-
PDF
- Split View
-
Views
-
Cite
Cite
Xu-Fang Chen, Li-Shen Qian, Hong-Hua Shi, Ya-Zhou Zhang, Min-Shu Song, Hang Sun, Jian-Guo Chen, Allelopathic potentials of surrounding vegetation on seedling establishment of alpine cushion Arenaria polytrichoides, Journal of Plant Ecology, Volume 17, Issue 2, April 2024, rtae026, https://doi.org/10.1093/jpe/rtae026
Close - Share Icon Share
Abstract
When facilitating other species and sustaining plant community structures and biodiversity, alpine cushion plants simultaneously experience negative feedback effects from surrounding vegetation. However, the impact of surrounding vegetation on cushion dynamics remains poorly understood, particularly in terms of allelopathic potentials. To investigate the allelopathic potentials of surrounding vegetation on seedling establishment of the typical cushion plant Arenaria polytrichoides Edgew. along an elevational gradient, we extracted potential allelopathic compounds and tested their impacts on seed germination and seedling growth of A. polytrichoides. In addition, exclusion experiments using activated carbon were conducted to further elucidate these effects. Our results demonstrate that surrounding vegetation exhibits certain allelopathic potentials on A. polytrichoides seedling establishment, with variations observed based on elevation, source and concentration of allelopathy compounds, as well as growing season. Specifically, low-elevation vegetation exerts pronounced suppression on seedling establishment. Conversely, higher-elevation vegetation generally shows no effect on seed germination but stimulates seedling growth through allelopathy mechanisms. Moreover, aboveground vegetation predominantly inhibits both seed germination and seedling growth in low-elevation communities; however, the effects of belowground vegetation depend on elevation and extract concentration levels. The identified allelopathic potentials of surrounding vegetation significantly influence the population dynamics of cushion A. polytrichoides by potentially accelerating population degeneration in lower-elevation communities while ensuring consistent population recruitment and expansion in higher-elevation communities.
摘要
高山垫状植物一方面可以促进其他物种的生存和发展、维持植物群落结构及物种多样性,同时,其自身也受到周围植被的反向负反馈作用。但是,这种反馈作用对于垫状植物自身种群动态的影响仍不清楚,尤其缺乏对周围植被是否通过潜在化感作用的形式影响垫状植物自身的认识。本研究中,我们首先通过人工提取不同海拔带内周围植被的潜在化感物质,并检测其对代表性高山垫状植物团状福禄草(Arenaria polytrichoides)种子萌发和幼苗生长的影响,来探索周围植被潜在的化感作用对团状福禄草种群更新早期阶段(种子萌发和幼苗生长)的影响。其次,通过添加活性炭以排除化感物质的手段来进一步检测周围植被的潜在化感效应。结果表明,周围植被对团状福禄草幼苗定植具有一定的化感作用,但其作用形式和强度同时受到海拔、化感物质来源及其浓度以及生长季节等因素的影响。具体而言,低海拔群落内的植被对团状福禄草种子萌发和幼苗生长均产生显著的抑制作用;相反,高海拔群落内的植被通常不会影响种子萌发,但却可以促进幼苗生长。此外,对于低海拔群落,来源于地上植被的化感物质对种子萌发和幼苗生长均产生了显著的抑制作用;然而,来源于地下植被的化感物质,其化感作用形式和强度取决于海拔及其浓度水平。基于研究发现,我们确定对于高山垫状植物团状福禄草,其所处群落中的周围植被可通过化感作用的形式对它们的种子萌发和幼苗定植等种群更新的关键阶段和过程产生重要影响,从而可能影响其种群动态过程。具体地,在低海拔植被群落内,周围植被可通过化感作用抑制种群更新从而加速种群衰退进程,而高海拔群落内的周围植被可通过化感作用促进其幼苗定植和生长,从而保证种群的正常更新和扩张。
INTRODUCTION
Plant–plant interactions play pivotal roles in maintaining alpine biodiversity (Cavieres et al. 2016; Kikvidze et al. 2015). For instance, facilitative interactions can restructure micro-community compositions (Badano et al. 2006), augment species abundance and diversity (Cavieres et al. 2016; Chen et al. 2015; Kikvidze et al. 2015) and foster aboveground biomass production (Callaway et al. 2002). Conversely, negative interactions also contribute to those ecological processes (Albrecht et al. 2010; Cipriotti and Aguiar 2015; Verdu et al. 2010). Generally, facilitative interactions occur within communities when one species benefits others by providing suitable microsites, thereby enhancing the survival and growth of other species (Brooker et al. 2008; Butterfield 2009; Gavini et al. 2020; Zhang and Tielbörger 2019). In contrast, negative interactions may arise from resource competition (Brooker et al. 2008; Burzle et al. 2018; Cavieres et al. 2007; Jensen et al. 2012; Maestre et al. 2009) or allelopathic effects induced by the release of specific secondary metabolites that can affect the growth, survival and/or reproduction of other species (Hierro and Callaway 2021; Rice 1984).
The allelopathic effect has been recognized as a crucial mechanism governing plant community structures and dynamics, and it plays significant roles in determining plant distribution patterns, diversity levels, invasion processes and community dynamics (Ens et al. 2009; Hager 2004; Hierro and Callaway 2021; Inderjit et al. 2011; Koocheki et al. 2013). Initially, it refers to the impact of one plant on neighboring plant(s) through the release of chemical compounds, known as allelochemicals, into the environment (Rice 1984). The effects can be neutral, positive or negative depending on factors such as compound composition, source, concentration, plant life history stages and/or growing seasons (Barto and Cipollini 2009; Deng et al. 2024; Gniazdowska and Bogatek 2005; Hierro and Callaway 2021; Inderjit et al. 2011; Koocheki et al. 2013; Zhang et al. 2015). Furthermore, these effects can vary under different environmental stresses (Maqbool et al. 2013 and references therein).
Allelochemicals can be released from various plant organs, including belowground root systems and aboveground parts, and subsequently emitted into the surrounding environments (Deng et al. 2024; Gniazdowska and Bogatek 2005; Koocheki et al. 2013; Rice 1984; Zhang et al. 2015). Indeed, diverse organic compounds may be exuded by plants during different life histories or phenological stages, potentially acting synergistically to induce allelopathic effects of either positive or negative nature (Gniazdowska and Bogatek 2005; Koocheki et al. 2013). Apart from influencing the growth, mortality and/or reproduction of established individuals, allelopathic effects have been predominantly observed in early life history stages of plants, such as seed germination, coleoptile elongation, radicle shoot development and root growth (Inderjit et al. 2011; Koocheki et al. 2013 and references therein).
