transportation; linkage to care for other family members).
As described above, each CBST module was delivered in the primary weekly session lasting 60–90 min. A follow-up session lasting 30 min was scheduled later in the week to assess homework completion and to help the family conduct additional practice of the skill taught in the primary session (e.g., helping the caregiver to fill out the behavioral contract with the youth for the day and to implement a reward or consequence).
Phase 3
Phase 3 (Weeks 21–24) consisted of termination planning and relapse prevention. During Phase 3, the CHW was permitted to either maintain session frequency at twice per week or eliminate the check-in session if indicated.
Treatment Fidelity
All CHWs providing services at the FQHC completed extensive training in their profession, including CHW competency training offered through the Michigan Community Health Worker Alliance. The two CHWs that provided RFC in the current study also received additional protocol-specific training during an 80 hr, 2-week long training period before providing the intervention. RFC training consisted of (1) diabetes education and information on the correlates and causes of the diabetes management problems addressed by RFC, (2) practice in conducting functional analyses and each assessment and CBST module, and (3) interpersonal and relationship skills to promote good therapeutic alliance with adolescents and families. To monitor fidelity to the RFC treatment model, quality assurance protocols were used that included use of a treatment manual with detailed session descriptions (available on request from the first author), weekly on-site clinical supervision from a master’s level supervisor at the FQHC with experience working with adults with diabetes, and weekly phone consultation with a child clinical psychologist who was member of the research team with extensive experience in behavioral diabetes management. All sessions were also audio recorded, and the CHW supervisor reviewed one randomly selected tape per CHW per month using a checklist of session content to ensure that session content was being delivered as planned. CHWs were assigned a full caseload (eight families) at the start of the trial and then received new cases based on their caseload as families completed treatment or dropped out.
Measures
Socioeconomic Status
SES was calculated using the MacArthur Scale of Subjective Socioeconomic Status (SSSS; Adler, Epel, Castellazzo, & Ickovics, 2000), a widely used scale of subjective social standing. The respondent selects the rung on a 10-rung ladder that represents their perception of their family’s social standing in society. The scale is coded such that higher scores indicated higher SES. Scores on the measure are related to employment grade, education, household and personal income, household wealth, satisfaction with standard of living, and feelings of financial security (Singh-Manoux, Adler, & Marmot, 2003). The adolescent’s caregiver completed the measure.
Glycemic Control
Glycemic control was calculated using HbA1c. Values were obtained using the Accubase test kit (DTI Laboratories, Inc.), which is FDA approved. The test uses a capillary tube blood collection method instead of venipuncture and is therefore suitable for home-based data collection by nonphlebotomists. High-performance liquid chromatography is used to analyze the blood sample.
Regimen Adherence
Adherence was measured through one subjective and one objective measure. The Diabetes Management Scale (DMS; (Schilling, Grey, & Knafl, 2002) is a self-report questionnaire. It is designed to measure a broad range of diabetes management behaviors, such as insulin management, dietary management, blood glucose monitoring, and symptom response. Each item asks “What percent of the time do you (take your insulin)?” The response scale is 0–100%. A total score is obtained by summing the items to reflect overall management behavior. A parent report version parallels the youth version and was used in the present study to obtain parent ratings of adolescent adherence. The measure has previously been shown to have good reliability and to be sensitive to change in intervention research (Ellis et al., 2012). Internal consistency (Cronbach’s alpha) for the parent report version was .81.and for the adolescent report version was .80.
Frequency of blood glucose testing, a specific adherence behavior, was also obtained directly from the adolescent’s blood glucose meter. For the present study, data were recorded regarding frequency of testing during the 14-day period immediately preceding data collection, and an average daily testing frequency was subsequently calculated.
Quality of Life
Quality of life was measured with the Diabetes Quality of Life-Youth scale (DQOL-Y), a 44-item scale designed to tap life satisfaction, diabetes impact, and diabetes-related worries in adolescents (Ingersoll & Marrero, 1991). Responses are on a five-point Likert scale. The total score was used in the present study with higher scores indicating higher-quality of life. The scale has been used both in large sample studies of adjustment in adolescents with T1D and in behavioral clinical trials and has been translated into several languages with adequate reliability and validity reported (Grey, Boland, Yu, Sullivan-Bolyai, & Tamborlane, 1998; Keller et al., 2017; Serlachius et al., 2016). Internal consistency (Cronbach’s alpha) across the three scales ranged from .73 to .91 in the current study.
