-
PDF
- Split View
-
Views
-
Cite
Cite
Hildur Helgadottir, Lars Ny, Gustav J Ullenhag, Johan Falkenius, Rasmus Mikiver, Roger Olofsson Bagge, Karolin Isaksson, Survival after introduction of adjuvant treatment in stage III melanoma: a nationwide registry-based study, JNCI: Journal of the National Cancer Institute, Volume 115, Issue 9, September 2023, Pages 1077–1084, https://doi.org/10.1093/jnci/djad081
Close - Share Icon Share
Abstract
Adjuvant treatments with PD-1 and BRAF+MEK inhibitors statistically significantly prolong recurrence-free survival in stage III cutaneous melanoma. Yet, the effect on overall survival is still unclear. Based on recurrence-free survival outcomes, these treatments have been approved and widely implemented. The treatments have considerable side effects and costs, and overall survival effect remains a highly anticipated outcome.
Clinical and histopathological parameters were obtained from the Swedish Melanoma Registry for patients diagnosed with stage III melanoma between 2016 and 2020. The patients were divided depending on if they were diagnosed before or from July 2018, based on the timepoint when adjuvant treatment was introduced in Sweden. Patients were followed up until the end of 2021. In this cohort study, melanoma-specific and overall survival were calculated using the Kaplan-Meier method and Cox-regression analyses.
There were 1371 patients diagnosed with stage III primary melanoma in Sweden in 2016-2020. The 2-year overall survival rates, comparing the 634 patients in the precohort and the 737 in the postcohort, were 84.3% (95% confidence interval [CI] = 81.4% to 87.3%) and 86.1% (95% CI = 83.4% to 89.0%), respectively, with an adjusted hazard ratio of 0.91 (95% CI = 0.70 to 1.19, P = .51). Further, no statistically significant overall or melanoma-specific survival differences were seen when comparing the precohort and the postcohort in different subgroups for age, sex, or tumor characteristics.
In this nationwide population-based and registry-based study, no survival benefit was detected in patients diagnosed before or after the implementation of adjuvant treatment in stage III melanoma. These findings encourage a careful assessment of the current recommendations on adjuvant treatment.
In recent years, adjuvant treatments have been implemented as standard of care in patients with fully resected high-risk cutaneous melanoma. Studies of adjuvant anti-CTLA-4 therapy with high-dose ipilimumab (10 mg/kg) in stage III melanoma demonstrated statistically significant recurrence-free survival compared with placebo (1,2). This led to a US Food and Drug Administration (FDA) approval in 2015. However, ipilimumab was not approved for this indication by other authorities, including the European Medicines Agency (EMA) and the Australian Therapeutic Goods Administration, mainly due to the severe toxicity that was reported. Further, anti-PD-1 adjuvant treatment with nivolumab in fully resected stage IIIB-C and IV (American Joint Committee on Cancer [AJCC] 7) showed, compared with ipilimumab (10 mg/kg), statistically significant recurrence-free and distant metastasis-free survival (CheckMate 238) (3). Based on the recurrence-free survival benefit, nivolumab was approved for the adjuvant indication by the FDA in December 2017 and by EMA in July 2018. Also, in the EORTC 1325/KEYNOTE-054 study, a recurrence-free survival benefit was reported for adjuvant pembrolizumab compared with placebo in stage III melanoma (4). Subsequently, adjuvant pembrolizumab was approved by EMA in October 2018 and by the FDA in February 2019. In parallel, the COMBI-AD study demonstrated superior recurrence-free survival in stage III patients treated with adjuvant BRAF+MEK inhibitors dabrafenib and trametinib, leading to FDA approval in May 2018 and EMA approval in July 2018 (5). Additionally, in December 2021 and July 2022, adjuvant treatment with the PD1-inhibitor pembrolizumab was approved by the FDA and EMA in stage IIB-C melanoma based on the KEYNOTE-716, with statistically significantly longer recurrence-free survival compared with placebo (6).
