-
PDF
- Split View
-
Views
-
Cite
Cite
Lisa M Fucito, Amanda M Palmer, Stephen R Baldassarri, A new perspective on mitigating lung cancer risks through smoking cessation and reduction, JNCI: Journal of the National Cancer Institute, Volume 116, Issue 6, June 2024, Pages 782–785, https://doi.org/10.1093/jnci/djae044
Close - Share Icon Share
Lung cancer persists as a leading cause of death and the primary cause of cancer death worldwide among men and women (1). Since the inaugural 1964 US Surgeon General’s report on cigarette smoking, extensive evidence underscores smoking as the chief cause of lung cancer (1,2). Beyond triggering lung cancer, smoking detrimentally impacts lung cancer prognosis among those diagnosed. Specifically, smoking promotes lung cancer recurrence, secondary cancer development, noncancerous adverse health consequences such as acute coronary events, cancer treatment toxicity and failure and reduces quality of life and overall survival (3,4). Smoking cessation, however, clinically diminishes lung cancer incidence, morbidity, and mortality (5,6).
Among US adults, cigarette smoking rates have reached a historic low of 11.5% (7), driven by strong tobacco control policies, effective smoking cessation interventions, and shifts in the tobacco product landscape (eg, the introduction of e-cigarettes) (8,9). Correspondingly, annual rates of new lung cancer cases and deaths have declined in parallel (10,11). These improvements in lung cancer risks are mainly credited to lower smoking prevalence as well as advances in lung cancer screening and treatment (12). Low-dose computer tomography (LDCT) scans enable earlier detection of lung cancers, which allows for more timely intervention (13). Likewise, new immunotherapies, targeted therapies, surgical techniques, and innovations in radiation therapy have expanded treatment options and improved survival for patients diagnosed with later stage lung cancers (14).
Nevertheless, lung cancer remains highly prevalent, and many adults either struggle to quit smoking despite the availability of effective treatments or are not interested in quitting (15). There is some evidence from clinical trials and cohort studies that reducing smoking may lower risk of developing lung cancer (16-18). Thus, a lingering, yet often controversial question, is whether tobacco harm reduction (THR) should be encouraged for people unable or unwilling to quit completely (19). Nicotine is the primary addictive chemical in cigarettes, but it is not the primary toxic chemical (20). The harmful effects of cigarettes stem primarily from tobacco, which becomes more toxic upon combustion (21). Reducing exposure to the combustion products of cigarettes is therefore crucial for mitigating lung cancer risks. THR can take several forms, including substituting cigarettes for use of pharmaceutical nicotine products (eg, nicotine patch, gum) or use of commercial, noncombustible tobacco products (eg, oral nicotine pouches, smokeless tobacco, or e-cigarettes), or simply reducing the amount of smoke exposure (eg, by reducing cigarettes smoked per day) (22).
In this issue of JNCI, Gutiérrez-Torres and colleagues (23) explored smoking behavior and lung cancer risk in a secondary analysis of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study (24,25), a large-scale trial of antioxidant supplementation for reducing lung cancer and adverse health outcomes in older Finnish men (aged 50-69 years) who smoked cigarettes. Gutiérrez-Torres et al. capitalized on this rich dataset to specifically test the effects of 1) changes in smoking intensity (number of cigarettes smoked per day) and 2) changes in smoking frequency (proportion of study visits where smoking was reported) on the likelihood of lung cancer diagnosis via cancer registry data. The authors observed the lowest lung cancer incidence among men who stopped smoking and remained abstinent and the highest incidence among men who smoked many cigarettes per day at all study visits. However, they also found that men who reduced their smoking quantity and/or frequency had lower lung cancer risk than their counterparts who did not reduce.
The Gutiérrez-Torres et al. (23) study had several strengths such as a large sample size, assessment and modeling of both changes in smoking quantity and frequency, and a strong retention rate that provided robust smoking endpoint data with low missingness. In addition, it used repeated assessment of cigarette smoking each year for nearly 10 years. Most prior studies of smoking reduction and lung cancer risk are limited to assessments of only a couple timepoints (26,27), which may underestimate the benefits of smoking cessation and/or reduction on lung cancer risk (28).
At the same time, the study highlights the challenges inherent in such research and remaining scientific gaps. Given the long latency period of lung cancer, accurately predicting the effect of smoking on risk requires extensive longitudinal studies, which are costly and difficult to conduct. In the ATBC trial, participants’ smoking was not assessed after the intervention. Thus, it is possible that the observed benefit of smoking reduction during the intervention period on later lung cancer risk might be due to those participants eventually quitting smoking post-intervention. Additional longitudinal studies are needed to clarify the effects of smoking cessation vs reduction on lung cancer incidence. Further, lung cancer is a complex, heterogenous disease that results from the interaction of lifestyle, environmental, and genetic factors (29). While reduced smoking at a population level may lower risk of lung cancer, its benefits for specific individuals could be minimal relative to complete cessation due to their unique risk profiles. Therefore, in alignment with emerging targeted lung cancer therapies, more research is essential for elucidating how to personalize and target lifestyle risk interventions more effectively to maximize lung cancer risk reduction. An efficient method for this purpose is to incorporate the systematic and repeated assessment of smoking in new and ongoing cancer prevention and treatment trials (30).