Cushion plants are recognized as crucial ecosystem engineers (Jones et al. 1994) that can facilitate the growth of other less stress-tolerant plants (Badano et al. 2006; Cavieres and Badano 2009; Cavieres et al. 2007; Chen et al. 2015; Kikvidze et al. 2015). Consequently, a substantial proportion of alpine biodiversity, including plants (Chen et al. 2015; Kikvidze et al. 2015), arthropods (Chen et al. 2021; Reid and Lortie 2012) and microbiomes (Chang et al. 2018; Roy et al. 2013; Wang et al. 2020), exclusively rely on the presence of cushion plants. For instance, cushion plants have been shown to increase overall regional species richness by up to 40% in the Northern Patagonian Andes in Argentina (Gavini et al. 2020), and increase local plant richness from 5% to 59% in the high Hengduan Mountains in southwestern China (Chen et al. 2015). Moreover, cushion plants can also regulate the attributes of the surrounding soil seed bank, thereby playing a crucial role in community dynamics and the conservation and recovery of degraded areas (Niknam et al. 2018). Therefore, any alterations in cushion plant populations could trigger a cascade of changes within the entire alpine community dynamics (Chen et al. 2023). Previous studies have reported that climate warming-induced expansion of lowland species has already resulted in certain shrinkage or degradation of communities dominated by cushion plants (Chen et al. 2023; Huang and Wang 1991; Zhao et al. 2011). However, limited research has been conducted on investigating the negative effects exerted by surrounding vegetation on cushion plants (but see Chen et al. 2024; Michalet et al. 2016; Schöb et al. 2014). Schöb et al. (2014) found that neighboring plants, known as ‘beneficiary plants’, can impose detrimental feedback effects on cushion plants, including physiological constraints and reduced reproductive outputs. Subsequently, Michalet et al. (2016) confirmed that these negative feedback effects intensify under increasing environmental stress. Recently, Chen et al. (2020a) found evidence suggesting that co-occurring surrounding plants may impede the seedling survival of cushion Arenaria polytrichoides Edgew., a typical cushion plant distributing in the Hengduan Mountains of SW China. Moreover, Chen et al. (2023) verified a higher mortality rate among A. polytrichoides individuals in lower-elevation communities with high surrounding vegetation cover (>95%), while those in higher-elevation communities with significantly lower vegetation cover (<15%) experienced a lower mortality rate. These findings suggest potential negative impacts of surrounding vegetation on cushion population recruitment within alpine ecosystems; however, none of these studies (Chen et al. 2020a, 2023; Michalet et al. 2016; Schöb et al. 2014) have investigated the allelopathic potentials of the neighboring vegetation.
Allelopathy potentially influences population recruitment by affecting the establishment of new seedlings. In this study, our objective was to investigate the allelopathic potentials of surrounding vegetation on seed germination and seedling growth of A. polytrichoides, a typical alpine cushion plant, across an altitudinal gradient. A recent study has reported degeneration in some populations of this species, and clarified some relevant driving mechanisms (Chen et al. 2023). However, the existence of allelopathy and its impact on seedling establishment and population dynamics under environmental stress remains elusive. Revealing these knowledge gaps will make a substantial contribution to comprehending the mechanisms underpinning the population dynamics of this pivotal cushion species. Specifically, we aimed to answer three questions: (i) Does surrounding vegetation affect the seedling establishment of A. polytrichoides through allelopathic potentials? If yes, (ii) How does it influence seed germination and seedling growth? And (iii) How does the allelopathic potential vary with elevation-associated environmental stress?
MATERIALS AND METHODS
Study system
Arenaria polytrichoides Edgew. (Caryophyllaceae) is a long-lived cushion-forming plant that inhabits the main Sino-Himalayan Mountains, ranging from ca. 3500 to 5300 m above sea level (a.s.l.) (http://www.efloras.org). As an exemplary ecosystem engineer (Jones et al. 1994), this species can effectively ameliorate micro-environments, thereby providing suitable microsites for both less stress-tolerant beneficiary plants and insects (Chen et al. 2015, 2021; Yang et al. 2010). Consequently, its population dynamics can have significant implications for alpine community structures (Chen et al. 2023), which might exert further influences on the soil erosion in alpine pastures and their associated services.
To collect vegetation materials, we selected three communities at different elevations in the Pujin pasture on the Baima snow mountains, which are located in the core biodiversity area of the Hengduan Mountains in southwestern China. The elevations range from 4380 m (28°26ʹ51″ N, 98°59ʹ52″ E), 4720 m (28°28ʹ41″ N, 99°00ʹ04″ E) to 4920 m (28°28ʹ50″ N, 99°00ʹ33″ E) with distinct environmental stresses experienced by each community. Specifically, the low-elevation community represents a dense alpine meadow with a vegetation cover exceeding 95%, dominated solely by sedge species. The middle-elevation community is characterized as an alpine meadow-screes transition zone with approximately 40% vegetation cover and a significant decrease in the abundance of sedge species compared to the low-elevation community. Additionally, dominant species include cushion A. polytrichoides and various taxa such as Saussurea, Saxifraga, Potentilla, Trollius and Veratrilla. The high-elevation community exhibits typical alpine screes vegetation, with less than 15% vegetation cover and a significantly decreased abundance of sedge species compared to lower-elevation communities. Apart from cushion species such as A. polytrichoides, A. oreophila, Potentilla articulata, Androsace delavayi, and Saxifraga finitima, no other distinct dominant plants were observed; however, Saussurea, Saxifraga, Draba, Astragalus, Rhodiola and Odontostemma species also thrive in this community. These changes in vegetation cover and species composition may indicate a shift in interspecific interactions within these communities—potentially transitioning from competition to facilitation as elevation increases (Chen et al. 2019). Furthermore, the abiotic stresses increase with increasing elevation (Chen et al. 2019; Wang 2006). For instance, soil nutrient availability along with soil and air temperatures all decrease as elevation increases (Chen et al. 2019, 2020b).
Field and laboratory experiments
The seeds of cushion A. polytrichoides used in this study were collected from populations at various elevations in the Pujin pasture in late September 2020. Notably, there were no significant differences in seed quality parameters, such as weight and germination percentage, among the diverse populations (Chen et al. 2020a, 2023). Consequently, a composite sample comprising all seeds from these diverse populations was employed for subsequent laboratory experiments.
Preliminary investigation—effects of vegetation extracts
Given the uncertainty of the existence of allelopathy in our study systems, we conducted a preliminary investigation to ascertain the impact of extracts from surrounding vegetation on seed germination and seedling growth of cushion A. polytrichoides. To accomplish this, we collected above- and belowground vegetation materials from the low- and middle-elevation communities on 3 October 2020. Our objective was to assess the collective allelopathic potential of the surrounding vegetation rather than focusing solely on specific plant species; therefore, early October was chosen as an appropriate time to collect vegetation materials encompassing most plant species within relevant communities after a complete growing season. The high-elevation community was subjectively overlooked in this preliminary investigation, partly due to the potential complete withering and dispersal of aboveground plant organs in such a late-growing season at such a high-elevation screes vegetation site. However, even in late October, the lower two communities exhibited well-preserved plant organs (personal observation).
