-
PDF
- Split View
-
Views
-
Cite
Cite
Axel P. N. Themmen, Anti-Müllerian Hormone: Its Role in Follicular Growth Initiation and Survival and as an Ovarian Reserve Marker, JNCI Monographs, Volume 2005, Issue 34, March 2005, Pages 18–21, https://doi.org/10.1093/jncimonographs/lgi026
Close - Share Icon Share
Abstract
In this paper the role in the ovary of anti-Müllerian hormone (AMH), a member of the transforming growth factor-β family of growth and differentiation factors, is reviewed. AMH has an inhibitory effect on primordial follicle recruitment and may also inhibit follicle-stimulating homone–dependent selection of follicles for dominance. In addition to its functional role in the ovary, AMH in serum is an excellent candidate marker as an indication of the ovarian reserve, not only in infertility clinic patients but also in women during and after cancer treatment.
INTRODUCTION
The ovary is a unique organ in the female body. To serve its two major functions, the production of female sex steroid hormones and the female gametes, cells in the ovary continuously undergo a developmental program in some respects similar to many processes during embryonal development. Another unique aspect is the cessation of ovarian function before the function of other organs is diminished during the aging process, causing both infertility and the physiological processes associated with the absence of female sex steroid hormones, i.e., menopause.
During fetal life, the ovary is populated by germ cells that after migration and proliferation enter the first phase of meiosis but do not finish the process [reviewed by McGee and Hsueh (1)]. These germ cells are enveloped by somatic cells forming so-called primordial follicles at around 20 weeks of gestation, whereas germ cells that fail to be enclosed are lost through apoptosis. As soon as primordial follicles have been formed, some enter the growing follicle pool: the enclosed oocyte increases in size, and the enveloping cells, now called granulosa cells, take a columnar shape and start to proliferate, the so-called recruitment process. This process continues throughout life until the primordial follicle pool is exhausted, culminating in menopause in women. The growing follicles will be lost through atresia, a process that involves apoptosis of the granulosa cells and the oocyte, unless the follicles are rescued by the pituitary gonadotropic hormone follicle-stimulating hormone (FSH). This rescue, also called selection, is possible only after puberty when the pituitary-gonadal endocrine axis has been activated. Those follicles that continue to grow under the control of FSH (multiple in species such as mouse or rat; normally only one in the case of the human), eventually are triggered to release their oocyte for fertilization by the other pituitary gonadotropin, luteinizing hormone (LH). During the ovulation process, the postponed final stages of meiosis in the oocyte are completed.
This process appears to be very “wasteful.” No new primordial follicles appear to be formed after the fetal period, although this classic view of ovarian function has recently been challenged by Johnson et al. (2), who showed, using a combination of cell biological techniques, that in the mouse ovary some neogenesis of primordial follicles may take place. However, it remains unclear to what extent this process may add to the extension of the lifetime of the primordial follicle stock. Of the approximately 7 million germ cells present in the human ovary during the fetal period, only approximately 500 will eventually ovulate and have a chance to be fertilized. Therefore, it is clearly essential that the recruitment and selection of follicles be under stringent control.
There are two major decision points that determine the future of a primordial follicle (Fig. 1): 1) recruitment and the start of follicle growth and of selection and 2) its rescue from atresia and further selection to become preovulatory (1).
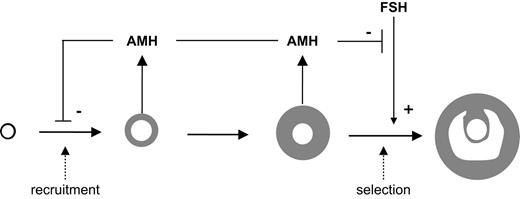
Model of the role of AMH in the ovary. Folliculogenesis is depicted by arrows between primordial, primary, preantral, small antral, and selected follicles, respectively. Recruitment and selection are indicated by the dashed arrows. AMH is produced by the small growing follicles and has an inhibitory effect on follicle recruitment and on FSH-dependent selection.