Importantly, the population of people who smoke and the nature of cigarette smoking have changed since completion of the ATBC study. We also have a better understanding of potential disparities in lung cancer (31,32). For instance, cigarette smoking is now disproportionately represented among adults from minoritized populations and those with medical and psychiatric comorbidities (7). Many individuals now smoke fewer cigarettes per day and/or use cigarettes in combination with other tobacco or nicotine products such as e-cigarettes (9). Some populations (eg, Black adults) have higher lung cancer incidence and mortality, even at lower levels of smoking (32). Longitudinal studies of cancer risk, however, have largely been conducted on samples that lack representativeness and diversity, limiting generalizability (33). Caution is warranted when generalizing these risk prediction findings to the broader population of adults who smoke. Studies that model the effects of smoking behavior changes over time on lung cancer risk in diverse samples are critically needed.
Although questions remain, the Gutiérrez-Torres et al. (23) study has clear implications that smoking abstinence is the ultimate goal for health. Adults who smoke should be encouraged to quit at every health encounter and assisted with access to evidence-based smoking cessation interventions to prevent lung cancer incidence and other smoking-related health risks and improve lung cancer prognosis (3). Several barriers, however, continue to limit smoking cessation treatment engagement such as cost, health-care access, lack of knowledge, stigma, and social and cultural factors (34,35). Reducing these obstacles requires a multifaceted public health approach that includes implementation studies to test optimal integration of tobacco treatment within routine health-care delivery.
If conventional tobacco treatment interventions are inaccessible, or an individual is unresponsive to them or unwilling to try them, THR approaches such as smoking reduction might be considered. Indeed, prior literature supports reducing to quit as a viable treatment approach for those who smoke cigarettes (36). Smoking reduction strategies may facilitate future quit attempts due to prompting motivation and lowering nicotine exposure and dependence (26). For those who are unable to achieve complete abstinence, maintaining a reduction in cigarette smoking could be an alternative good clinical outcome. In health-care settings, THR may be especially well received by patients as it supports their autonomy in the quit process. Notably, smoking reduction should be presented to patients with the eventual goal of complete abstinence, as this outcome promotes the highest risk reduction for future cancers (see Figure 1). Nevertheless, evidence-based smoking reduction interventions are limited, and further research is necessary to investigate the long-term sustainability of reduced smoking (37).
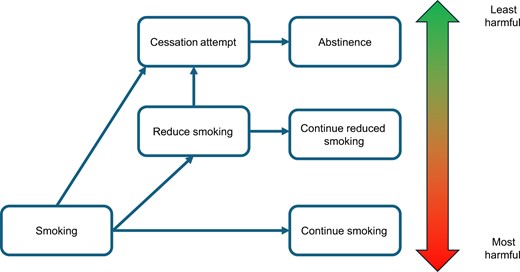
The hypothesized spectrum of lung cancer risk in the context of continued smoking, smoking reduction, and smoking abstinence.
What about other potential THR strategies such as substituting cigarette smoking for noncombustible tobacco products such as e-cigarettes, heat-not-burn tobacco, oral nicotine pouches, and/or smokeless tobacco? (19) It would be expected that substituting noncombustible forms of tobacco and nicotine for cigarettes should reduce lung cancer risk, but this hypothesis needs to be tested. The availability of these products also raises more questions. As an example, for lung cancer risk reduction, is it better for adults to switch completely from cigarettes to e-cigarette or reduce their smoking without concomitant use of other products? Similarly, are lung cancer risks lower for noninhalable forms of tobacco such as oral nicotine pouches and smokeless tobacco than e-cigarettes? Additionally, what are the lung cancer risks of these products in people with no smoking histories? There is strong interest in whether e-cigarettes have oncologic potential, but no firm evidence to date. Longitudinal studies examining patterns of multiple tobacco use and transitions as well as cancer-related toxicity and health outcomes will be helpful in answering these questions. Together, these data are critical for informing the potential implementation of these THR approaches as integral components of comprehensive cancer prevention and control strategies and tobacco treatment within healthcare settings.
Data availability
No new data were generated or analyzed for this editorial.
Author contributions
Lisa Fucito, PhD (Conceptualization; Project administration; Writing—original draft; Writing—review & editing), Amanda Palmer, PhD (Conceptualization; Writing—original draft; Writing—review & editing), Stephen Baldassarri, MD, MHS (Conceptualization; Writing—original draft; Writing—review & editing).
Funding
The authors were supported by National Institutes of Health funding as follows: LMF (P50CA196530, P30CA016359); AMP (P30CA138313); SRB (P50CA196530; K23DA045957).
Conflicts of interest
Unrelated to this article, Dr Fucito has received funding from Imbrium Therapeutics LLC for serving on an advisory board. All other authors have no disclosures to report.
Acknowledgments
The funders had no role in the writing of this editorial.