Specifically, three points (approximately 30 cm × 30 cm) were randomly selected within each chosen community. For aboveground vegetation materials, all plant organs within each point were collected and mixed together for each community. For belowground materials, including roots and decaying matter, plant materials from approximately 0 to 10 cm below the surface were collected and mixed for each community. Although allelochemicals may also be released by soil microbes (Inderjit 2005), our primary objective in this preliminary investigation was to confirm whether allelochemicals can be released by surrounding vegetation (plants) and identify the specific parts responsible for their release; therefore, any adhering soils on the collected plant materials were carefully removed. All plant materials were finely chopped into small pieces (less than 0.5 cm) to facilitate subsequent extraction of potential allelochemicals. To ensure comprehensive extraction of all potential allelochemicals from the aforementioned vegetation materials, which can be released through various mechanisms (Inderjit et al. 2011 and references therein), we employed two types of solvents—distilled water and 75% ethanol—as intermediaries for extracting potential allelochemicals. Half of these materials (approximately 50 g dry mass) were soaked in conical flasks containing 500 mL distilled water at around 25°C for a duration of 48 h, while the other half was soaked in conical flasks containing 75% ethanol under similar conditions. The conical flasks were placed on an electronic lab shaker and gently shaken at a speed of 90 rpm/min for a duration of 10 min every 4 h. Subsequently, all plant materials underwent a filtration process followed by evaporation using a rotary evaporator under reduced pressure conditions at a temperature of approximately 30°C until extracts obtained. The extracts were stored in opaque glass bottles with airtight seals at a temperature of 4°C until they were used. To the best of our knowledge, this study represents the first attempt to investigate the allelopathic potential of surrounding vegetation on alpine cushion plants within the entire Hengduan Mountains region. Unfortunately, prior to this study, there was a lack of available information regarding the concentration of allelopathic compounds released by plants into the surrounding vegetation in our study area. However, it is known that compositions and concentrations of allelopathic compounds can vary among communities due to changes in plant composition and related ecological processes (Inderjit et al. 2011 and references therein). Therefore, it may not be necessary to use identical extract concentrations when studying allelopathic effects in different systems. The primary objective of this section was to investigate whether extracts released from the surrounding vegetation exhibit any allelopathic potential on cushion plants and determine if these allelopathic effects are influenced by changes in compound concentrations. Following recommendations by Inderjit and Nilsen (2003) that laboratory studies on allelopathy should include a range of concentrations (also see Zhang et al. 2015), we prepared four different extract solutions with concentrations of 20, 50, 100 and 250 mg/L. Although these concentrations may exceed naturally occurring levels of plant-released chemicals, their inclusion is crucial for effectively detecting allelopathic potentials within the context of this study.
The seeds and subsequent seedlings were cultivated in 10 cm diameter Petri dishes containing filter paper under controlled conditions of a 12-h night (dark)/12-h day (light) cycle with full light availability (7000 lx) in an incubator, at a temperature range of 10/15°C. This temperature range has been reported as the most suitable for seed germination and seedling growth of A. polytrichoides by Chen et al. (2020a, 2023). Each vegetation part (above- and belowground) from each community (low and middle) was randomly assigned two Petri dishes, each divided into three grids, with 30 fully matured seeds allocated to each grid. Therefore, there were six replicates per extract concentration for each relevant extract source, resulting in a total of 34 Petri dishes and 102 grids used in this study (Supplementary Fig. S1). Each grid of the experimental Petri dishes was soaked with 5 mL solution containing the corresponding extract concentration to ensure adequate seed soaking, while control Petri dishes received 5 mL distilled water at the beginning of the experiment. To prevent drought stress, an additional volume of either extract solution or distilled water was added every time the filter paper became dry at approximately 5-day intervals throughout the experiment; thus, eight applications were made overall. It is worth noting that although absolute extracts increased progressively with subsequent additions during this process within experimental groups, their relative concentrations remained consistent across treatments; therefore, any observed differences in seed and/or seedling performance can be reasonably attributed to variations in extract concentration treatments employed here. Germinated seeds were recorded daily until no new seedlings appeared for at least 5 consecutive days over a period lasting up to 40 days from initiation. The five visually longest (in order to minimize the potential impact of germination lag) seedlings in each Petri dish grid were selected for measuring their length (from the base of the shoot to the apex of the leaf) and values were averaged per Petri dish grid for subsequent analysis.
Further investigation—effects of activated carbon
Elucidating the specific mechanisms and effects of allelopathy remains a contemporary challenge due to difficulties in distinguishing between allelopathy and resource competition, as well as other processes that can influence plant–plant interactions (Hierro and Callaway 2021; Lau et al. 2008). Furthermore, laboratory investigations of allelopathy may be confounded by methodological artifacts (Inderjit and Nilsen 2003). Despite potentially introducing some ambiguity in assessing the effects of allelopathy (Lau et al. 2008), the addition of activated carbon (ActC) effectively absorbs exudates released from plants or soil microbes, making it a commonly utilized approach for reducing relevant allelopathic effects (Kheirabadi et al. 2020; Lyytinen and Lindström 2019). To further investigate the allelopathic potential of surrounding vegetation on cushion seedling recruitment, we employed activated carbon (hereafter referred to as ‘ActC’) to exclude potential allelochemicals.
We conducted a comparative analysis of seed germination and seedling growth in vegetation materials with ‘ActC-mixed’ and ‘ActC-free’ compositions. It is well documented that the composition and concentration of allelochemicals released by plants can vary across different plant life history stages or growing seasons (Barto and Cipollini 2009; Gniazdowska and Bogatek 2005; Inderjit et al. 2011; Koocheki et al. 2013 and references therein). Although seeds of cushion A. polytrichoides typically germinate during the early to middle-growing season, it is possible for allelochemicals released from vegetation in different growing seasons to persist in the soils, exerting their effects on subsequent growth seasons. To investigate the allelopathic potentials of surrounding vegetation at different elevations and during various growing seasons, we collected vegetation materials from three selected communities.
Specifically, on 5 July, 20 August and 22 September 2021, corresponding to the early-, middle- and late-growing seasons, respectively, three points (approximately 30 cm × 30 cm) were randomly selected within each community with a minimum distance of three meters between any two points. Vegetation turfs (approximately 30 cm × 30 cm × 5 cm) were collected from each point. The vegetation turfs from the same community were thoroughly mixed and air-dried for a duration of 1 month after removing stones larger than 0.5 cm in diameter. To account for potential allelochemicals released by certain soil microbiomes (Inderjit et al. 2011; Koocheki et al. 2013), other adhering soils present in the vegetation turfs were retained. Subsequently, the collected vegetation turfs from each community during each growing season were pulverized and equally divided into two duplicates. One duplicate solely consisted of crushed vegetation turf materials while the other was mixed at a ratio of 2:1 with activated carbon. These vegetation turf materials served as a culture medium to assess the allelopathic potentials of surrounding vegetation on seed germination and seedling growth of cushion A. polytrichoides. For this purpose, flowerpots (10 cm × 10 cm × 12 cm) were filled with the aforementioned culture mediums—six flowerpots filled with ActC-free vegetation turf medium (ActC-free treatment) and another six filled with ActC-mixed vegetation turf medium (ActC-mixed treatment). Each flowerpot was filled with approximately 120 g of relevant mediums, resulting in a total of 108 flowerpots across both treatments (Supplementary Fig. S2). The flowerpots were adequately watered with distilled water and maintained at room temperature (with an average temperature of approximately 22°C) for a duration of 10 days to allow for the sufficient release of potential allelochemicals in the‘ActC-free treatment’. In the‘ActC-mixed treatment’, activated carbon was used to ensure adequate absorption of allelochemicals. Subsequently, 30 fully matured seeds of A. polytrichoides were randomly selected and sown into each flowerpot, where they were cultivated under the aforementioned conditions. Distilled water was provided every 3 days to prevent drought stress. Germinated seeds were recorded daily until no new seedlings appeared for at least 5 consecutive days or until all 30 seeds had germinated completely. Following this, regular watering was continued until the end of the 90-day experiment duration. At that point, we selected five visually longest seedlings from each flowerpot (in order to minimize the potential impact of germination lag) and measured their length (from the base of the shoot to the apex of the leaf). These values were averaged per flowerpot for subsequent analysis.