With a longer follow-up, improved distant metastasis-free survival and overall survival were demonstrated with ipilimumab compared with placebo (2). Noteworthy, the study participants were included in the years 2008-2011, which was before the approval of checkpoint inhibitory or targeted therapy in the metastatic setting. Hence, in the occurrence of disease-relapse at this timepoint, treatment options were still very limited. Conversely, longer follow-up in both the CheckMate 238 and also the SWOG S1404 study (adjuvant pembrolizumab vs ipilimumab 10 mg/kg or high-dose Interferon alfa (IFNalpha)-2b in stage III-IV melanoma) did not show any overall survival benefit (7,8). The lack of translation of the recurrence-free survival benefit to an overall survival benefit has been attributed to both the study design, with active comparators, and also to the entrance of effective treatments in the metastatic setting (9-15). Further, for the 2 studies with placebo comparators, KEYNOTE-054 and COMBI-AD, overall survival results have still not been published. Hence, adjuvant treatments with PD-1 and BRAF+MEK inhibitors have been widely implemented based on the recurrence-free survival benefit. However, in light of the considerable side effects and costs of the treatment, the effect on overall survival compared with no adjuvant treatment remains a highly anticipated outcome. In this nationwide registry-based study from Sweden, we report melanoma-specific and overall survival in stage III melanoma patients diagnosed before or after the national implementation of adjuvant treatments for this indication.
Methods
Patients and follow-up
This study is based on the Swedish Melanoma Registry (SweMR) that covers nearly all (99%) primary cutaneous melanomas diagnosed in Sweden (16). By law, clinicians and pathologists are obliged to report all cancer diagnoses to the Swedish National Cancer Registry, thereby ensuring high-quality reported data. Every patient is also linked by their unique individual Swedish personal identity number to the records of the national cause of death registry, a high-quality virtually complete register of all deaths in Sweden (17).
The current population of Sweden is 10.5 million inhabitants, and the majority are Caucasian of Scandinavian descent. There has been a growing immigrant population that constituted less than 1% of inhabitants in the year 1960, and presently 13% are born outside northern Europe. However, of the melanomas diagnosed in the years 1990-2007, only 1% occurred in individuals with an origin outside northern Europe (18,19). Race is a variable that is not included in Swedish registries. Sweden currently has an annual age standardized (world) melanoma incidence of 23.3 per 100 000 inhabitants and has the sixth-highest melanoma incidence of the world’s countries, preceded by Australia, New Zealand, Denmark, the Netherlands, and Norway (20).
For the purpose of this study, all patients diagnosed with stage III melanoma in the years 2016-2020 were identified. Staging was according to the AJCC staging manual for melanoma, 8th edition (21). Data were retrieved for the age and sex of the patients, site, histopathological subtype, Breslow thickness and ulceration of the primary melanoma, type of locoregional spread (occult or clinically detected lymph node metastases or in transit or satellite metastasis), and numbers of affected lymph nodes. The patients were divided depending on if they were diagnosed before or from July 2018, the timepoint of the European Medicines Agency approval for adjuvant nivolumab and for adjuvant dabrafenib and trametinib (precohort and postcohort). Follow-up was until the end of 2021. During the study period, from 2016 to 2021, treatment recommendations and drugs available in the setting of metastatic unresectable melanoma have been essentially similar, with the BRAF and MEK inhibitor combination approved in 2014, PD-1 monotherapy approved in 2015, and PD-1/CTLA-4 inhibitor combinations approved in 2016 (9,12-14). The Swedish Ethical Review Boards approved the study (Dnr 99160/1999).
Statistical analyses
Baseline characteristics were compared with the χ2 test for categorical variables and the Mann-Whitney U-test for continuous variables. For the continuous variables, the median and interquartile range (IQR), defined as the spread between the 25th and 75th percentiles of the data, were calculated. The primary endpoints were melanoma-specific and overall survival calculated from the date of diagnosis until the date of the event or the date of censoring. In this cohort study, the Kaplan-Meier method and Cox regression analyses (with adjustments for age, sex, and tumor stage) were applied to compare survival in the precohort and postcohort. The results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs) and P values based on log-rank test. The level of statistical significance was .05, and all P values were 2-tailed. All analyses were performed using IBM SPSS statistics version 22.0 (IBM Corp, Armonk, NY, USA) and R Statistical Software (v4.0.3 R Core Team 2020).