Intervention Satisfaction
Family satisfaction with the RFC intervention was measured in two ways. First, caregivers completed semi-structured, face-to-face exit interviews with the research assistant at the 7-month data collection visit designed to elicit their feedback on RFC's overall appropriateness and utility as well as its delivery structure and format (i.e., program length, session length and frequency, delivery by CHWs). As with quantitative measures, exit interviews were completed with families regardless of their treatment completion status. Exit interviews were audio recorded and later transcribed by a professional transcription service. Second, caregivers were asked to provide a global quantitative rating of their satisfaction with the RFC program and its helpfulness with improving diabetes management on a scale from 1 to 10, with 1 being “not at helpful” and 10 being “extremely helpful.” Objective data on treatment dose were obtained from treatment charts kept by the CHWs.
Data Analyses
Quantitative analyses were intent to treat and all randomized participants were included with the exception of the three study removals. Quantitative analyses were completed with SPSS, version 24 (IBM, 2017). Paired t-tests were used to evaluate the pretest to posttest effect of RFC and SC on adolescents’ glycemic control, diabetes management, and quality of life using directional hypotheses. Effect sizes were calculated using Cohen’s d (d = Mdiff/SDdiff) (Cohen, 1992). Missing data at follow-up were estimated using the missing values analysis procedure and Expectation-Maximization (EM) estimation within SPSS. In total, three SC and one RFC cases were estimated because of missing data (4 of 46 cases or 9%).
Qualitative analyses were conducted by two graduate students under the guidance of one of the authors (A.I.C.). Data from 22 exit interviews (one family randomized to RFC did not complete follow-up data collection) were analyzed using the framework matrix analysis method (Gale, Heath, Cameron, Rashid, & Redwood, 2013), which allows for efficient, systematic thematic analysis of qualitative data (Fereday & Muir-Cochrane, 2006). The initial coding matrix was based on content areas derived from the exit interview guide. Two coders independently coded each transcript by ‘charting’ them into the matrix. Coders met weekly to review and compare their matrices. Together, coders identified themes and summarized caregivers’ experiences in RFC.
All discrepancies were resolved through discussion, resulting in a final matrix that was coded to consensus. Consensus coding is a standard practice in qualitative health research (Bradley, Curry, and Devers (2007).
Results
Sample characteristics are summarized in Table I. The sample was composed primarily of African-American adolescents (79%) with a mean age of 14.26 years (SD = 2.39). The mean HbA1c of the sample at baseline was 11.50% (1.94%). The majority of youth (64%) used basal–bolus injection therapy to manage their diabetes, 26% used insulin pumps, and the rest (10%) used conventional injection therapy (two to three injections per day of short and intermediate acting insulin in various combinations). In total, 45% of the adolescents resided in single-parent homes, and the mean score on the SSSS measure was 5.09 (SD = 1.79) suggesting moderately low subjective SES. There were no significant differences at baseline between families assigned to RFC or the control group on adolescent gender, family income, number of caregivers in the home, or HbA1c. There were significant differences between groups based on adolescent age, with adolescents randomized to SC older than those in RFC [M = 15.02 vs. 13.46, t = 2.35, p = .023].
| . | % (N) or M (SD) . | ||
|---|---|---|---|
| Total sample . | RFC . | Control . | |
| Adolescents | |||
| Age (years) | 14.26 (2.39) | 13.46 (2.24) | 15.02 (2.32) |
| Female gender | 62% (29) | 74% (17) | 50% (12) |
| Race | |||
| African-American/Black | 79% (37) | 83% (19) | 75% (18) |
| White/Caucasian | 17% (8) | 17% (4) | 17% (4) |
| Other | 4% (2) | 0% (0) | 8% (2) |
| Age of diagnosis (years) | 7.57 (3.66) | 7.55 (3.43) | 7.59 (3.94) |
| Duration of diabetes (years) | 6.69 (3.63) | 5.91 (3.27) | 7.43 (3.86) |
| Insulin delivery regimen | |||
| Basal–bolus injection therapy | 64% (30) | 61% (14) | 67% (16) |
| Insulin infusion pump | 25% (12) | 26% (6) | 25% (6) |
