-
PDF
- Split View
-
Views
-
Cite
Cite
Annelie Johansson, Huma Dar, Anna Nordenskjöld, Gizeh Perez-Tenorio, Nicholas P Tobin, Christina Yau, Christopher C Benz, Laura J Esserman, Laura J van ‘t Veer, Bo Nordenskjöld, Olle Stål, Tommy Fornander, Linda S Lindström, Differential long-term tamoxifen therapy benefit by menopausal status in breast cancer patients: secondary analysis of a controlled randomized clinical trial, JNCI: Journal of the National Cancer Institute, Volume 117, Issue 5, May 2025, Pages 868–878, https://doi.org/10.1093/jnci/djae268
Close - Share Icon Share
Abstract
Estrogen receptor–positive breast cancer patients have a long-term risk of distant metastatic disease, and premenopausal patients have a higher risk. Randomized studies with long-term follow-up are essential to understand treatment benefit. We elucidated the long-term tamoxifen therapy benefit by menopausal status in the Stockholm tamoxifen trials with 20 years complete follow-up.
Secondary analysis of 1242 estrogen receptor–positive and HER2-negative patients that were randomly assigned to 2-5 years of 40 mg adjuvant tamoxifen or no endocrine therapy. Distant recurrence-free interval in tamoxifen-treated vs endocrine untreated patients was assessed by Kaplan–Meier, Cox proportional hazards regression, and time-varying analyses.
In premenopausal patients, a statistically significant tamoxifen benefit was observed for lymph node–negative (adjusted hazard ratio [HR] = 0.46, 95% confidence interval [CI] = 0.24 to 0.87), progesterone receptor–positive (adjusted HR = 0.61, 95% CI = 0.41 to 0.91), and genomic low-risk tumors (adjusted HR = 0.47, 95% CI = 0.26 to 0.85) but only lasted beyond 10 years for genomic low-risk tumors. Postmenopausal patients showed long-term benefit for all good-prognosis markers including low-grade (adjusted HR = 0.55, 95% CI = 0.41 to 0.73), lymph node–negative (adjusted HR = 0.44, 95% CI = 0.30 to 0.64), progesterone receptor–positive (adjusted HR = 0.60, 95% CI = 0.44 to 0.80), Ki-67 low (adjusted HR = 0.51, 95% CI = 0.38 to 0.68), and genomic low-risk tumors (adjusted HR = 0.53, 95% CI = 0.37 to 0.74), and regardless of tumor size (≤20 mm: adjusted HR = 0.55, 95% CI = 0.39 to 0.77; >20 mm: adjusted HR = 0.64, 95% CI = 0.44 to 0.94). Premenopausal patients with no poor-prognosis tumor characteristics (clinical marker score = 0) showed early benefit and postmenopausal long-term benefit.
Our study suggests differential tamoxifen benefit by menopausal status. Improved long-term endocrine therapy prediction in premenopausal patients is needed and could involve molecular markers because standard tumor characteristics cannot predict benefit beyond 10 years.
Introduction
Breast cancer is the most common female cancer worldwide and the leading cause of cancer death in women.1 Patients diagnosed with estrogen receptor–positive breast cancer have a steady and long-term risk of fatal disease with time to distant metastasis ranging from months to decades after primary diagnosis.2-7 The risk of breast cancer increases with age; however, approximately 30% of breast cancers are diagnosed in premenopausal women.8 Moreover, premenopausal breast cancer patients have a higher risk of dying from their disease compared with postmenopausal patients.9-11 Although aggressive tumor characteristics are enriched in younger breast cancer patients, age itself is an independent risk factor.12-14 This has led to the proposal of a biologically distinct phenotype in young breast cancer patients, but the complex biology behind the poor prognosis is largely unknown.15-18 Premenopausal patients have a long life expectancy and concerns related to their risk of recurrence for several decades after primary diagnosis. It is thus important to clearly establish the long-term treatment benefit in this patient group. Long-term endocrine therapy benefit prediction remains a challenge in breast cancer, given the large heterogeneity in the metastatic capacity and time to disease recurrence. For this, clinical randomized trials are essential, but as of now, there is a general lack of trials with long-term follow-up.
Tamoxifen is widely used as endocrine therapy for estrogen receptor–positive breast cancer and reduces the risk of distant metastatic disease in premenopausal and postmenopausal patients.6,19-23 The choice of endocrine therapies in European and American treatment guidelines are based on the patient’s clinical risk estimated from patient’s age and clinically used tumor characteristics including tumor size, histological tumor grade, lymph node status, progesterone receptor status, HER2 status, and Ki-67 levels, in addition to patient’s tolerability and treatment side effects.19-21,24 Indeed, in the perspective of 5-10 years after primary diagnosis, these tumor characteristics are prognostic3,25,26 and used to predict the benefit from additional chemotherapy,19,21 but whether they can predict benefit from endocrine therapy is debated, especially in the long-term perspective beyond 10 years from primary diagnosis.6
The aim of this study was to elucidate differences in the long-term benefit from tamoxifen therapy in premenopausal and postmenopausal estrogen receptor–positive and HER2-negative breast cancer patients by recently annotated clinically used tumor characteristics, a genomic risk signature (the 70-gene signature27), and by a summary clinical marker score in the Stockholm tamoxifen (STO) trials with 20 years complete follow-up. The STO trials enable the unique comparison to a control group of patients randomly assigned to no endocrine therapy and enrolled patients in a population-based manner from the same geographic area, which provided detailed information on menopausal status. To our knowledge, this is the first study comparing the long-term tamoxifen therapy benefit by clearly defined menopausal status rather than using age as a proxy, which better reflects the patient’s hormonal milieu.