Results
There were 1371 patients diagnosed with stage III primary melanoma in Sweden in the years 2016-2020: 820 males and 551 females (Table 1). The median age at diagnosis was 66 years (IQR = 53-75 years), the median Breslow thickness was 3.1 mm (IQR = 1.8-5.0 mm), and the majority 1100 (80.2%) had occult (sentinel node detected) lymph node metastasis. There were 634 patients in the precohort (diagnosed January 2016 to June 2018) and 737 in the postcohort (diagnosed July 2018 to December 2020). No statistically significant differences were seen in the precohort and postcohort related to the patients’ sex, age (continuous or categorical [≤ or >75 years] variable), site, histopathological subtype, Breslow thickness (continuous variable), tumor ulceration, or the number of lymph node metastases. Statistically significant differences were observed with thinner melanomas (categorical variable, P = .002) and more in transit or satellite metastasis (P = .011) in the precohort. In the precohort, a statistically significantly higher fraction of patients had stage IIIA melanoma compared with the postcohort (P = .020). In 50 patients (3.6%), substaging in specific stage III groups (IIIA, IIIB, IIIC, or IIID) was not possible, mainly because information on the primary tumor thickness or ulceration status was missing (Table 1).
Baseline characteristics of stage III melanoma cases diagnosed before or after July 2018, based on the timepoint when adjuvant treatment was introduced in Swedena
| . | Precohort, diagnosis . | Postcohort, diagnosis . | P . |
|---|---|---|---|
| . | Jan. 2016-June 2018 . | July 2018-Dec. 2020 . | . |
| Characteristics . | n = 634 . | n = 737 . | . |
| Sex | .114 | ||
| Male | 394 (62.1%) | 426 (57.8%) | |
| Female | 240 (37.9%) | 311 (42.2%) | |
| Age, median (IQR), y | 66.0 (51.2-75.0) | 66.0 (54.0-74.0) | .502 |
| Age, y | .958 | ||
| 0-74 | 474 (74.8%) | 553 (75.0%) | |
| 75+ | 160 (25.2%) | 184 (25.0%) | |
| Site of primary melanoma | .556 | ||
| Extremities | 267 (42.1%) | 305 (41.4%) | |
| Head or neck | 65 (10.3%) | 64 (8.7%) | |
| Trunk | 276 (43.5%) | 343 (46.5%) | |
| Palm or subungual | 26 (4.1%) | 25 (3.4%) | |
| Subtype of primary melanoma | .834 | ||
| Superficial spreading melanoma | 292 (46.1%) | 348 (47.2%) | |
| Lentigo maligna melanoma | 7 (1.1%) | 10 (1.4%) | |
| Nodular melanoma | 211 (33.3%) | 248 (33.6%) | |
| Acral lentiginous melanoma | 23 (3.6%) | 27 (3.7%) | |
| Other | 61 (9.6%) | 70 (9.5%) | |
| Not reported | 40 (6.31%) | 34 (4.6%) | |
| Breslow thickness, median (IQR), mm | 3.00 (1.70-5.00) | 3.20 (1.90-5.20) | .154 |
| Breslow thickness | .002 | ||
| 0.1-0.7 | 23 (3.6%) | 10 (1.4%) | |
| 0.8-1.0 | 22 (3.4%) | 13 (1.8%) | |
| 1.1-2.0 | 166 (26.2%) | 179 (24.3%) | |
| 2.1-4.0 | 171 (27.0%) | 255 (34.6%) | |