FACTORS INVOLVED IN RECRUITMENT
The molecular mechanisms that regulate recruitment of the primordial follicles are largely unknown. The rate by which recruitment occurs is dependent on the size of the primordial follicle stock, as shown by the observed exponential decay with age in the number of remaining follicles in the ovary (3). In addition, several growth factors have been identified that are involved in the initiation of growth or its inhibition. Thus, stem cell factor, a growth factor of great importance during migration and proliferation of the primordial germ cells (4), has also been found to stimulate primordial follicle growth, as have other growth factors such as leukemia inhibitory factor (LIF), insulin, and basic fibroblast growth factor (bFGF) (5–8). Growth factors of the transforming growth factor-β (TGF-β) family are also involved. Bone morphogenetic factor-4 (BMP4) stimulates the primordial follicle survival and the primordial-to-primary follicle transition in in vitro culture (6). In addition, absence of GDF9, an oocyte-specific TGF-β family member, results in a block of folliculogenesis at the primary follicle stage (9), although GDF9 does not appear to be involved in the actual primordial follicle recruitment itself (7).
ANTI-MÜLLERIAN HORMONE
Recently, another member of the TGF-β family, anti-Müllerian hormone (AMH), has been found to have an important role in the regulation of the recruitment process. AMH was originally identified as the fetal testis factor that signals the regression of the müllerian ducts in the male fetus. However, AMH is also expressed in the ovary (Fig. 2). All growing follicles up to the antral stage in the mouse (10) or to a size of approximately 6 mm in the human (10,11) express AMH. Indeed, the first columnar cells in a transitional primordial follicle are also positive for AMH immunoreactivity. This pattern of AMH protein expression indicates that AMH may have a special role to play in the regulation of follicle growth: it is expressed immediately after recruitment and ceases in the follicles that have been selected for ovulation. To investigate the possible role of AMH in follicle recruitment, the follicle dynamics in AMHnull(12) mice were determined. In ovaries of 4-month-old AMHnull mice, almost threefold more growing follicles were found, accompanied by a decrease in the number of primordial follicles [Fig. 3; (10)]. The increased number of growing follicles was already seen at 25 days, before the start of estrous cycling in the mice, and the increased recruitment caused a premature exhaustion of the primordial follicle pool in 13-month-old AMHnull mice (Fig. 3). These results indicated that AMH might have an inhibitory effect on follicle recruitment. Indeed, AMH inhibits primordial follicle recruitment in in vitro cultures of neonatal ovaries (13). Thus, AMH produced by the small growing follicles exerts a paracrine effect on the primordial follicle, inhibiting their recruitment into the growing follicle pool. As indicated in Fig. 1, AMH also attenuates the effects of FSH on growing follicles. In granulosa cell cultures, AMH inhibits the FSH-dependent induction of aromatase activity and LH receptor expression (14). Using follicles from 14-day-old mice, AMH was shown to inhibit FSH-dependent follicle growth (15). Furthermore, FSH treatment of AMHnull mice resulted in more and further developed ovarian follicles (15).
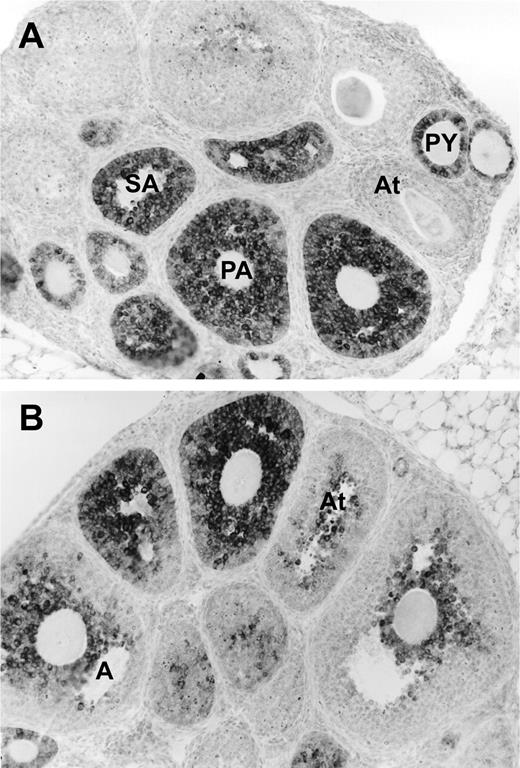
AMH expression in mouse ovaries. AMH was detected using a monoclonal antibody that recognizes rat, mouse, and human AMH (11). A) AMH is present in primary (PY), preantral (PA), and small antral (SA) follicles. B) AMH decreases in antral follicles (A) and is absent in large follicles and atretic follicles (At).