Methods
Patients
The STO trials (STO-2, STO-3, STO-5) enrolled 3930 women diagnosed with invasive breast cancer of clinical low and high risk in a population-based manner from 1976 to 1997 (see CONSORT diagram in Figure 1).4,7,28-32 Premenopausal status was defined as having had a menstrual cycle in the last 6 months, and likewise, postmenopausal status was defined as not having had a menstrual cycle in the last 6 months. All postmenopausal patients were randomly assigned to at least 2 years of adjuvant tamoxifen (40 mg orally daily) vs no endocrine therapy. Premenopausal patients in STO-5 were randomly assigned to 2 years of adjuvant tamoxifen (40 mg orally daily) and/or goserelin (3.6 mg subcutaneously every 28 days) vs no endocrine therapy.31,32 Patients included in the goserelin-only random assignment arm (n = 130) were excluded given that a previous study showed differential long-term treatment benefit from tamoxifen and goserelin.30 Furthermore, premenopausal patients in STO-2 were not randomly assigned to tamoxifen vs no endocrine therapy and were thus excluded from analysis (n = 251). In addition to the random assignment to tamoxifen therapy vs no endocrine therapy, postmenopausal patients in the STO-2 trial were randomly assigned to adjuvant chemotherapy vs postoperative locoregional radiotherapy. Also, premenopausal patients in STO-5 with lymph node–positive status were allocated to adjuvant chemotherapy, and patients with 4 or more positive lymph nodes to adjuvant chemotherapy and postoperative locoregional radiotherapy. Further details are provided in Supplementary Methods. A total of 1242 estrogen receptor–positive and HER2-negative patients were included in this study: 381 premenopausal and 861 postmenopausal.

CONSORT diagram of patients from the Stockholm tamoxifen (STO) trials included in this study. aPremenopausal patients in STO-5 were randomly assigned to tamoxifen only or tamoxifen and goserelin in combination. bPremenopausal patients in STO-2 not randomly assigned to tamoxifen vs no endocrine therapy were excluded. cPremenopausal patients in STO-5 randomly assigned to goserelin only were excluded. FFPE = formalin-fixed paraffin-embedded; ER = estrogen receptor; pN = number of positive lymph nodes; pT = tumor size.
Twenty-years follow-up until December 31, 2016, was available for all patients from Swedish high-quality national and regional registries of high validity and essentially complete coverage.33-36 Informed consent was obtained before random assignment, and the trial was approved by the Karolinska Institutet regional ethics committee with the Stockholm Regional Cancer Center as the trial center. The STO trials were approved and initiated before the practice of trial registration in Sweden; thus, the registration number is unavailable.
Tumor size and tumor grade
Tumor size was measured as the diameter according to clinical guidelines, and tumor grading was performed according to the Nottingham Histologic Score system (Elston grade).37
Immunohistochemistry
Immunohistochemical analysis of estrogen receptor, progesterone receptor, HER2, and Ki-67 were reannotated in 2014 (STO-3) and 2020 (STO-2 and STO-5) and followed standard recommended clinical protocols (details in Supplementary Methods). A total of 2250 primary formalin-fixed paraffin-embedded tumor blocks with sufficient invasive tumor cells were collected and analyzed (see Figure 1).7,38,39 The percentage of cancer cells positive for estrogen receptor, progesterone receptor, HER2, and Ki-67 was scored by experienced breast cancer pathologists. According to the Swedish National Guidelines,40 estrogen receptor positivity and progesterone receptor positivity was defined by a threshold of 10% or greater, HER2 positivity was defined as intensity of 3 or more by immunohistochemistry, and Ki-67 was measured in the whole stained tumor slide and categorized as low (<15%) and medium to high (≥15%).
Genomic risk
Tumors were classified as being of genomic low risk or high risk using the 70-gene signature molecular risk prediction tool (MammaPrint, Agendia Inc, Irvine, CA, USA) on RNA extracted from the primary formalin-fixed paraffin-embedded tumor blocks, as described previously.27,39,41
Clinical marker score
A clinical marker score was defined as the number of aggressive characteristics associated with poor prognosis, namely, large tumor size, tumor grade 3, lymph node–positive, progesterone receptor–negative status, and Ki-67 medium to high. Patients with a score of zero have none of these characteristics, whereas patients with a score of 5 have all. Clinical marker score was categorized as 0, 1, 2, and 3 or higher.
Statistical analyses
Tamoxifen therapy benefit was assessed by univariate Kaplan–Meier analysis (log-rank test), multivariable Cox proportional hazards regression, and multivariable time-varying analysis using flexible parametric survival (details in Supplementary Methods).42 All tumor characteristics were divided into 2 categories separated by characteristics generally associated with good or poor prognosis (ie, small or large tumor size, tumor grade 1-2 or 3, lymph node negative or positive, progesterone receptor positive or negative, Ki-67 low or medium to high, genomic low or high risk). All analyses were based on intention to treat, and the endpoint was distant recurrence-free interval (DRFI),43,44 including distant metastasis or fatal breast cancer (in patients with missing date of distant metastasis, n = 22) as event. Multivariable analyses were adjusted for age, random assignment year, tumor size, tumor grade, lymph node status, progesterone receptor status, Ki-67 status, chemotherapy, radiotherapy, and type of surgery (breast-conserving surgery or mastectomy).
Analyses were done in R version 3.5.2 and SAS version 9.4. All statistical tests were 2-sided, and a P value less than .05 was considered statistically significant.