| Conventional injection therapy | 11% (5) | 13% (3) | 8% (2) |
| HbA1c (%) | 11.50 (1.94) | 11.73 (2.01) | 11.27(1.88) |
| Caregivers | |||
| Age (years) | 41.70 (7.45) | 40.01 (6.44) | 43.31 (8.10) |
| Female gender | 96% (45) | 96% (22) | 96% (23) |
| Race | |||
| African-American/Black | 79% (37) | 83% (19) | 75% (18) |
| White/Caucasian | 19% (9) | 17% (4) | 21% (5) |
| Other | 2% (1) | 0% (0) | 4% (1) |
| Relationship to adolescent | |||
| Biological parent | 92% (43) | 100% (23) | 83% (20) |
| Legal guardian | 8% (4) | 0% (0) | 17% (4) |
| Marital status | |||
| Single parent | 45% (21) | 35% (8) | 54% (13) |
| Two parents | 55% (26) | 65% (15) | 46% (11) |
| Family income (in USD) | 44,362 (34,161) | 49,739 (36,236) | 39,528 (33,681) |
| McArthur Socioeconomic Index | 5.09 (1.79) | 5.27 (2.03) | 4.91 (1.54) |
| . | % (N) or M (SD) . | ||
|---|---|---|---|
| Total sample . | RFC . | Control . | |
| Adolescents | |||
| Age (years) | 14.26 (2.39) | 13.46 (2.24) | 15.02 (2.32) |
| Female gender | 62% (29) | 74% (17) | 50% (12) |
| Race | |||
| African-American/Black | 79% (37) | 83% (19) | 75% (18) |
| White/Caucasian | 17% (8) | 17% (4) | 17% (4) |
| Other | 4% (2) | 0% (0) | 8% (2) |
| Age of diagnosis (years) | 7.57 (3.66) | 7.55 (3.43) | 7.59 (3.94) |
| Duration of diabetes (years) | 6.69 (3.63) | 5.91 (3.27) | 7.43 (3.86) |
| Insulin delivery regimen | |||
| Basal–bolus injection therapy | 64% (30) | 61% (14) | 67% (16) |
| Insulin infusion pump | 25% (12) | 26% (6) | 25% (6) |
| Conventional injection therapy | 11% (5) | 13% (3) | 8% (2) |
| HbA1c (%) | 11.50 (1.94) | 11.73 (2.01) | 11.27(1.88) |
| Caregivers | |||
| Age (years) | 41.70 (7.45) | 40.01 (6.44) | 43.31 (8.10) |
| Female gender | 96% (45) | 96% (22) | 96% (23) |
| Race | |||
| African-American/Black | 79% (37) | 83% (19) | 75% (18) |
| White/Caucasian | 19% (9) | 17% (4) | 21% (5) |
| Other | 2% (1) | 0% (0) | 4% (1) |
| Relationship to adolescent | |||
| Biological parent | 92% (43) | 100% (23) | 83% (20) |
| Legal guardian | 8% (4) | 0% (0) | 17% (4) |
| Marital status | |||
| Single parent | 45% (21) | 35% (8) | 54% (13) |
| Two parents | 55% (26) | 65% (15) | 46% (11) |
| Family income (in USD) | 44,362 (34,161) | 49,739 (36,236) | 39,528 (33,681) |
| McArthur Socioeconomic Index | 5.09 (1.79) | 5.27 (2.03) | 4.91 (1.54) |
Note. Hemoglobin A1c = HbA1c; RFC = Reach for Control.
| . | % (N) or M (SD) . | ||
|---|---|---|---|
| Total sample . | RFC . | Control . | |
| Adolescents | |||
| Age (years) | 14.26 (2.39) | 13.46 (2.24) | 15.02 (2.32) |
| Female gender | 62% (29) | 74% (17) | 50% (12) |
| Race | |||
| African-American/Black | 79% (37) | 83% (19) | 75% (18) |
| White/Caucasian | 17% (8) | 17% (4) | 17% (4) |
| Other | 4% (2) | 0% (0) | 8% (2) |
| Age of diagnosis (years) | 7.57 (3.66) | 7.55 (3.43) | 7.59 (3.94) |
| Duration of diabetes (years) | 6.69 (3.63) | 5.91 (3.27) | 7.43 (3.86) |
| Insulin delivery regimen | |||
| Basal–bolus injection therapy | 64% (30) | 61% (14) | 67% (16) |
| Insulin infusion pump | 25% (12) | 26% (6) | 25% (6) |
| Conventional injection therapy | 11% (5) | 13% (3) | 8% (2) |
| HbA1c (%) | 11.50 (1.94) | 11.73 (2.01) | 11.27(1.88) |
| Caregivers | |||
| Age (years) | 41.70 (7.45) | 40.01 (6.44) | 43.31 (8.10) |
| Female gender | 96% (45) | 96% (22) | 96% (23) |
| Race | |||
| African-American/Black | 79% (37) | 83% (19) | 75% (18) |
| White/Caucasian | 19% (9) | 17% (4) | 21% (5) |
| Other | 2% (1) | 0% (0) | 4% (1) |
| Relationship to adolescent | |||
| Biological parent | 92% (43) | 100% (23) | 83% (20) |
| Legal guardian | 8% (4) | 0% (0) | 17% (4) |
| Marital status | |||
| Single parent | 45% (21) | 35% (8) | 54% (13) |
| Two parents | 55% (26) | 65% (15) | 46% (11) |
| Family income (in USD) | 44,362 (34,161) | 49,739 (36,236) | 39,528 (33,681) |
| McArthur Socioeconomic Index | 5.09 (1.79) | 5.27 (2.03) | 4.91 (1.54) |
| . | % (N) or M (SD) . | ||
|---|---|---|---|
| Total sample . | RFC . | Control . | |
| Adolescents | |||
| Age (years) | 14.26 (2.39) | 13.46 (2.24) | 15.02 (2.32) |
| Female gender | 62% (29) | 74% (17) | 50% (12) |
| Race | |||
| African-American/Black | 79% (37) | 83% (19) | 75% (18) |
| White/Caucasian | 17% (8) | 17% (4) | 17% (4) |
| Other | 4% (2) | 0% (0) | 8% (2) |
| Age of diagnosis (years) | 7.57 (3.66) | 7.55 (3.43) | 7.59 (3.94) |
| Duration of diabetes (years) | 6.69 (3.63) | 5.91 (3.27) | 7.43 (3.86) |