Data analyses
The effects of potential allelopathic compounds on seed germination and seedling growth were assessed using linear mixed-effects models. In the experiment for extracting allelopathic compounds, we considered the source (above- and belowground), extracting medium (aqueous or ethanol), concentration, community (low and middle) and their interactions as fixed effects, with Petri dish identity as a random effect. In the ActC treatment experiment, we considered the community (low, middle and high), growing season (July, August and September), ActC treatment (‘ActC-mixed’ and ‘ActC-free’) and their interactions as fixed effects, with replicate as a random effect. To examine the allelopathic potentials of surrounding vegetation influenced by all these factors, initial models were conducted using synthesized data including all relevant information (factors). Subsequently, separate models were performed to test the influence of other factors within specific communities, extracting mediums or growing seasons. Seed germination data were standardized between 0 and 1 using the formula where X represents the relevant value of seed germination. On the other hand, seedling growth data (height) were log10 transformed to meet the assumptions of parametric analyses.
The analyses described above were performed using R v.4.1.1 (R Core Team 2021). For linear mixed-effects modeling, the ‘lme4’ package developed by Bates et al. (2015) was utilized. Post hoc tests were conducted to examine pairwise differences between treatments, employing the ‘Tukey’ method as implemented in the ‘emmeans’ package (Lenth 2022). All figures presented in this study were generated using the ‘ggplot2’ package (Wickham 2016), and figure layouts were designed using Adobe Illustrator 2021.
RESULTS
The surrounding vegetation indeed exhibited certain allelopathic potentials on the seed germination and seedling growth of cushion A. polytrichoides. However, these potentials were found to be dependent on elevation (low and middle), vegetation component (above- and belowground), allelochemical extracting solvent (distilled water and alcohol), concentration, as well as their interactions (Table 1). Specifically, when extracted with distilled water, allelochemicals released from aboveground vegetation in the low-elevation community significantly inhibited seed germination across all concentrations; conversely, no effect was observed for allelochemicals released from belowground vegetation (Fig. 1a). However, in the middle-elevation community, inhibitory effects were only detected at a concentration of 50 mg/L for belowground-released allelochemicals and a concentration of 250 mg/L for aboveground-released allelochemicals (Fig. 1c).
Effect of potential allelopathic compounds released from surrounding vegetation on the seed germination percentage of cushion Arenaria polytrichoides
| . | SS . | MS . | NumDF . | DenDF . | F value . | Pr(>F) . |
|---|---|---|---|---|---|---|
| Community | 21.96 | 21.96 | 1 | 204 | 77.87 | 4.99 × 10−16 |
| Allelochemical source | 50.40 | 25.20 | 2 | 204 | 89.36 | 1.34 × 10−28 |
| Extracting medium | 4.69 | 4.69 | 1 | 204 | 16.63 | 6.50 × 10−05 |
| Allelochemical concentration | 13.89 | 4.63 | 3 | 204 | 16.42 | 1.35 × 10−09 |
| Com × AlleloS | 17.41 | 8.71 | 2 | 204 | 30.88 | 1.93 × 10−12 |
| Com × ExtrM | 5.04 | 5.04 | 1 | 204 | 17.87 | 3.58 × 10−05 |
| AlleloS × ExtrM | 0.21 | 0.10 | 2 | 204 | 0.36 | 0.69 |
| Com × AlleloC | 23.70 | 7.90 | 3 | 204 | 28.01 | 3.27 × 10−15 |
| AlleloS × AlleloC | 6.69 | 2.23 | 3 | 204 | 7.91 | 5.14 × 10−05 |
| ExtrM × AlleloC | 10.83 | 3.61 | 3 | 204 | 12.80 | 1.07 × 10−07 |
| Com × AlleloS × ExtrM | 1.67 | 0.83 | 2 | 204 | 2.96 | 0.054 |
| Com × AlleloS × AlleloC | 7.16 | 2.39 | 3 | 204 | 8.46 | 2.53 × 10−05 |
| Com × ExtrM × AlleloC | 5.84 | 1.95 | 3 | 204 | 6.91 | 1.89 × 10−04 |
| AlleloS × ExtrM × AlleloC | 2.05 | 0.68 | 3 | 204 | 2.42 | 0.07 |
| Com × AlleloS × ExtrM × AlleloC | 0.82 | 0.27 | 3 | 204 | 0.97 | 0.41 |
| . | SS . | MS . | NumDF . | DenDF . | F value . | Pr(>F) . |
|---|---|---|---|---|---|---|
| Community | 21.96 | 21.96 | 1 | 204 | 77.87 | 4.99 × 10−16 |
| Allelochemical source | 50.40 | 25.20 | 2 | 204 | 89.36 | 1.34 × 10−28 |
| Extracting medium | 4.69 | 4.69 | 1 | 204 | 16.63 | 6.50 × 10−05 |
| Allelochemical concentration | 13.89 | 4.63 | 3 | 204 | 16.42 | 1.35 × 10−09 |
| Com × AlleloS | 17.41 | 8.71 | 2 | 204 | 30.88 | 1.93 × 10−12 |
| Com × ExtrM | 5.04 | 5.04 | 1 | 204 | 17.87 | 3.58 × 10−05 |
| AlleloS × ExtrM | 0.21 | 0.10 | 2 | 204 | 0.36 | 0.69 |
| Com × AlleloC | 23.70 | 7.90 | 3 | 204 | 28.01 | 3.27 × 10−15 |
| AlleloS × AlleloC | 6.69 | 2.23 | 3 | 204 | 7.91 | 5.14 × 10−05 |
| ExtrM × AlleloC | 10.83 | 3.61 | 3 | 204 | 12.80 | 1.07 × 10−07 |
| Com × AlleloS × ExtrM | 1.67 | 0.83 | 2 | 204 | 2.96 | 0.054 |
| Com × AlleloS × AlleloC | 7.16 | 2.39 | 3 | 204 | 8.46 | 2.53 × 10−05 |
| Com × ExtrM × AlleloC | 5.84 | 1.95 | 3 | 204 | 6.91 | 1.89 × 10−04 |
| AlleloS × ExtrM × AlleloC | 2.05 | 0.68 | 3 | 204 | 2.42 | 0.07 |
| Com × AlleloS × ExtrM × AlleloC | 0.82 | 0.27 | 3 | 204 | 0.97 | 0.41 |
Abbreviations: AlleloC = allelochemical concentration (0, 20, 50, 100 and 250 mg/L), AlleloS = allelochemical source (above- or belowground), Com = community (low, middle and high), ExtrM = extracting medium (ethanol or aqueous).