Results
Patients
A total of 1242 (381 premenopausal patients and 861 postmenopausal) patients with estrogen receptor–positive and HER2-negative tumors from the STO trials were included in this study (see Figure 1). The average age was 46.6 years (range = 26-55 years) for premenopausal and 61.2 years (range = 45-73 years) for postmenopausal patients. Premenopausal patients were more likely to have lymph node–positive disease (51.4% vs 30.9%) and progesterone receptor–positive tumors (90.6% vs 71.1%) (see Table 1). No statistically significant difference was seen by tumor size, tumor grade, Ki-67 status, or genomic risk.
Patient and primary tumor characteristics of estrogen receptor–positive and HER2-negative breast cancer patients in the Stockholm tamoxifen trials
| Patient and primary tumor characteristics . | Premenopausal patients (n = 381) . | Postmenopausal patients (n = 861) . | Pa . |
|---|---|---|---|
| Age, No. (%), y | — | ||
| 45 and younger | 132 (34.6) | 2 (0.2) | |
| 46-50 | 175 (45.9) | 17 (2.0) | |
| 51-55 | 74 (19.4) | 126 (14.6) | |
| 56-60 | 0 (0.0) | 242 (28.1) | |
| 61-65 | 0 (0.0) | 249 (28.9) | |
| Older than 65 | 0 (0.0) | 225 (26.1) | |
| Tumor size, No. (%) | .786 | ||
| pT ≤ 20 mm | 264 (70.0) | 604 (70.9) | |
| pT > 20 mm | 113 (30.0) | 248 (29.1) | |
| Unknown | 4 | 9 | |
| Tumor grade, No. (%) | .269 | ||
| 1 | 62 (16.3) | 163 (19.2) | |
| 2 | 245 (64.5) | 550 (64.7) | |
| 3 | 73 (19.2) | 137 (16.1) | |
| Unknown | 1 | 11 | |
| Lymph node status, No. (%) | <.001 | ||
| Negative | 185 (48.6) | 595 (69.1) | |
| Positive | 196 (51.4) | 266 (30.9) | |
| Unknown | 0 | 0 | |
| Progesterone receptor status, No. (%) | <.001 | ||
| Positive | 345 (90.6) | 607 (71.1) | |
| Negative | 36 (9.4) | 247 (28.9) | |
| Unknown | 0 | 7 | |
| Ki-67 status, No. (%) | .466 | ||
| Low <15% | 281 (74.7) | 636 (76.8) | |
| Medium to high ≥15% | 95 (25.3) | 192 (23.2) | |
| Unknown | 5 | 33 | |
| Genomic risk, No. (%) | .183 | ||
| Low | 213 (72.0) | 509 (67.5) | |
| High | 83 (28.0) | 245 (32.5) | |
| Unknown | 85 | 107 | |
| Clinical marker score No. (%) | .716 | ||
| 0 | 103 (27.0) | 233 (27.1) | |
| 1 | 123 (32.3) | 301 (35.0) | |
| 2 | 95 (24.9) | 192 (22.3) | |
| ≥3 | 60 (15.7) | 135 (15.7) | |
| Unknown | 0 | 0 |
| Patient and primary tumor characteristics . | Premenopausal patients (n = 381) . | Postmenopausal patients (n = 861) . | Pa . |
|---|---|---|---|
| Age, No. (%), y | — | ||
| 45 and younger | 132 (34.6) | 2 (0.2) | |
| 46-50 | 175 (45.9) | 17 (2.0) | |
| 51-55 | 74 (19.4) | 126 (14.6) | |
| 56-60 | 0 (0.0) | 242 (28.1) | |
| 61-65 | 0 (0.0) | 249 (28.9) | |
| Older than 65 | 0 (0.0) | 225 (26.1) | |
| Tumor size, No. (%) | .786 | ||
| pT ≤ 20 mm | 264 (70.0) | 604 (70.9) | |
| pT > 20 mm | 113 (30.0) | 248 (29.1) | |
| Unknown | 4 | 9 | |
| Tumor grade, No. (%) | .269 | ||
| 1 | 62 (16.3) | 163 (19.2) | |
| 2 | 245 (64.5) | 550 (64.7) | |
| 3 | 73 (19.2) | 137 (16.1) | |
| Unknown | 1 | 11 | |
| Lymph node status, No. (%) | <.001 | ||
| Negative | 185 (48.6) | 595 (69.1) | |
| Positive | 196 (51.4) | 266 (30.9) | |
| Unknown | 0 | 0 | |
| Progesterone receptor status, No. (%) | <.001 | ||
| Positive | 345 (90.6) | 607 (71.1) | |
| Negative | 36 (9.4) | 247 (28.9) | |
| Unknown | 0 | 7 | |
| Ki-67 status, No. (%) | .466 | ||
| Low <15% | 281 (74.7) | 636 (76.8) | |
| Medium to high ≥15% | 95 (25.3) | 192 (23.2) | |
| Unknown | 5 | 33 | |
| Genomic risk, No. (%) | .183 | ||
| Low | 213 (72.0) | 509 (67.5) | |
| High | 83 (28.0) | 245 (32.5) | |
| Unknown | 85 | 107 | |
| Clinical marker score No. (%) | .716 | ||
| 0 | 103 (27.0) | 233 (27.1) | |
| 1 | 123 (32.3) | 301 (35.0) | |
| 2 | 95 (24.9) | 192 (22.3) | |
| ≥3 | 60 (15.7) | 135 (15.7) | |
| Unknown | 0 | 0 |
P value calculated by Fisher exact test.