| Insulin delivery regimen | |||
| Basal–bolus injection therapy | 64% (30) | 61% (14) | 67% (16) |
| Insulin infusion pump | 25% (12) | 26% (6) | 25% (6) |
| Conventional injection therapy | 11% (5) | 13% (3) | 8% (2) |
| HbA1c (%) | 11.50 (1.94) | 11.73 (2.01) | 11.27(1.88) |
| Caregivers | |||
| Age (years) | 41.70 (7.45) | 40.01 (6.44) | 43.31 (8.10) |
| Female gender | 96% (45) | 96% (22) | 96% (23) |
| Race | |||
| African-American/Black | 79% (37) | 83% (19) | 75% (18) |
| White/Caucasian | 19% (9) | 17% (4) | 21% (5) |
| Other | 2% (1) | 0% (0) | 4% (1) |
| Relationship to adolescent | |||
| Biological parent | 92% (43) | 100% (23) | 83% (20) |
| Legal guardian | 8% (4) | 0% (0) | 17% (4) |
| Marital status | |||
| Single parent | 45% (21) | 35% (8) | 54% (13) |
| Two parents | 55% (26) | 65% (15) | 46% (11) |
| Family income (in USD) | 44,362 (34,161) | 49,739 (36,236) | 39,528 (33,681) |
| McArthur Socioeconomic Index | 5.09 (1.79) | 5.27 (2.03) | 4.91 (1.54) |
Note. Hemoglobin A1c = HbA1c; RFC = Reach for Control.
Intervention Feasibility and Acceptability
Treatment Dose
Of the 23 families who received RFC, 9 (39%) dropped out of treatment before receiving the full 6-month dose. Five of these families dropped out during the assessment process and did not complete a treatment plan. There were no significant difference between RFC treatment completers and those who dropped out on any demographic or medical characteristic. The mean dose of treatment for treatment completers was 16.6 primary sessions (SD = 4.9) and 1.6 follow-up sessions (SD = 1.0). For dropouts, the mean dose of treatment was 4.7 primary sessions (SD = 2.8) and 0.3 follow-up sessions (SD = 0.50). In contrast to the completion rate for primary sessions, which suggested that treatment completers received most of the expected treatment dose (20 possible sessions), completion rates for follow-up sessions suggested that little of the expected treatment dose (16 sessions) was delivered, even to treatment completers.
In qualitative interviews, half the caregivers (50%) reported that they found the length of the program (6 months) to be acceptable, whereas a third (36%) found it too long and the remainder found it too short. In total, 59% reported that the twice weekly session frequency was acceptable, but 27% reported that they found this frequency to be too high. The majority (75%) found the length of treatment sessions to be acceptable.
Satisfaction
On the quantitative item assessing satisfaction with RFC, the mean score was 8.12 (SD = 2.18) on a 1–10 scale, indicating high satisfaction. All caregivers interviewed (100%) found the location of the intervention (i.e., home and community) to be convenient and none reported a preference for an alternative delivery location such as the hospital.
Three themes were identified from qualitative interviews regarding aspects of treatment that were found to be positive. First, caregivers reported that they found the RFC program helpful because it provided them with new information and skills particular to diabetes management.
It was some information that wasn’t taught, I don’t think, in clinic visits that the one-on-one, the home-based, was able to address more detailed questions that I had and things that was going on in my home that I was able to address.
Because when we were in the program it improved her numbers. It showed us different practices and showed us different skills to emulate to better help take care of her diabetes.
It gave us some tools that we didn’t think of, you know? And she’s been diabetic for pretty much her whole life, so it helped.
In particular, caregivers reported that learning how to review and analyze blood glucose patterns for their specific relationships to the adolescent’s diabetes management behaviors helped them gain a better understanding of their adolescent’s highs and lows Caregivers stated that RFC session helped them identify specific diabetes management problems responsible for a period of high or low blood glucose values and then generate solutions with their adolescent. Developing this skill encouraged them to take a more active role in the management of their adolescent’s diabetes.
Looking at the numbers for the day and for the week, the inconsistency of high and low, and being taught how to look out for, and question what went on. What did you eat? How was you feeling?