Effect of potential allelopathic compounds released from surrounding vegetation on the seed germination percentage of cushion Arenaria polytrichoides
| . | SS . | MS . | NumDF . | DenDF . | F value . | Pr(>F) . |
|---|---|---|---|---|---|---|
| Community | 21.96 | 21.96 | 1 | 204 | 77.87 | 4.99 × 10−16 |
| Allelochemical source | 50.40 | 25.20 | 2 | 204 | 89.36 | 1.34 × 10−28 |
| Extracting medium | 4.69 | 4.69 | 1 | 204 | 16.63 | 6.50 × 10−05 |
| Allelochemical concentration | 13.89 | 4.63 | 3 | 204 | 16.42 | 1.35 × 10−09 |
| Com × AlleloS | 17.41 | 8.71 | 2 | 204 | 30.88 | 1.93 × 10−12 |
| Com × ExtrM | 5.04 | 5.04 | 1 | 204 | 17.87 | 3.58 × 10−05 |
| AlleloS × ExtrM | 0.21 | 0.10 | 2 | 204 | 0.36 | 0.69 |
| Com × AlleloC | 23.70 | 7.90 | 3 | 204 | 28.01 | 3.27 × 10−15 |
| AlleloS × AlleloC | 6.69 | 2.23 | 3 | 204 | 7.91 | 5.14 × 10−05 |
| ExtrM × AlleloC | 10.83 | 3.61 | 3 | 204 | 12.80 | 1.07 × 10−07 |
| Com × AlleloS × ExtrM | 1.67 | 0.83 | 2 | 204 | 2.96 | 0.054 |
| Com × AlleloS × AlleloC | 7.16 | 2.39 | 3 | 204 | 8.46 | 2.53 × 10−05 |
| Com × ExtrM × AlleloC | 5.84 | 1.95 | 3 | 204 | 6.91 | 1.89 × 10−04 |
| AlleloS × ExtrM × AlleloC | 2.05 | 0.68 | 3 | 204 | 2.42 | 0.07 |
| Com × AlleloS × ExtrM × AlleloC | 0.82 | 0.27 | 3 | 204 | 0.97 | 0.41 |
| . | SS . | MS . | NumDF . | DenDF . | F value . | Pr(>F) . |
|---|---|---|---|---|---|---|
| Community | 21.96 | 21.96 | 1 | 204 | 77.87 | 4.99 × 10−16 |
| Allelochemical source | 50.40 | 25.20 | 2 | 204 | 89.36 | 1.34 × 10−28 |
| Extracting medium | 4.69 | 4.69 | 1 | 204 | 16.63 | 6.50 × 10−05 |
| Allelochemical concentration | 13.89 | 4.63 | 3 | 204 | 16.42 | 1.35 × 10−09 |
| Com × AlleloS | 17.41 | 8.71 | 2 | 204 | 30.88 | 1.93 × 10−12 |
| Com × ExtrM | 5.04 | 5.04 | 1 | 204 | 17.87 | 3.58 × 10−05 |
| AlleloS × ExtrM | 0.21 | 0.10 | 2 | 204 | 0.36 | 0.69 |
| Com × AlleloC | 23.70 | 7.90 | 3 | 204 | 28.01 | 3.27 × 10−15 |
| AlleloS × AlleloC | 6.69 | 2.23 | 3 | 204 | 7.91 | 5.14 × 10−05 |
| ExtrM × AlleloC | 10.83 | 3.61 | 3 | 204 | 12.80 | 1.07 × 10−07 |
| Com × AlleloS × ExtrM | 1.67 | 0.83 | 2 | 204 | 2.96 | 0.054 |
| Com × AlleloS × AlleloC | 7.16 | 2.39 | 3 | 204 | 8.46 | 2.53 × 10−05 |
| Com × ExtrM × AlleloC | 5.84 | 1.95 | 3 | 204 | 6.91 | 1.89 × 10−04 |
| AlleloS × ExtrM × AlleloC | 2.05 | 0.68 | 3 | 204 | 2.42 | 0.07 |
| Com × AlleloS × ExtrM × AlleloC | 0.82 | 0.27 | 3 | 204 | 0.97 | 0.41 |
Abbreviations: AlleloC = allelochemical concentration (0, 20, 50, 100 and 250 mg/L), AlleloS = allelochemical source (above- or belowground), Com = community (low, middle and high), ExtrM = extracting medium (ethanol or aqueous).
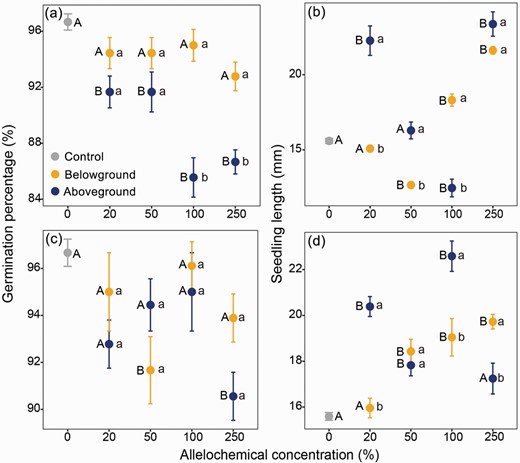
Effect of aqueous extracts derived from aboveground or belowground vegetation in low-elevation (a, b) and middle-elevation (c, d) communities on seed germination percentage (a, c) and seedling growth (b, d) of cushion Arenaria polytrichoides. Superscript lowercase letters indicate significant differences between above- and belowground vegetation at specific extract concentrations; while uppercase letters indicate significant differences between the control treatment (cultivated in distilled water) and extracts of varying concentrations.
Furthermore, the extracts exhibited varying effects on seedling growth, which were also influenced by elevation, vegetation part, allelochemical extracting medium, concentration and their interactions (Supplementary Table S1). In comparison to seedlings cultivated in distilled water, extracts derived from aboveground vegetation of the low-elevation community significantly enhanced seedling growth at both lower (20 mg/L) and higher (250 mg/L) concentrations (z = −10.70, P < 0.001 and z = −12.23, P < 0.001 for lower and higher concentrations, respectively), while having no effect at 50 mg/L (z = −1.26, P = 0.996). Conversely, extracts released from belowground vegetation showed no effect at 20 mg/L (z = 1.01, P = 1.00), inhibitory effect at 50 mg/L (z = 6.34, P < 0.001), but stimulatory effect at higher concentrations (P < 0.001, Fig. 1b). For the middle-elevation community, both above- and belowground vegetation-derived extracts generally stimulated seedling growth (P < 0.01), except for those obtained from belowground vegetation at a concentration of 20 mg/L, as well as those obtained from aboveground vegetation at a concentration of 250 mg/L (P > 0.05, Fig. 1d).
Ethanol extraction of allelochemicals from the low-elevation vegetation exhibited inhibitory effects on seed germination, particularly at higher extract concentrations. This inhibitory effect was most prominent when utilizing aboveground vegetation-derived extracts (Fig. 2a). However, within the middle-elevation community, solely aboveground vegetation-derived extracts demonstrated a significant inhibitory effect on seed germination (Fig. 2c).
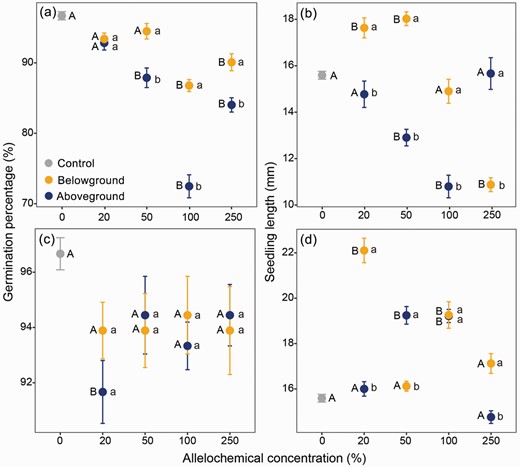
Effect of ethanol extracts derived from aboveground or belowground vegetation in low-elevation (a, b) and middle-elevation (c, d) communities on seed germination percentage (a, c) and seedling growth (b, d) of cushion Arenaria polytrichoides. Superscript lowercase letters indicate significant differences between above- and belowground vegetation at specific extract concentrations, while uppercase letters indicate significant differences between the control treatment (cultivated in distilled water) and extracts of varying concentrations.