Patient and primary tumor characteristics of estrogen receptor–positive and HER2-negative breast cancer patients in the Stockholm tamoxifen trials
| Patient and primary tumor characteristics . | Premenopausal patients (n = 381) . | Postmenopausal patients (n = 861) . | Pa . |
|---|---|---|---|
| Age, No. (%), y | — | ||
| 45 and younger | 132 (34.6) | 2 (0.2) | |
| 46-50 | 175 (45.9) | 17 (2.0) | |
| 51-55 | 74 (19.4) | 126 (14.6) | |
| 56-60 | 0 (0.0) | 242 (28.1) | |
| 61-65 | 0 (0.0) | 249 (28.9) | |
| Older than 65 | 0 (0.0) | 225 (26.1) | |
| Tumor size, No. (%) | .786 | ||
| pT ≤ 20 mm | 264 (70.0) | 604 (70.9) | |
| pT > 20 mm | 113 (30.0) | 248 (29.1) | |
| Unknown | 4 | 9 | |
| Tumor grade, No. (%) | .269 | ||
| 1 | 62 (16.3) | 163 (19.2) | |
| 2 | 245 (64.5) | 550 (64.7) | |
| 3 | 73 (19.2) | 137 (16.1) | |
| Unknown | 1 | 11 | |
| Lymph node status, No. (%) | <.001 | ||
| Negative | 185 (48.6) | 595 (69.1) | |
| Positive | 196 (51.4) | 266 (30.9) | |
| Unknown | 0 | 0 | |
| Progesterone receptor status, No. (%) | <.001 | ||
| Positive | 345 (90.6) | 607 (71.1) | |
| Negative | 36 (9.4) | 247 (28.9) | |
| Unknown | 0 | 7 | |
| Ki-67 status, No. (%) | .466 | ||
| Low <15% | 281 (74.7) | 636 (76.8) | |
| Medium to high ≥15% | 95 (25.3) | 192 (23.2) | |
| Unknown | 5 | 33 | |
| Genomic risk, No. (%) | .183 | ||
| Low | 213 (72.0) | 509 (67.5) | |
| High | 83 (28.0) | 245 (32.5) | |
| Unknown | 85 | 107 | |
| Clinical marker score No. (%) | .716 | ||
| 0 | 103 (27.0) | 233 (27.1) | |
| 1 | 123 (32.3) | 301 (35.0) | |
| 2 | 95 (24.9) | 192 (22.3) | |
| ≥3 | 60 (15.7) | 135 (15.7) | |
| Unknown | 0 | 0 |
| Patient and primary tumor characteristics . | Premenopausal patients (n = 381) . | Postmenopausal patients (n = 861) . | Pa . |
|---|---|---|---|
| Age, No. (%), y | — | ||
| 45 and younger | 132 (34.6) | 2 (0.2) | |
| 46-50 | 175 (45.9) | 17 (2.0) | |
| 51-55 | 74 (19.4) | 126 (14.6) | |
| 56-60 | 0 (0.0) | 242 (28.1) | |
| 61-65 | 0 (0.0) | 249 (28.9) | |
| Older than 65 | 0 (0.0) | 225 (26.1) | |
| Tumor size, No. (%) | .786 | ||
| pT ≤ 20 mm | 264 (70.0) | 604 (70.9) | |
| pT > 20 mm | 113 (30.0) | 248 (29.1) | |
| Unknown | 4 | 9 | |
| Tumor grade, No. (%) | .269 | ||
| 1 | 62 (16.3) | 163 (19.2) | |
| 2 | 245 (64.5) | 550 (64.7) | |
| 3 | 73 (19.2) | 137 (16.1) | |
| Unknown | 1 | 11 | |
| Lymph node status, No. (%) | <.001 | ||
| Negative | 185 (48.6) | 595 (69.1) | |
| Positive | 196 (51.4) | 266 (30.9) | |
| Unknown | 0 | 0 | |
| Progesterone receptor status, No. (%) | <.001 | ||
| Positive | 345 (90.6) | 607 (71.1) | |
| Negative | 36 (9.4) | 247 (28.9) | |
| Unknown | 0 | 7 | |
| Ki-67 status, No. (%) | .466 | ||
| Low <15% | 281 (74.7) | 636 (76.8) | |
| Medium to high ≥15% | 95 (25.3) | 192 (23.2) | |
| Unknown | 5 | 33 | |
| Genomic risk, No. (%) | .183 | ||
| Low | 213 (72.0) | 509 (67.5) | |
| High | 83 (28.0) | 245 (32.5) | |
| Unknown | 85 | 107 | |
| Clinical marker score No. (%) | .716 | ||
| 0 | 103 (27.0) | 233 (27.1) | |
| 1 | 123 (32.3) | 301 (35.0) | |
| 2 | 95 (24.9) | 192 (22.3) | |
| ≥3 | 60 (15.7) | 135 (15.7) | |
| Unknown | 0 | 0 |
P value calculated by Fisher exact test.
Twenty-year tamoxifen therapy benefit in premenopausal and postmenopausal patients
For all patients in the STO trials, a statistically significantly improved DRFI from tamoxifen therapy vs control was seen in univariate Kaplan–Meier analysis (log-rank P ≤ .001) and multivariable Cox proportional hazard regression analysis (adjusted hazard ratio [HR] = 0.66, 95% confidence interval [CI] = 0.54 to 0.81) (see Figure S1A). Stratification by menopausal status showed no statistically significant difference in DRFI over the 20 years of follow-up in premenopausal patients, whereas a statistically significantly improved DRFI from tamoxifen therapy vs control was seen in postmenopausal patients (log-rank P < .001; adjusted HR = 0.59, 95% CI = 0.46 to 0.76; see Figure S1, B and C).