Well, with her trying to find out the patterns that, you know, if at a certain point of the day, were they this because – and figure out why they were that way. That was helpful. [And how was it helpful for her?] Cause it pinpointed what she was doing wrong.
Yeah, so we don’t know what the problem is we can’t fix it, so we should always identify if he having some highs and lows we should always be able to write the problem down and look at which ways we can go and fix it, and make a goal, set a goal.
A second theme of caregivers’ feedback pertained to increased accountability and responsibility for both their adolescent and themselves. Specifically, caregivers stated the presence of someone other than a family member (i.e., the CHW) discussing diabetes care with the adolescent was particularly helpful to changing family interactions.
Just somebody else bringing it to [adolescent’s name] attention other than me. So really identifying it, somebody else other than me, and encouraging [name] to make some changes.
Probably the thing that helped her was just, for lack of a better word, the annoyance of having a third party come in and look at the things.
Caregivers also reported that with the CHWs’ support and guidance, they were better able to hold their adolescents accountable for diabetes care activities.
I took ownership on everything. And anything went wrong, I just blamed myself. And, I think that’s just came from what I’ve been doing for years as a parent. And I’ve been backing off…. and now I hold him accountable to his behavior and what he does not do correct for his diabetes.
In addition, caregivers reported that behavioral contracts helped them to encourage their adolescent to take a more active role in completing daily diabetes care.
The behavioral contract was all good because I thought that gave us something to work with to give him an incentive to want to change his numbers.
There were so many times where she’s like, ‘I already did! I’m going to!’ And I’d be like, ‘Alright, fine.’ And I’d let it go. But with that [behavioral contract session], she came right up to me every night, and we did our Lantus, and it actually helped a lot.
Finally, caregivers indicated that the CHWs encouraged them to assume more involvement in actively monitoring their children’s diabetes care.
It just made me more cognizant of what was going on with her blood sugar, jst getting back into that management role as the parent.
Even though she is as old as she is, you would think she had it all programmed to do it without the help, but I learned that even though that she still needs the help, more the supervision I guess would be in that as well.
The third theme identified in qualitative analyses was improved parent–adolescent communication. Caregivers reported finding their adolescent to be more straightforward about their emotions regarding diabetes, which led to improved communication. This openness helped families approach diabetes management together as a team.
I get frustrated very quickly cause she’s a yeller, and it taught me in a way to just kind of take it down and not go right back… it kind of helped a lot when it came to communicating wise of, ‘I’m not feeling good, and this is why.’ Which she wouldn’t talk to about before, and now she is. So that was helpful.
And when we worked together as a team then I could help remind her, you know, you need to do this and how to do it, and so I saw a decrease in her numbers being so high all the time.
Use of CHWs
In qualitative interviews, most caregivers found CHWs to be effective treatment providers, describing them as “professional,” “understanding,” “resourceful,” “informative,” and “knowledgeable.” However, interviews for families who dropped out of treatment did reflect some dissatisfaction with the type of treatment provider. Two of nine caregivers whose families dropped out of treatment reported that they felt their CHWs did not have a good understanding of diabetes and a third caregiver reported that the family dropped out because of interpersonal conflict with the CHW. Another caregiver who dropped out of treatment reported that their adolescent was resistant to engaging in treatment overall.
Intervention Effectiveness
Table II shows Ms and SDs on outcome measures by treatment arm. Adolescents assigned to RFC experienced a 0.70% reduction in their HbA1c from baseline to follow-up during the course of treatment. This improvement was statistically significant (p = .05). Furthermore, a decrease of 0.5% or greater is considered clinically significant and has been linked to reductions in diabetes complications (Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, 2008). In contrast, adolescents who continued to receive SC experienced a 0.11% increase in their HbA1c during the course of the study. Adolescents assigned to RFC demonstrated improvements in diabetes regimen adherence based on self-report on the DMS, caregiver report on the DMS, and objective data from blood glucose meters (increase of 0.30 tests per day). These improvements were not statistically significant, however (p = .22, .27, and .10, respectively). In comparison, adolescents who continued to receive SC showed improvements in adherence based on self-report on the DMS, but these changes were not statistically significant (p = .16). For those in SC, adherence decreased based on caregiver report on the DMS and data from blood glucose meters. Finally, adolescents assigned to RFC reported improved diabetes-related quality of life. This improvement was statistically significant (p = .001). In contrast, adolescents who received SC reported an improvement in their quality of life during the study, but this was not significant (p = .146). No study-related adverse effects of the intervention, such as increased rates of low blood glucose or increased family conflict, were identified during the trial.