The extracts released from aboveground vegetation in the low-elevation community exhibited general inhibitory effects on seedling growth, particularly at intermediate concentrations (50 and 100 mg/L); while no significant effect was observed at neither 20 nor 250 mg/L concentrations (Fig. 2b). Interestingly, the extracts released from belowground vegetation in the low-elevation community showed a significant stimulatory effect on seedling growth at lower concentration (20 and 50 mg/L), but conversely inhibited seedling growth at higher concentrations (250 mg/L; Fig. 2b). For the middle-elevation community, aboveground vegetation extracts displayed stimulatory effects on seedling growth at intermediate concentrations (50 and 100 mg/L) but had no effect at either low (20 mg/L) or high (250 mg/L) concentrations (Fig. 2d). Meanwhile, aboveground vegetation extracts stimulated seedling growth significantly in both low and high concentration range of 20–100 mg/L but not in and intermediate range of concentration between them (50–250 mg/L; Fig. 2d).
The seed germination of the cushion A. polytrichoides was significantly influenced by activated carbon (ActC), with effects varying depending on elevation, growing season, activated carbon and their interactions (Table 2). Specifically, when ActC was mixed with vegetation collected from low-elevation communities during all growing seasons, there was a significant increase in the seed germination percentage (z = 3.20, P = 0.017 for July; z = 3.67, P = 0.003 for August; and z = 3.83, P = 0.002 for September; Fig. 3). This indicates that surrounding vegetation in low-elevation communities consistently inhibits the seed germination of cushion A. polytrichoides. In contrast, ActC addition only increased the percentage of seed germination in late-growing season (September) for vegetation from middle-elevation communities (z = 4.96, P < 0.001), while it had no effect during early-growing season (July) and mid-growing season (August) (z = −1.20, P = 0.837 for July and z = −1.33, P = 0.777 for August; Fig. 3). Similarly, ActC addition only increased the percentage of seed germination during early-growing season (July) for high-elevation community vegetation (z = −4.40, P < 0.001), but had no effect during mid-growing season (August) (z = 2.44, P = 0.141) and late-growing season (September) (z = −2.69, P = 0.077) (Fig. 3).
Effect of surrounding vegetation mixed with or without activated carbon on seed germination percentage of cushion Arenaria polytrichoides
| . | SS . | MS . | NumDF . | DenDF . | F value . | Pr(>F) . |
|---|---|---|---|---|---|---|
| Community | 14.56 | 7.28 | 2 | 90 | 17.46 | 3.90 × 10−07 |
| Growing season | 10.35 | 5.18 | 2 | 90 | 12.42 | 1.73 × 10−05 |
| Activated carbon | 3.23 | 3.23 | 1 | 90 | 7.75 | 6.54 × 10−3 |
| Com × GS | 8.26 | 2.06 | 4 | 90 | 4.95 | 1.18 × 10−3 |
| Com × ActC | 13.86 | 6.93 | 2 | 90 | 16.63 | 7.16 × 10−07 |
| GS × ActC | 5.20 | 2.60 | 2 | 90 | 6.24 | 2.90 × 10−3 |
| Com × GS × ActC | 14.05 | 3.51 | 4 | 90 | 8.43 | 8.04 × 10−6 |
| . | SS . | MS . | NumDF . | DenDF . | F value . | Pr(>F) . |
|---|---|---|---|---|---|---|
| Community | 14.56 | 7.28 | 2 | 90 | 17.46 | 3.90 × 10−07 |
| Growing season | 10.35 | 5.18 | 2 | 90 | 12.42 | 1.73 × 10−05 |
| Activated carbon | 3.23 | 3.23 | 1 | 90 | 7.75 | 6.54 × 10−3 |
| Com × GS | 8.26 | 2.06 | 4 | 90 | 4.95 | 1.18 × 10−3 |
| Com × ActC | 13.86 | 6.93 | 2 | 90 | 16.63 | 7.16 × 10−07 |
| GS × ActC | 5.20 | 2.60 | 2 | 90 | 6.24 | 2.90 × 10−3 |
| Com × GS × ActC | 14.05 | 3.51 | 4 | 90 | 8.43 | 8.04 × 10−6 |
Abbreviations: ActC = activated carbon, Com = community, GS = growing season.
Effect of surrounding vegetation mixed with or without activated carbon on seed germination percentage of cushion Arenaria polytrichoides
| . | SS . | MS . | NumDF . | DenDF . | F value . | Pr(>F) . |
|---|---|---|---|---|---|---|
| Community | 14.56 | 7.28 | 2 | 90 | 17.46 | 3.90 × 10−07 |
| Growing season | 10.35 | 5.18 | 2 | 90 | 12.42 | 1.73 × 10−05 |
| Activated carbon | 3.23 | 3.23 | 1 | 90 | 7.75 | 6.54 × 10−3 |
| Com × GS | 8.26 | 2.06 | 4 | 90 | 4.95 | 1.18 × 10−3 |
| Com × ActC | 13.86 | 6.93 | 2 | 90 | 16.63 | 7.16 × 10−07 |
| GS × ActC | 5.20 | 2.60 | 2 | 90 | 6.24 | 2.90 × 10−3 |
| Com × GS × ActC | 14.05 | 3.51 | 4 | 90 | 8.43 | 8.04 × 10−6 |
| . | SS . | MS . | NumDF . | DenDF . | F value . | Pr(>F) . |
|---|---|---|---|---|---|---|
| Community | 14.56 | 7.28 | 2 | 90 | 17.46 | 3.90 × 10−07 |
| Growing season | 10.35 | 5.18 | 2 | 90 | 12.42 | 1.73 × 10−05 |
| Activated carbon | 3.23 | 3.23 | 1 | 90 | 7.75 | 6.54 × 10−3 |
| Com × GS | 8.26 | 2.06 | 4 | 90 | 4.95 | 1.18 × 10−3 |
| Com × ActC | 13.86 | 6.93 | 2 | 90 | 16.63 | 7.16 × 10−07 |
| GS × ActC | 5.20 | 2.60 | 2 | 90 | 6.24 | 2.90 × 10−3 |
| Com × GS × ActC | 14.05 | 3.51 | 4 | 90 | 8.43 | 8.04 × 10−6 |
Abbreviations: ActC = activated carbon, Com = community, GS = growing season.