Furthermore, a multivariable time-varying analysis was performed to assess how the tamoxifen therapy benefit varies over the 20 years of follow-up, contrasting tamoxifen-treated patients with endocrine untreated patients (see Figure S2). For all patients, a statistically significant long-lasting tamoxifen therapy benefit was observed up to at least 20 years (adjusted HR = 0.61, 95% CI = 0.41 to 0.90 at year 20). Stratification by menopausal status showed no significant tamoxifen therapy benefit in premenopausal patients over the 20 years of follow-up, whereas a long-lasting benefit was observed in postmenopausal patients up to at least 20 years (adjusted HR = 0.48, 95% CI = 0.29 to 0.78 at year 20). The risk of distant metastatic disease was highest the first 10 years for premenopausal and postmenopausal patients and remained elevated throughout the 20 years of follow-up (see Figure S3). There was a clear difference in the risk between postmenopausal patients in the tamoxifen-treated and control arm, but this was not seen in premenopausal patients.
In summary, a differential tamoxifen therapy benefit in premenopausal and postmenopausal patients was observed, with a significant long-term benefit in postmenopausal patients only.
Twenty-year tamoxifen therapy benefit by clinically used tumor characteristics in premenopausal and postmenopausal patients
Further analyses assessed the association between clinically used tumor characteristics and long-term tamoxifen therapy benefit in premenopausal and postmenopausal patients. In premenopausal patients, a statistically significantly improved DRFI from tamoxifen therapy vs control was seen for lymph node–negative disease in univariate Kaplan–Meier analysis (log-rank P = .033; Figure S4). Furthermore, multivariable Cox proportional hazards regression analysis showed that premenopausal patients treated with tamoxifen therapy had a statistically significantly improved DRFI compared with control for lymph node–negative (adjusted HR = 0.46, 95% CI = 0.24 to 0.87), progesterone receptor–positive (adjusted HR = 0.61, 95% CI = 0.41 to 0.91), and genomic low-risk tumors (adjusted HR = 0.47, 95% CI = 0.26 to 0.85; Figure 2). No statistically significant difference in DRFI was observed by any of the other tumor characteristics.
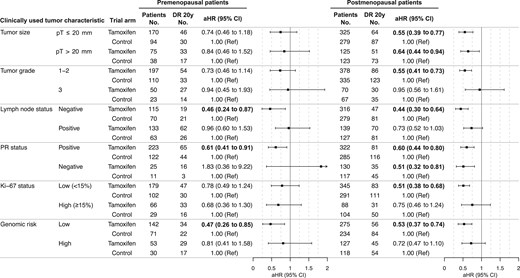
Twenty-year tamoxifen therapy benefit by clinically used tumor characteristics. Multivariable Cox proportional hazards regression analysis of the 20-year distant recurrence-free interval for premenopausal and postmenopausal breast cancer patients with estrogen receptor–positive and HER2-negative tumors, comparing patients randomly assigned to tamoxifen vs no endocrine therapy (control). Adjusted for age, random assignment year, tumor size, tumor grade, lymph node status, progesterone receptor status, Ki-67 status, type of surgery, chemotherapy, and radiotherapy. Bold indicates a significant P value less than .05. aHR = adjusted hazard ratio; CI = confidence interval; DR = distant recurrence; PR = progesterone receptor; Ref = referent.
In postmenopausal patients, a statistically significantly improved DRFI from tamoxifen therapy was seen for all good prognosis tumor characteristics in the univariate Kaplan–Meier (log-rank P ≤ .001 for all; Figure S5) and multivariable Cox proportional hazards regression analyses, including small tumor size (adjusted HR = 0.55, 95% CI = 0.39 to 0.77), tumor grade 1-2 (adjusted HR = 0.55, 95% CI = 0.41 to 0.73), lymph node–negative (adjusted HR = 0.44, 95% CI = 0.30 to 0.64), progesterone receptor–positive (adjusted HR = 0.60, 95% CI = 0.44 to 0.80), Ki-67–low (adjusted HR = 0.51, 95% CI = 0.38 to 0.68), and genomic low-risk tumors (adjusted HR = 0.53, 95% CI = 0.37 to 0.74; Figure 2). In addition, postmenopausal patients treated with tamoxifen therapy showed a significantly improved DRFI compared with endocrine untreated patients for the poor prognosis tumor characteristics large tumor size (log-rank P = .006; adjusted HR = 0.64, 95% CI = 0.44 to 0.94), lymph node–positive status (log-rank P = .035; adjusted HR = 0.73, 95% CI = 0.52 to 1.03), and progesterone receptor–negative status (log-rank P = .029; adjusted HR = 0.51, 95% CI = 0.32 to 0.81; Figure 2 and Figure S5).
Given the many clinical parameters that could potentially influence the early and/or long-term benefit from tamoxifen therapy, a summary score including the clinically used tumor characteristics could be helpful in assessing patient benefit in the clinic. Therefore, the number of poor prognosis characteristics was summarized into a clinical marker score to understand whether the accumulation of these is predictive of the long-term tamoxifen therapy benefit, including large tumor size, tumor grade 3, lymph node–positive status, progesterone receptor–negative status, or high Ki-67 levels. The distribution of the clinical marker score was similar between premenopausal and postmenopausal patients (see Table 1). In agreement with previous analyses, premenopausal patients with clinical marker score 0 only showed a statistically significantly improved DRFI from tamoxifen therapy vs control in univariate Kaplan–Meier (log-rank P = .040) and multivariable Cox proportional hazards regression analysis (adjusted HR = 0.34, 95% CI = 0.13 to 0.91; Figure S6 and Figure 3). In postmenopausal patients, a significantly improved DRFI from tamoxifen therapy was observed in univariate and multivariable analyses for clinical marker score 0 (log-rank P = .005; adjusted HR = 0.38, 95% CI = 0.18 to 0.77) and clinical marker score 1 (log-rank P = .008; adjusted HR = 0.54, 95% CI = 0.35 to 0.85) but not for clinical marker score 2 or 3 or higher (Figure S6 and Figure 3).