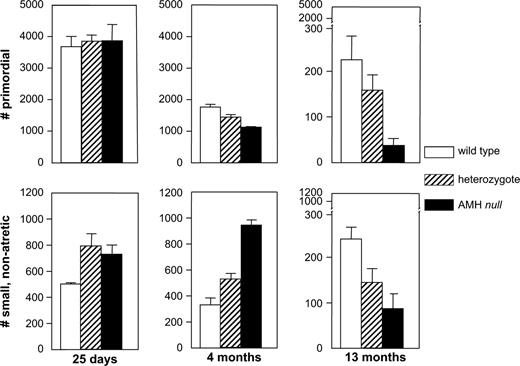
Follicle dynamics in wild-type, heterozygous, and AMHnull mouse ovaries. In the top row, the numbers of primordial follicles is given, i.e,. the size of the remaining pool. In the bottom row, the number of small, nonatretic follicles is depicted, representing the growing pool of follicles. Figure based on data taken from Durlinger et al. (10).
Thus, AMH is one of the factors that indicate the size of the growing follicle pool. It controls the size of the pool by inhibiting both its growth (recruitment) and its decline (selection).
ANTI-MÜLLERIAN HORMONE AS A MARKER OF FERTILITY
The role of AMH as an intraovarian signal for the size of the growing follicle pool may have an important benefit for the clinic. One of the questions asked in fertility clinics is how to determine the size of the ovarian reserve, which represents both the quantity and quality of the ovarian follicle pool. Decline of ovarian reserve is called ovarian aging. Besides aspects such as oocyte quality, the number of primordial follicles that are left in the ovary is also an important parameter for ovarian reserve (16). However, the size of the primordial follicle stock in women is difficult to measure directly, although it appears that the number of growing follicles is correlated to the size of primordial follicle stock from which they are recruited (17,18). Therefore, a marker that reflects all follicles that have made the transition from the primordial follicle pool to the growing pool may be a good indirect marker of the quantitative aspects of the ovarian reserve. Various techniques have been used to determine the ovarian reserve. Antral follicle count (AFC) using vaginal ultrasonography gives an indication of the number of growing follicles and therefore, by extension, of the size of the primordial follicle pool. FSH and inhibin B levels have also been proposed to serve as predictors of the ovarian reserve. There are strong indications that AMH may serve as a good candidate marker for the determination of the ovarian reserve. AMH is also expressed in human follicles immediately after recruitment right up to the selection stage (4- to 6-mm diameter) (11). Levels in serum of AMH in normal cycling women decline with age and become undetectable in menopausal women (19). In addition, AMH in serum showed an excellent correlation with AFC (r = 0.66/0.71; n = 41) (19).
The usefulness of AMH levels in serum as a measure of ovarian reserve was tested further in women undergoing in vitro fertilization (IVF) treatment. Hormonal parameters and AFC using transvaginal ultrasonography were determined on the 3rd day of the menstrual cycle in 119 IVF patients, not more than 3 months preceding IVF treatment (20). The measured parameters were analyzed after division of the patients into two groups on the basis of the number of oocytes retrieved after IVF treatment: normal responders (four or more retrieved oocytes) and poor responders (fewer than four retrieved oocytes and cancellations of IVF treatment). Ovaries of normal-responding women contained significantly more growing antral follicles than did ovaries of women with a poor response to IVF treatment. In addition, levels in serum of AMH in these poor-responding women were lower than in normal-responding women (Table 1). Levels of AMH in serum strongly correlated with the AFH, the number of follicles retrieved, age, inhibin B, and FSH. In addition, logistic regression analysis in predictive models of poor or normal response showed that both AFH and AMH levels in serum were equally important for prediction, since the calculated area under the receiver operating characteristic (ROC) curve (ROCAUC) was 0.86 and 0.85, respectively (20). The ROCAUC determines the sensitivity of the diagnostic test and may vary between 0.5 (no discriminative power) and 1.0 (perfect discrimination). These data support the observations made in another study where also a correlation was found between AMH levels in serum and retrieved oocytes in women undergoing IVF treatment (21)
Results of ovarian reserve test in IVF-treated patients, divided in normal and poor responders