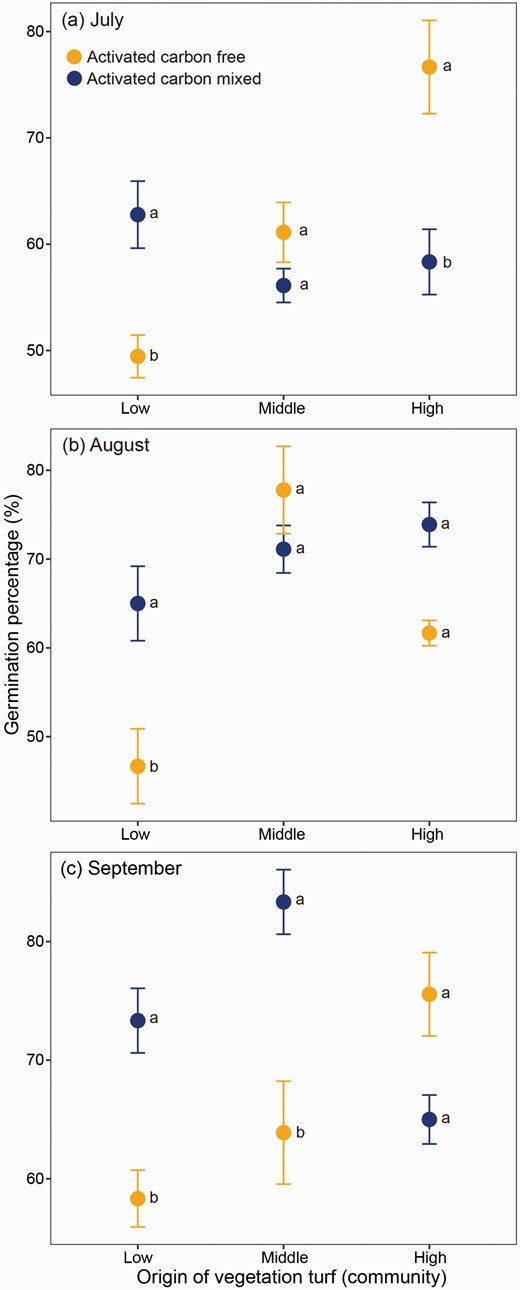
Effect of surrounding vegetation from different communities on the seed germination percentage of cushion Arenaria polytrichoides assessed in the presence or absence of activated carbon. Vegetation samples were collected from communities of low (4380 m), middle (4720 m) and high (4920 m) altitudes during early-growing (a, July), middle-growing (b, August) and late-growing (c, September) seasons.
The presence of activated carbon (ActC) also exerted certain effects on the seedling growth of cushion A. polytrichoides, but these effects were only significantly influenced by the community and the interaction between the community and ActC (Supplementary Table S2). Specifically, when mixed with ActC, vegetation collected from low-elevation communities consistently promoted seedling growth throughout the entire growing season (z = 3.79, P = 0.002 for July; z = 3.71, P = 0.003 for August; and z = 3.83, P = 0.002 for September; Fig. 4). Vegetation collected from middle-elevation community enhanced seedling growth during both early- and late-growing seasons (z = −3.18, P = 0.018 for July; z = −3.18, P = 0.018 for September), while no significant effect was observed during the middle season (z = −1.87, P = 0.421; Fig. 4). However, vegetation in the high-elevation community did not show any significant impact on seedling growth throughout the entire growing season (z = −1.16, P = 0.856 for July; z = −1.69, P = 0.536 for August; and z = −1.15, P = 0.861 for September; Fig. 4).
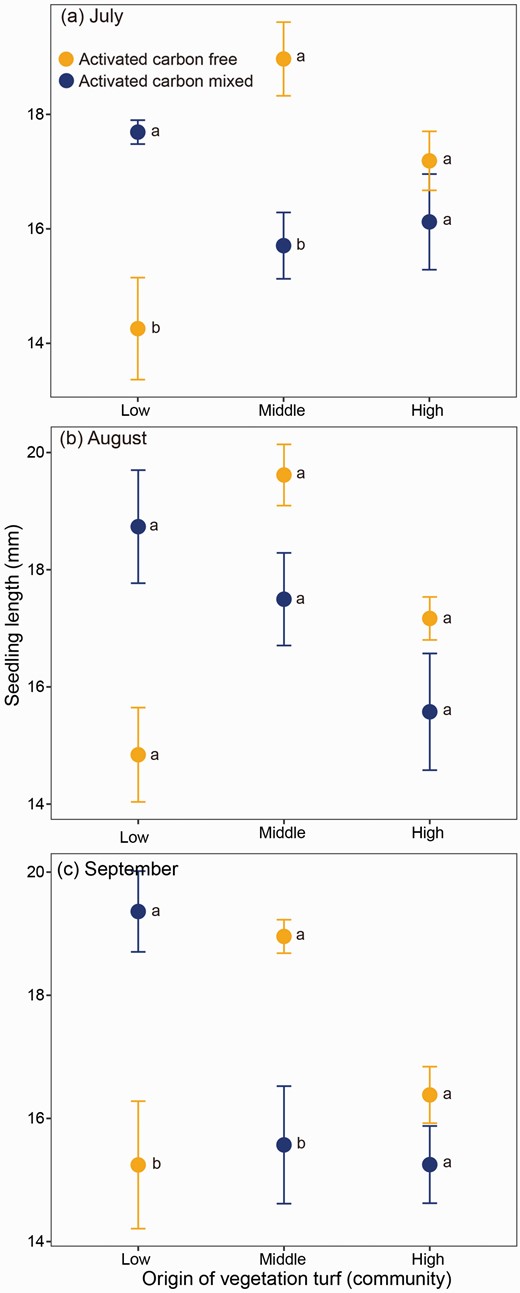
Effect of surrounding vegetation from different communities on the seedling growth of cushion Arenaria polytrichoides assessed in the presence or absence of activated carbon. Vegetation samples were collected from communities of low (4380 m), middle (4720 m) and high (4920 m) altitudes during early-growing (a, July), middle-growing (b, August) and late-growing (c, September) seasons.
DISCUSSION
We observed that the surrounding vegetation in the low-elevation community generally exhibited inhibitory effects on both seed germination and seedling growth of cushion A. polytrichoides. First, extracts significantly reduced the percentage of seed germination and inhibited seedling growth (Figs 1a, b and 2a, b). Second, the addition of activated carbon (ActC), which effectively reduces potential allelochemicals (Kheirabadi et al. 2020; Lyytinen and Lindström 2019), was shown to enhance both the percentage of seed germination and the length of seedlings (Figs 3 and 4). These findings strongly indicate that the surrounding vegetation indeed exerts negative allelopathic potentials on both seed germination and seedling growth of cushion A. polytrichoides.
Previous studies have confirmed that allelopathic effects tend to vary depending on the source (leaves or roots) and concentration of the extract (Deng et al. 2024; Gniazdowska and Bogatek 2005; Koocheki et al. 2013; Zhang et al. 2015). Different species may release distinct allelopathic compounds, resulting in varying allelopathic effects (Li et al. 2021; Xu et al. 2023). For example, Zhang et al. (2015) observed that low concentrations of extracts from Potentilla acaulis (Rosaceae) had a growth-promoting effect, while higher concentrations inhibited seedling growth of neighboring plants. The low-elevation community in this study is a typical alpine meadow with dense vegetation cover (>95%), where the proportion of juveniles or young individuals of cushion A. polytrichoides is significantly lower compared to high-elevation communities, indicating substantial challenges for the successful establishment of A. polytrichoides seedlings in situ (Chen et al. 2020a, 2023). Chen et al. (2020a, 2023) attributed this phenomenon to intense competition from surrounding plants without investigating whether allelopathy-based competition exists or not. Our findings in this study suggest that surrounding vegetation may suppress cushion seedling recruitment by releasing allelopathic compounds into the environment. Moreover, the aboveground vegetation-derived allelochemicals can exert stronger inhibitory effects on seedling recruitment than those released from belowground vegetation (roots).