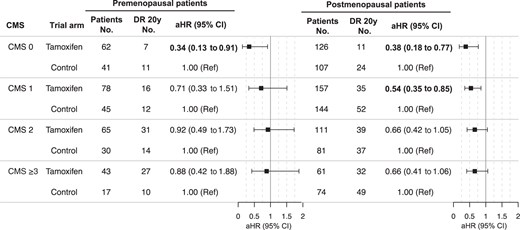
Twenty-year tamoxifen therapy benefit by clinical marker score. Multivariable Cox proportional hazards regression analysis of the 20-year distant recurrence-free interval for premenopausal and postmenopausal breast cancer patients with estrogen receptor–positive and HER2-negative tumors, comparing patients randomly assigned to tamoxifen vs no endocrine therapy (control). Analyses in premenopausal patients adjusted for age, random assignment year, and type of surgery, and analyses in postmenopausal patients adjusted for age, random assignment year, type of surgery, chemotherapy, and radiotherapy. Bold indicates a statistically significant P value less than .05. aHR = adjusted hazard ratio; CI = confidence interval; CMS = clinical marker score; DR = distant recurrence; Ref = referent.
In summary, tamoxifen therapy benefit was observed for a few of the clinically used tumor characteristics in premenopausal patients, whereas a benefit was seen for all good prognosis tumor characteristics in postmenopausal patients. In general, the univariate and multivariable analyses showed similar results, with a few exceptions, which could be because the clinically used tumor characteristics are to some extent correlated.
Time-varying analysis of tamoxifen therapy benefit by clinically used tumor characteristics in premenopausal and postmenopausal patients
Multivariable time-varying analysis was further performed to assess how the tamoxifen therapy benefit varies over the 20 years of follow-up for the clinically used tumor characteristics in premenopausal and postmenopausal patients, contrasting tamoxifen-treated patients with endocrine untreated patients. In premenopausal patients, a statistically significant benefit was observed up to 10 years for patients with lymph node–negative tumors (adjusted HR = 0.48, 95% CI = 0.23 to 0.99 at year 10), up to 5 years for progesterone receptor–positive tumors (adjusted HR = 0.62, 95% CI = 0.41 to 0.92 at year 5), and up to 15 years for genomic low-risk tumors (adjusted HR = 0.44, 95% CI = 0.19 to 0.98 at year 15; Figure 4). Analysis in progesterone receptor–negative premenopausal patients was not conducted because of sample size (90.6% were progesterone receptor positive).
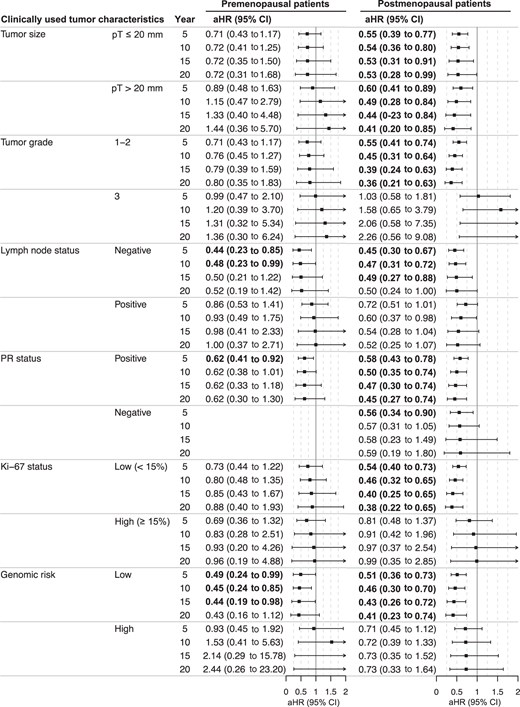
Time-varying analysis of tamoxifen therapy benefit by clinically used tumor characteristics. Analysis by flexible parametric modeling of the distant recurrence-free interval estimating hazard ratios at year 5, 10, 15, and 20 for premenopausal and postmenopausal breast cancer patients with estrogen receptor–positive and HER2-negative tumors, comparing patients randomly assigned to tamoxifen vs no endocrine therapy (control). Adjusted for age, random assignment year, tumor size, tumor grade, lymph node status, progesterone receptor status, Ki-67 status, type of surgery, chemotherapy, and radiotherapy. Bold indicates a significant P value less than .05. aHR = adjusted hazard ratio; CI = confidence interval; PR = progesterone receptor.
In postmenopausal patients, time-varying analysis contrasting tamoxifen-treated patients with endocrine untreated patients showed a statistically significant long-lasting tamoxifen benefit for all good prognosis tumor characteristics, including for patients with small tumor size (adjusted HR = 0.53, 95% CI = 0.28 to 0.99 at year 20), lower tumor grade (adjusted HR = 0.36, 95% CI = 0.21 to 0.63 at year 20), lymph node–negative (adjusted HR = 0.49, 95% CI = 0.27 to 0.88 at year 15), progesterone receptor–positive (adjusted HR = 0.45, 95% CI = 0.27 to 0.74 at year 20), Ki-67–low (adjusted HR = 0.38, 95% CI = 0.22 to 0.65 at year 20), and genomic low-risk tumors (adjusted HR = 0.41, 95% CI = 0.23 to 0.74 at year 20; Figure 4). In addition, a significant long-term tamoxifen therapy benefit up to at least 20 years was seen for postmenopausal patients with large tumor size (adjusted HR = 0.41, 95% CI = 0.20 to 0.85 at year 20) and a short-term benefit up to 5 years for progesterone receptor–negative tumors (adjusted HR = 0.56, 95% CI = 0.34 to 0.90 at year 5; Figure 4).