RFC and Standard Care Effects on Diabetes Management, Health Status, and Quality of Life
| . | . | M (SD) . | . | . | Effect size d . | 95% confidence interval . | |||
|---|---|---|---|---|---|---|---|---|---|
| Variable . | Condition . | Baseline . | Follow-up . | t . | p . | Lower . | Upper . | ||
| HbA1c | |||||||||
| RFC | 11.73 (2.01) | 11.03 (2.10) | 1.69 | .05 | 0.35 | −0.23 | 0.93 | ||
| SC | 11.28 (1.88) | 11.39 (2.12) | −0.29 | .39 | −0.06 | −0.63 | 0.51 | ||
| DMS-A | |||||||||
| RFC | 74.69 (19.79) | 76.51 (15.32) | −0.79 | .22 | −0.17 | −0.75 | 0.41 | ||
| SC | 69.63 (18.56) | 72.39 (15.91) | −1.02 | .16 | −0.21 | −0.78 | 0.36 | ||
| DMS-C | |||||||||
| RFC | 73.97 (17.53) | 75.22 (18.54) | −0.62 | .27 | −0.13 | −0.71 | 0.45 | ||
| SC | 68.93 (20.66) | 68.37 (18.10) | 0.21 | .42 | 0.04 | −0.53 | 0.61 | ||
| BGT | |||||||||
| RFC | 3.19 (1.73) | 3.50 (1.81) | −1.32 | .10 | −0.18 | −0.76 | 0.40 | ||
| SC | 2.50(1.20) | 2.07 (1.68) | 1.30 | .15 | 0.31 | −0.84 | 0.30 | ||
| DQOL-Y | |||||||||
| RFC | 3.80 (0.56) | 4.00 (0.50) | −3.70 | .001 | −0.77 | −1.37 | −0.17 | ||
| SC | 3.64 (0.67) | 3.73 (0.63) | −1.08 | .15 | −0.22 | −0.79 | 0.35 | ||
| . | . | M (SD) . | . | . | Effect size d . | 95% confidence interval . | |||
|---|---|---|---|---|---|---|---|---|---|
| Variable . | Condition . | Baseline . | Follow-up . | t . | p . | Lower . | Upper . | ||
| HbA1c | |||||||||
| RFC | 11.73 (2.01) | 11.03 (2.10) | 1.69 | .05 | 0.35 | −0.23 | 0.93 | ||
| SC | 11.28 (1.88) | 11.39 (2.12) | −0.29 | .39 | −0.06 | −0.63 | 0.51 | ||
| DMS-A | |||||||||
| RFC | 74.69 (19.79) | 76.51 (15.32) | −0.79 | .22 | −0.17 | −0.75 | 0.41 | ||
| SC | 69.63 (18.56) | 72.39 (15.91) | −1.02 | .16 | −0.21 | −0.78 | 0.36 | ||
| DMS-C | |||||||||
| RFC | 73.97 (17.53) | 75.22 (18.54) | −0.62 | .27 | −0.13 | −0.71 | 0.45 | ||
| SC | 68.93 (20.66) | 68.37 (18.10) | 0.21 | .42 | 0.04 | −0.53 | 0.61 | ||
| BGT | |||||||||
| RFC | 3.19 (1.73) | 3.50 (1.81) | −1.32 | .10 | −0.18 | −0.76 | 0.40 | ||
| SC | 2.50(1.20) | 2.07 (1.68) | 1.30 | .15 | 0.31 | −0.84 | 0.30 | ||
| DQOL-Y | |||||||||
| RFC | 3.80 (0.56) | 4.00 (0.50) | −3.70 | .001 | −0.77 | −1.37 | −0.17 | ||
| SC | 3.64 (0.67) | 3.73 (0.63) | −1.08 | .15 | −0.22 | −0.79 | 0.35 | ||
Note. BGT = blood glucose testing; DMS-A = Diabetes Management Scale-Adolescent; DMS-C = Diabetes Management Scale-Caregiver; DQOL-Y = Diabetes Quality of Life-Youth scale; Hemoglobin A1c = HbA1c; RFC = Reach for Control; SC = Standard Care.