Interestingly, diverse allelopathic potentials were observed in higher-elevation communities. In the middle-elevation community, extracts released from the surrounding vegetation during early- and mid-growing seasons exhibited no impact on seed germination; however, those released during late-growing season significantly reduced seed germination percentage (Fig. 3). Conversely, extracts from both early- and late-growing seasons significantly promoted seedling growth (Fig. 4). Furthermore, except for certain concentrations of aboveground extracts (Figs 1c and 2c), similar effects on seed germination were observed across all extract types, whereas generally stimulatory effects on seedling growth were evident (Figs 1d and 2d). Vegetation in the high-elevation community had minimal influence on both seed germination and seedling growth, with the exception of aboveground vegetation potentially suppressing seed germination (Figs 3 and 4). These findings are consistent with previous studies (Barto and Cipollini 2009; Deng et al. 2024; Zhang et al. 2015) and highlight the dependence of allelopathic potentials on extract source and concentration. The vegetation coverage decreases progressively along an elevational gradient, from over 95% in the low-elevation community to approximately 40% in the middle-elevation community, and less than 15% in the high-elevation community, indicating reduced interspecific competition along an elevational gradient. In comparison to the findings observed in the low-elevation community, our results from higher-elevation communities suggest that allelopathic compounds released by surrounding vegetation may undergo alterations under increasing environmental stresses. On the one hand, it is imperative for higher-elevation communities with lower vegetation coverage and density to exhibit diminished release of allelopathic compounds within a given area, considering the potential release of allelopathic compounds from specific quantities of vegetation materials. On the other hand, our study sites also demonstrate a decrease in species composition and abundance with increasing elevation (see ‘Study system’ section), which could potentially result in variations in both allelopathic compound types and concentrations. Additionally, declining air and soil temperatures observed at higher elevations (Chen et al. 2019) might suppress plant metabolic processes (Fernandez-Marin et al. 2020), leading to decreased rates of allelochemical release. Consequently, both the composition and concentration of allelochemicals may vary with increasing elevation, thereby influencing their respective allelopathic potentials (Gniazdowska and Bogatek 2005; Inderjit et al. 2011; Koocheki et al. 2013).
Additionally, we also observed that the allelopathic potential of surrounding vegetation on seed germination and/or seedling growth could vary between growing seasons (Figs 3 and 4). In the alpine ecosystems of our study region, most plants have a growing season from late June to mid-September, while there also are numerous plants with diverse phenological attributes. Early flowering plants, such as species from Cruciferae and Primulaceae, typically initiate growth and flowering immediately after snow melt in late May or early June; whereas late flowering plants may commence growth and flowering in late August or even early October, such as species from Gentianaceae. As mentioned earlier, allelochemicals can be released by different species or at different life history stages (Gniazdowska and Bogatek 2005; Koocheki et al. 2013; Zhang et al. 2015). Considering that the species compositions and relevant phenological attributes may differ between growing seasons (Liu et al. 2021), leading to variations in released allelochemicals, it is not surprising that heterogenous allelopathic potentials were found between growing seasons in this study.
Previous studies have indicated that populations of Arenaria at higher elevations exhibit sustained recruitment and expansion, while populations at lower elevations undergo degeneration (Chen et al. 2020a, 2023). The degeneration of cushion-dominant communities/vegetation has been previously observed through field observations (Huang and Wang 1991) and species niche modeling research (Zhao et al. 2011), yet the underlying driving mechanisms remain elusive. In addition to recent findings (Chen et al. 2020a, 2023), this study provides supplementary explanations. Within the low-elevation community, our results confirm that allelopathy-based competition partially inhibits germination and seedling growth. Furthermore, due to its slow growth rate, A. polytrichoides cushions face strong competition from surrounding dense vegetation comprising faster-growing species in terms of resource acquisition and allocation. For example, the presence of shading from surrounding vegetation could potentially lead to an increase in resource allocation toward shoot growth rather than root development in seedlings. Consequently, this may result in higher seedling mortality during unpredictable severe drought events in subsequent life processes (Chen et al. 2020a, 2023). As a consequence, the lower-elevation population of cushion A. polytrichoides is facing recruitment challenges and currently undergoing population degeneration (Chen et al. 2023). In contrast, at higher elevations, allelopathic suppression on seed germination decreases or even disappears; however, certain allelochemicals might promote seedling growth (Figs 1d and 2d). These stimulatory effects on growth are crucial for seedlings to acquire a high overwintering capacity since rapid growth facilitates resource accumulation such as carbohydrates and various nutrients, thereby establishing a robust overwintering ability (Chen et al. 2023; Luscher et al. 2001). Such explanations are plausible considering that prevailing interspecific interactions between cushions and other non-cushion plants commonly transition from competition at lower elevations to facilitation at higher elevations (Chen et al. 2019).
CONCLUSIONS
This study, for the first time, provides evidence suggesting that the surrounding vegetation may exert certain allelopathic potentials on seedling establishment of alpine cushion A. polytrichoides. Generally, allelopathy suppresses seedling establishment in low-elevation communities, partly explaining cushion degeneration in such communities (Chen et al. 2023). However, allelopathy generally does not affect seed germination but may promote seedling growth in high-elevation communities, potentially resulting in higher seedling establishment and consistent population recruitment and expansion. Nonetheless, several limitations should be acknowledged in this study. First, we did not identify the specific allelochemicals responsible for either inhibitory or stimulatory effects or determine the exact plant species releasing these allelochemicals. Considering that sedge species dominate the low-elevation community and exhibit negative allelopathic potentials on cushion plants, it is plausible to hypothesize that these sedge plants are primarily responsible for releasing effective allelochemicals. Second, caution should be exercised when interpreting our results due to potential ambiguity associated with using activated carbon as a means to exclude possible allelopathic compounds; this method may inadvertently alter micro-environmental conditions with relevant media (Lau et al. 2008). Therefore, further studies conducted under field conditions are urgently needed to address these issues.
Supplementary Material
Supplementary material is available at Journal of Plant Ecology online.
Table S1: Effect of potential allelopathic compounds released from surrounding vegetation on the seedling growth of cushion Arenaria polytrichoides.
Table S2: Effect of surrounding vegetation mixed with or without activated carbon on seed germination percentage of cushion Arenaria polytrichoides.
Figure S1: Schematic diagram illustrating the experimental design employed to investigate the effects of surrounding vegetation extracts on seed germination and seedling growth of Arenaria polytrichoides.
Figure S2: Schematic diagram illustrating the experimental design employed to investigate the effects of activated carbon on seed germination and seedling growth in Arenaria polytrichoides.
Funding
This work was supported by the Second Tibetan Plateau Scientific Expedition and Research Program (2019QZKK0502), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA20050203), the Yunnan Applied Basic Research Project (202001AT070060, 202301AS070058) and the Young Academic and Technical Leader Raising Foundation of Yunnan Province (202205AC160053).
Acknowledgements
The authors thank Baima Snow Mountains, Deqen, Diqing, Yunnan, Alpine Subnival Ecosystem Observation and Research Station of Yunnan Province for providing the logistic support. We also thank Ping Wu for her assistance with allelopathic compounds extracting and Lu Sun for his assistance with R codes presentation.
Conflict of interest statement.
The authors declare that they have no conflict of interest.