Time-varying analyses showed a statistically significant tamoxifen therapy benefit for clinical marker score 0 up to 5 years in premenopausal patients (adjusted HR = 0.31, 95% CI = 0.10 to 0.98 at year 5; Figure S7). No statistically significant benefit was seen in premenopausal patients with a higher clinical marker score (1, 2, or 3 or higher). Furthermore, postmenopausal patients with clinical marker score 0 had a statistically significant benefit up to at least 20 years (adjusted HR = 0.22, 95% CI = 0.08 to 0.65 at year 20) and patients with clinical marker score 1 up to 10 years (adjusted HR = 0.56, 95% CI = 0.33 to 0.95 at year 10; Figure S7).
In summary, the tamoxifen therapy benefit in premenopausal patients remained beyond 10 years only for genomic low-risk tumors, whereas a significant long-lasting tamoxifen therapy benefit was seen for all good prognosis tumor characteristics in postmenopausal patients.
Discussion
In this study, a differential long-term benefit from tamoxifen therapy was observed between premenopausal and postmenopausal estrogen receptor–positive and HER2-negative breast cancer patients. Clinical low-risk postmenopausal patients had a long-term benefit. However, for premenopausal patients, the benefit was seen for few of the good prognosis tumor characteristics, and importantly, and not previously shown, the benefit was only seen to last beyond 10 years for genomic low-risk tumors. In summary, the findings from this study suggest that the clinically used tumor characteristics could not identify premenopausal patients with long-term tamoxifen therapy benefit. Clearly, there is a need for improved long-term treatment predictive markers, especially for premenopausal breast cancer patients, together with improved treatment strategies to address the challenges of late disease recurrence.
Estrogen receptor–positive breast cancer patients have a steady and long-term risk of distant metastatic disease that extends decades beyond primary diagnosis.2-7 Tamoxifen therapy reduces the risk, and our previous findings suggest long-term benefit mainly for low-risk patients.4,30 Importantly, differences in survival are observed after 10 years; thus, long-term follow-up studies are essential to understand the benefit from endocrine therapy. However, there is a general lack of long-term studies. The STO trials with complete and high-quality 20-year follow-up allowed for this unique assessment of the long-term tamoxifen therapy benefit. The population-based recruitment of patients from the same geographic area reduces unknown variability between the 2 patient groups (premenopausal vs postmenopausal), and menopausal status was clearly defined. If, instead, 50 years had been used as a proxy for menopausal status, nearly 20% of the premenopausal patients would have been misclassified.
In this study, tamoxifen therapy benefit was seen for patients with lymph node–negative disease regardless of menopausal status; however, the benefit was suggested to last up to 10 years in premenopausal and at least 20 years for postmenopausal patients. Additionally, long-term tamoxifen therapy benefit for low tumor grade and low Ki-67 levels was only seen for postmenopausal patients. This suggests differences in terms of cell proliferation and differentiation, as observed previously in young vs older patients45 (ie, histological grading and Ki-67 status have a differential impact by menopausal status for the long-term tamoxifen therapy benefit). Interestingly, no statistically significant difference in Ki-67 status was seen between premenopausal and postmenopausal patients in the STO trials. Furthermore, this study supports a role of progesterone receptor for tamoxifen therapy benefit in premenopausal and postmenopausal breast cancer patients, which has been reported previously.46-49 A high proportion (>90%) of progesterone receptor–positive tumors was observed in premenopausal patients, which is in agreement with other studies.50
The longest benefit from tamoxifen in premenopausal patients was seen for genomic low-risk tumors, namely, up to 15 years from primary diagnosis. Half of premenopausal genomic low-risk patients and two-thirds of premenopausal genomic high-risk patients were lymph node positive (ie, these 2 important prognostic and potentially tamoxifen therapy predictive factors are not highly correlated). This indicates that underlying molecular processes drive the development of metastatic disease, which are not necessarily reflected equally well by clinical tumor characteristics. Indeed, the molecular biology of premenopausal breast cancer has been explored previously; however, it is yet to be understood if more information beyond molecular subtypes is informative.15-18 This study suggests that the 70-gene signature could be helpful in the prediction of the long-term tamoxifen therapy benefit, but this could also include other molecular markers contributing with further insights into the molecular mechanisms such as the Breast Cancer Index and the Sensitivity to Endocrine Therapy for stage II-III breast cancer (SET2,3) index.51,52 However, they are yet to be explored in a long-term setting in premenopausal patients.
The clinically used tumor characteristics were also summarized in a clinical marker score to understand whether the accumulation of poor-prognosis tumor characteristics is predictive of long-term tamoxifen therapy benefit. A summary score could be helpful in the clinical assessment of patient benefit. Indeed, tamoxifen therapy benefit was observed for a low clinical marker score. However, for patients with clinical marker score 0, the benefit was suggested to be short-term (up to 5 years) for premenopausal patients and long-term (at least 20 years) for postmenopausal patients.