RFC and Standard Care Effects on Diabetes Management, Health Status, and Quality of Life
| . | . | M (SD) . | . | . | Effect size d . | 95% confidence interval . | |||
|---|---|---|---|---|---|---|---|---|---|
| Variable . | Condition . | Baseline . | Follow-up . | t . | p . | Lower . | Upper . | ||
| HbA1c | |||||||||
| RFC | 11.73 (2.01) | 11.03 (2.10) | 1.69 | .05 | 0.35 | −0.23 | 0.93 | ||
| SC | 11.28 (1.88) | 11.39 (2.12) | −0.29 | .39 | −0.06 | −0.63 | 0.51 | ||
| DMS-A | |||||||||
| RFC | 74.69 (19.79) | 76.51 (15.32) | −0.79 | .22 | −0.17 | −0.75 | 0.41 | ||
| SC | 69.63 (18.56) | 72.39 (15.91) | −1.02 | .16 | −0.21 | −0.78 | 0.36 | ||
| DMS-C | |||||||||
| RFC | 73.97 (17.53) | 75.22 (18.54) | −0.62 | .27 | −0.13 | −0.71 | 0.45 | ||
| SC | 68.93 (20.66) | 68.37 (18.10) | 0.21 | .42 | 0.04 | −0.53 | 0.61 | ||
| BGT | |||||||||
| RFC | 3.19 (1.73) | 3.50 (1.81) | −1.32 | .10 | −0.18 | −0.76 | 0.40 | ||
| SC | 2.50(1.20) | 2.07 (1.68) | 1.30 | .15 | 0.31 | −0.84 | 0.30 | ||
| DQOL-Y | |||||||||
| RFC | 3.80 (0.56) | 4.00 (0.50) | −3.70 | .001 | −0.77 | −1.37 | −0.17 | ||
| SC | 3.64 (0.67) | 3.73 (0.63) | −1.08 | .15 | −0.22 | −0.79 | 0.35 | ||
| . | . | M (SD) . | . | . | Effect size d . | 95% confidence interval . | |||
|---|---|---|---|---|---|---|---|---|---|
| Variable . | Condition . | Baseline . | Follow-up . | t . | p . | Lower . | Upper . | ||
| HbA1c | |||||||||
| RFC | 11.73 (2.01) | 11.03 (2.10) | 1.69 | .05 | 0.35 | −0.23 | 0.93 | ||
| SC | 11.28 (1.88) | 11.39 (2.12) | −0.29 | .39 | −0.06 | −0.63 | 0.51 | ||
| DMS-A | |||||||||
| RFC | 74.69 (19.79) | 76.51 (15.32) | −0.79 | .22 | −0.17 | −0.75 | 0.41 | ||
| SC | 69.63 (18.56) | 72.39 (15.91) | −1.02 | .16 | −0.21 | −0.78 | 0.36 | ||
| DMS-C | |||||||||
| RFC | 73.97 (17.53) | 75.22 (18.54) | −0.62 | .27 | −0.13 | −0.71 | 0.45 | ||
| SC | 68.93 (20.66) | 68.37 (18.10) | 0.21 | .42 | 0.04 | −0.53 | 0.61 | ||
| BGT | |||||||||
| RFC | 3.19 (1.73) | 3.50 (1.81) | −1.32 | .10 | −0.18 | −0.76 | 0.40 | ||
| SC | 2.50(1.20) | 2.07 (1.68) | 1.30 | .15 | 0.31 | −0.84 | 0.30 | ||
| DQOL-Y | |||||||||
| RFC | 3.80 (0.56) | 4.00 (0.50) | −3.70 | .001 | −0.77 | −1.37 | −0.17 | ||
| SC | 3.64 (0.67) | 3.73 (0.63) | −1.08 | .15 | −0.22 | −0.79 | 0.35 | ||
Note. BGT = blood glucose testing; DMS-A = Diabetes Management Scale-Adolescent; DMS-C = Diabetes Management Scale-Caregiver; DQOL-Y = Diabetes Quality of Life-Youth scale; Hemoglobin A1c = HbA1c; RFC = Reach for Control; SC = Standard Care.
Discussion
Families of children with special health care needs such as T1D consistently report difficulties obtaining health care and related services, with these disparities most evident among minority families (Rosen-Reynoso et al., 2016). There have been repeated calls for improvements in care coordination for such children, including more integrated behavioral and medical care (Bethell et al., 2014; Strickland et al., 2015). The purpose of the present study was to adapt an evidence-based intervention for adolescents with CPMC for use in a community setting delivered by CHWs and to conduct a preliminary evaluation of the feasibility and efficacy of the new intervention.
In total, 88% of eligible families who were contacted were willing to participate in the study, suggesting that families of adolescents with poorly controlled diabetes were open to the idea of receiving a home-based, family intervention delivered by a CHW. In addition, quantitative ratings and exit interviews suggested that caregiver satisfaction for those families who received RFC was high. Specific aspects of the program that caregivers viewed positively included the opportunity to practice and rehearse diabetes care skills in the home, working with their adolescent to improve communication around diabetes care, and support and encouragement to increase their own involvement in their adolescent’s care. In addition, families generally found CHWs to be professional and informative. CHWs have previously been used to successfully provide behavioral intervention services to high-risk children and families with chronic health conditions such as asthma (Krieger, Takaro, Song, Beaudet, & Edwards, 2009; Margellos-Anast, Gutierrez, & Whitman, 2012); the present study extends those findings to another chronic health condition.