| . | Total (n = 119) . | Normal responders (n = 84) . | Poor responders (n = 35) . | P . |
|---|---|---|---|---|
| Age (years) | 33.8 (22.3–44.0) | 33.8 (24.4–44.0) | 36.3 (22.3–43.3) | .09 |
| No. of oocytes (n = 96)* | 7 (1–28) | 9 (4–28) | 2 (1–3) | Not applicable |
| Antral follicles (n) | 8 (0–35) | 11 (0–35) | 4 (0–15) | .001 |
| AMH (μg/L) | 0.9 (0.0–6.2) | 1.4 (0.0–6.2) | 0.2 (0.0–1.7) | .001 |
| . | Total (n = 119) . | Normal responders (n = 84) . | Poor responders (n = 35) . | P . |
|---|---|---|---|---|
| Age (years) | 33.8 (22.3–44.0) | 33.8 (24.4–44.0) | 36.3 (22.3–43.3) | .09 |
| No. of oocytes (n = 96)* | 7 (1–28) | 9 (4–28) | 2 (1–3) | Not applicable |
| Antral follicles (n) | 8 (0–35) | 11 (0–35) | 4 (0–15) | .001 |
| AMH (μg/L) | 0.9 (0.0–6.2) | 1.4 (0.0–6.2) | 0.2 (0.0–1.7) | .001 |
Number of patients is 77 in the normal responder group (seven cancellations due to high response) and 19 in the poor responder group (including 16 cancellations). Data taken and adapted from van Rooij et al. (20).
Results of ovarian reserve test in IVF-treated patients, divided in normal and poor responders
| . | Total (n = 119) . | Normal responders (n = 84) . | Poor responders (n = 35) . | P . |
|---|---|---|---|---|
| Age (years) | 33.8 (22.3–44.0) | 33.8 (24.4–44.0) | 36.3 (22.3–43.3) | .09 |
| No. of oocytes (n = 96)* | 7 (1–28) | 9 (4–28) | 2 (1–3) | Not applicable |
| Antral follicles (n) | 8 (0–35) | 11 (0–35) | 4 (0–15) | .001 |
| AMH (μg/L) | 0.9 (0.0–6.2) | 1.4 (0.0–6.2) | 0.2 (0.0–1.7) | .001 |
| . | Total (n = 119) . | Normal responders (n = 84) . | Poor responders (n = 35) . | P . |
|---|---|---|---|---|
| Age (years) | 33.8 (22.3–44.0) | 33.8 (24.4–44.0) | 36.3 (22.3–43.3) | .09 |
| No. of oocytes (n = 96)* | 7 (1–28) | 9 (4–28) | 2 (1–3) | Not applicable |
| Antral follicles (n) | 8 (0–35) | 11 (0–35) | 4 (0–15) | .001 |
| AMH (μg/L) | 0.9 (0.0–6.2) | 1.4 (0.0–6.2) | 0.2 (0.0–1.7) | .001 |
Number of patients is 77 in the normal responder group (seven cancellations due to high response) and 19 in the poor responder group (including 16 cancellations). Data taken and adapted from van Rooij et al. (20).
Recently, the use of AMH as a marker of ovarian reserve was further investigated in young women after treatment for cancer in childhood (22). In this study, it was shown that young cancer survivors suffered a partial loss of the ovarian reserve, which was accompanied by an decrease in AMH in serum, increase in FSH in serum, and a decrease in ovarian volume but not of AFC. These results support further investigation of AMH as a marker of ovarian reserve, also in (young) women undergoing cancer therapy.
References
McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles.
Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary.
Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause.
Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis.
Nilsson EE, Skinner MK. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition.
Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development.
Nilsson EE, Skinner MK. Growth and differentiation factor-9 stimulates progression of early primary but not primordial rat ovarian follicle development.
Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries.
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis.
Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary.
Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment.
Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development.
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary.
di Clemente N, Goxe B, Rémy JJ, Cate RL, Josso N, Vigier B, et al. Inhibitory effect of AMH upon aromatase activity and LH receptors of granulosa cells of rat and porcine immature ovaries.
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary.
te Velde ER, Pearson PL. The variability of female reproductive ageing.
Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility.
Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses.
de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging.
van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve.
Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles.