Although tamoxifen has been shown to be effective in premenopausal and postmenopausal breast cancer patients,6,22 our results suggest that the clinically used tumor characteristics cannot predict long-term tamoxifen therapy benefit in premenopausal patients alone. Instead, clinical assessment in premenopausal patients might need to be complemented with molecular markers to understand which patients will benefit from tamoxifen and thus can be spared a more aggressive treatment including chemotherapy and/or ovarian function suppression. As of today, it is not clear when to offer tamoxifen or ovarian function suppression to premenopausal patients, as we have discussed previously.30 Underlying mechanisms that could explain the differential benefit by menopausal status are complex and likely to include several factors. For instance, the treatment benefit and the optimal dose and duration likely depend on a patient’s age, estrogen levels, metabolism, genetics, and lifestyle factors.53 Future studies investigating interpatient variability might reduce side effects, improve compliance, and save lives. Moreover, biomarkers to identify which patients benefit more from other endocrine therapies, extended endocrine therapy and/or additional chemotherapy, and the long-term effects from these would be valuable to tailor the right treatment to the right patient.54-57
This study has limitations and advantages. Sample size of this subset of the STO trials, despite originating from large individual trials, becomes limited in some of the subgroup analyses, especially for premenopausal patients, and thus caution should be taken in the interpretation. Furthermore, the research question included in the study was not originally planned, making this a secondary analysis. However, the high-quality and long-term follow-up and the inclusion of a control arm make results from this study important to consider. Other advantages are the clear definition of menopausal status instead of using age as a proxy and the population-based recruitment of patients from the same geographic area in Sweden, which reduced unknown variability. Differences in trial design include random assignment of clinical high-risk postmenopausal patients to chemotherapy, whereas lymph node–positive premenopausal patients received chemotherapy as standard of care. Note that all premenopausal and approximately 75% of the postmenopausal patients in this study received 2 years of tamoxifen therapy, whereas today’s standard is at least 5 years. This could be helpful for clinicians and soothing for patients unable to endure long duration of endocrine therapy. However, as also the dose differs from today’s standard, it is difficult to directly compare the at least 2 years of 40 mg once daily as given in the STO trials with current treatment guidelines of at least 5 years of 20 mg once daily, including how this affects side effects such as the risk of endometrial cancer. As of today, the optimal dose and duration of endocrine therapy have yet not been established and require further research, and these may differ between premenopausal and postmenopausal patients.
In conclusion, this study suggests a differential long-term tamoxifen therapy benefit by menopausal status and highlights the need to improve long-term endocrine therapy benefit prediction beyond the clinically used tumor characteristics. As shown, this could involve molecular risk prediction tools and be of particular importance for premenopausal patients. An intriguing but challenging question is to understand whether patients with late risk would benefit from additional treatment when the benefit from the primary treatment wears off. Further insight into the molecular mechanisms driving late metastatic development can improve treatment guidance for breast cancer patients reflecting their individual long-term risk profile.
Acknowledgments
The authors thank all the patients who participated in the STO trials and the Stockholm Breast Cancer Study Group that enabled this study. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Author contributions
Annelie Johansson, MSc, PhD (Conceptualization; Data curation; Formal analysis; Investigation; Project administration; Resources; Software; Supervision; Visualization; Writing—original draft; Writing—review & editing), Huma Dar, MSc, PhD (Conceptualization; Data curation; Formal analysis; Resources; Software; Supervision; Visualization; Writing—review & editing), Anna Nordenskjöld, MD, PhD (Resources; Supervision; Writing—review & editing), Gizeh Perez-Tenorio, MSc, PhD (Data curation; Resources; Supervision; Writing—review & editing), Nicholas P. Tobin, MSc, PhD (Supervision; Writing—review & editing), Christina Yau, PhD (Resources; Supervision; Writing—review & editing), Christopher C. Benz, MD (Resources; Supervision; Writing—review & editing), Laura J. Esserman, MD (Resources; Supervision; Writing—review & editing), Laura J van ‘t Veer, MSc, PhD (Resources; Supervision; Writing—review & editing), Bo Nordenskjöld, MD, PhD (Conceptualization; Data curation; Resources; Supervision; Writing—review & editing), Olle Stål, MSc, PhD (Conceptualization; Data curation; Resources; Supervision; Writing—review & editing), Tommy Fornander, MD, PhD (Conceptualization; Data curation; Funding acquisition; Investigation; Project administration; Resources; Supervision; Writing—review & editing), and Linda S. Lindström, MSc, PhD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Project administration; Resources; Software; Supervision; Visualization; Writing—original draft; Writing—review & editing).
Supplementary material
Supplementary material is available at JNCI: Journal of the National Cancer Institute online.
Funding
This work was supported by the Swedish Research Council (Vetenskapsrådet, grant number 2020-02466 and 2023-03009 to LSL); Swedish Research Council for Health, Working life and Welfare, (FORTE, grant number 2019-00477 to LSL); ALF medicine (grant number FoUI-974882 to LSL); Gösta Milton Donation Fund (Stiftelsen Gösta Miltons donationsfond, to LSL); Swedish Cancer Society (Cancerfonden, grant number 222081 and 220552SIA to LSL, 222216 to OS and 200802 to NPT); Stockholm Cancer Society (Cancerföreningen i Stockholm, grant number 221233 and 201212 to LSL, 181093 to TF and 224112 to NPT); King Gustav V Jubilee Clinical Research Foundation (grant number 2021-356 to AN); California Breast Cancer Research Program (CBCRP, grant number 180B-0065 to LJE); and National Institutes of Health (NIH, grant number: U01-CA196406 to LJE).
Conflicts of interest
LV is a cofounder, stockholder, and part-time employee of Agendia. All remaining authors declare no potential conflicts of interest.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study.