In qualitative interviews, treatment dose (frequency and length of sessions) was reported to be acceptable, and only a subset of families, even those who dropped out, noted any significant concerns regarding this aspect of RFC. However, as compared with our MST-HC trials, where the intervention was delivered by a master’s level mental health professional, a higher rate of treatment dropout before completion of the full (6 months) program was documented (39 vs. 25%). In addition, while primary weekly sessions were completed at an acceptable rate, most families did not complete the additional weekly check-in sessions, despite the fact that session frequency was identified as a problem by only 27% of families. This finding was unexpected, given that the earlier MST intervention was successfully delivered at a mean dose of approximately two sessions per week (Ellis et al., 2012), but this issue may have been better anticipated had families been included in the initial adaptation process as stakeholders. Anecdotally, FQHC supervisory staff reported that families often canceled, rescheduled or no-showed for these sessions or reported being “too busy” to schedule a second session during the week. Therefore, delivering the check-in session by phone or dropping it from program content could be considered in future implementations of RFC. In addition, although limited program weaknesses were articulated in qualitative interviews, adolescent resistance to treatment participation—and the CHW’s ability to engage resistant adolescents and/or caregivers who disagreed with treatment recommendations—appeared to be related to treatment dropout for a subset of families. This may suggest that increasing CHW training in motivating and/or building relationships with challenging adolescents and families is an area for future program improvement.
Findings from the pilot clinical trial suggest that adolescents who received RFC had statistically significant and clinically meaningful improvements in glycemic control (0.7%). A 0.5% decrease in HbA1c has been linked to decreases in rates of diabetes complications (Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, 2008). HbA1c for adolescents receiving standard medical care were unchanged or slightly worse over the 6-month study period, reflecting the well-known negative trajectories for most youth with poorly controlled diabetes over time (Drotar et al., 2013; Hilliard, Wu, Rausch, Dolan, & Hood, 2013; King et al., 2012; Rohan et al., 2014). While frequency of blood glucose testing improved in the RFC group, these improvements were not large enough to be statistically significant. However, adolescents receiving RFC reported statistically significant increases in diabetes-related quality of life over the 6-month study window. Individuals’ self-rated experiences of health have been increasingly acknowledged as valuable markers of well-being beyond objective measures of health status (CDC Healthy People, 2020). Abualula et al. (2016) note that despite recent consensus statements on the care of youth with T1D that encourage the assessment of both health status and quality of life when monitoring outcomes in adolescents with T1D, most behavioral interventions targeting this group have focused solely on improving HbA1c. Others have also noted the challenges inherent in optimizing both HbA1c and quality of life for adolescents with T1D (Ingerski et al., 2010) and suggested that the quest for tight diabetes management has the potential for negatively impacting quality of life. Therefore, the ability of RFC to positively affect both these end points is noteworthy. Although not measured in this pilot study, prior work by our group using intensive home-based family treatment with youth with CPMC suggests that such interventions increase caregiver involvement in care (Ellis, Yopp, et al., 2006) and reduce youth diabetes-related stress (Ellis et al., 2005). Such changes could have in part accounted for RFC’s effects on Quality of Life (QOL) as well and could be further investigated in future studies.
Limitations of the study include the small sample size and lack of follow-up to monitor stability of treatment effects; findings require replication in a larger trial. Research assistants were not blind to intervention condition because of the need to complete exit interviews with treatment families. As the RFC intervention involved multiple components and intervened in multiple systems (child, family, school, health care), understanding which components were delivered most frequently or were responsible for treatment effects could help in reducing the intervention’s length and/or cost. Additional studies with larger sample sizes or different designs (e.g. multiphase optimization strategy [MOST]; Collins, Kugler & Gwadz, 2016) are needed to answer these questions. As the study was an initial test of the adapted RFC intervention, it did not address critical dissemination issues including a detailed investigation of barriers and facilitators of intervention adoption at the level of the interventionists, supervisor, or agency. In addition, as the study was conducted only in one agency, local factors related to intervention success, such as the agency’s prior use of CHWs, may have influenced study outcomes. Treatment fidelity was monitored through weekly group supervision focused on treatment manual adherence and review of session audiotapes but was not formally assessed because of the absence of an existing RFC fidelity measure at the time the study was initiated. Such a measure should be developed in the future, with a focus on developing a measure that can easily be used in a community setting. Future dissemination trials could also help to determine what types of CHW, supervisor, or organizational characteristics support high RFC treatment fidelity.
In conclusion, there continues to be a dearth of effective behavioral interventions for youth with T1D and CPMC that have been widely adopted in real-world settings. Additional research is needed to determine how to best disseminate programs that can improve health status and well-being to such adolescents and their families.
Funding
This work was supported by funding from the National Institute of Diabetes Digestive and Kidney Disease of the National Institutes of Health (R34 DK102091-01, PI).
Conflicts of interest: None declared.