| >4.0 | 239 (37.7%) | 264 (35.8%) | |
| Not reported | 13 (2.1%) | 16 (2.2%) | |
| Tumor ulceration | .336 | ||
| No | 304 (47.9%) | 330 (44.8%) | |
| Yes | 314 (49.5%) | 381 (51.7%) | |
| Not reported | 16 (2.52%) | 26 (3.53%) | |
| Node (N) category | .621 | ||
| N1 | 434 (68.5%) | 522 (70.8%) | |
| N2 | 146 (23.0%) | 155 (21.0%) | |
| N3 | 54 (8.5%) | 60 (8.1%) | |
| Type of locoregional spread | .011 | ||
| Occult lymph node (LN) metastasis | 498 (78.5%) | 602 (81.7%) | |
| Clinically detected LN metastasis | 84 (13.2%) | 99 (13.4%) | |
| In transit or satellite metastasis | 46 (7.3%) | 36 (4.9%) | |
| Not reported | 6 (1.0%) | 0 (0.0%) | |
| Tumor stage (AJCC 8) | .020 | ||
| IIIA | 144 (22.7%) | 119 (16.1%) | |
| IIIB | 121 (19.1%) | 173 (23.5%) | |
| IIIC | 325 (51.3%) | 398 (54.0%) | |
| IIID | 21 (3.3%) | 20 (2.7%) | |
| Not reported | 23 (3.6%) | 27 (3.7%) |
| . | Precohort, diagnosis . | Postcohort, diagnosis . | P . |
|---|---|---|---|
| . | Jan. 2016-June 2018 . | July 2018-Dec. 2020 . | . |
| Characteristics . | n = 634 . | n = 737 . | . |
| Sex | .114 | ||
| Male | 394 (62.1%) | 426 (57.8%) | |
| Female | 240 (37.9%) | 311 (42.2%) | |
| Age, median (IQR), y | 66.0 (51.2-75.0) | 66.0 (54.0-74.0) | .502 |
| Age, y | .958 | ||
| 0-74 | 474 (74.8%) | 553 (75.0%) | |
| 75+ | 160 (25.2%) | 184 (25.0%) | |
| Site of primary melanoma | .556 | ||
| Extremities | 267 (42.1%) | 305 (41.4%) | |
| Head or neck | 65 (10.3%) | 64 (8.7%) | |
| Trunk | 276 (43.5%) | 343 (46.5%) | |
| Palm or subungual | 26 (4.1%) | 25 (3.4%) | |
| Subtype of primary melanoma | .834 | ||
| Superficial spreading melanoma | 292 (46.1%) | 348 (47.2%) | |
| Lentigo maligna melanoma | 7 (1.1%) | 10 (1.4%) | |
| Nodular melanoma | 211 (33.3%) | 248 (33.6%) | |
| Acral lentiginous melanoma | 23 (3.6%) | 27 (3.7%) | |
| Other | 61 (9.6%) | 70 (9.5%) | |
| Not reported | 40 (6.31%) | 34 (4.6%) | |
| Breslow thickness, median (IQR), mm | 3.00 (1.70-5.00) | 3.20 (1.90-5.20) | .154 |
| Breslow thickness | .002 | ||
| 0.1-0.7 | 23 (3.6%) | 10 (1.4%) | |
| 0.8-1.0 | 22 (3.4%) | 13 (1.8%) | |
| 1.1-2.0 | 166 (26.2%) | 179 (24.3%) | |
| 2.1-4.0 | 171 (27.0%) | 255 (34.6%) | |
| >4.0 | 239 (37.7%) | 264 (35.8%) | |
| Not reported | 13 (2.1%) | 16 (2.2%) | |
| Tumor ulceration | .336 | ||
| No | 304 (47.9%) | 330 (44.8%) | |
| Yes | 314 (49.5%) | 381 (51.7%) | |
| Not reported | 16 (2.52%) | 26 (3.53%) | |
| Node (N) category | .621 | ||
| N1 | 434 (68.5%) | 522 (70.8%) | |
| N2 | 146 (23.0%) | 155 (21.0%) | |
| N3 | 54 (8.5%) | 60 (8.1%) | |
| Type of locoregional spread | .011 | ||
| Occult lymph node (LN) metastasis | 498 (78.5%) | 602 (81.7%) | |
| Clinically detected LN metastasis | 84 (13.2%) | 99 (13.4%) | |
| In transit or satellite metastasis | 46 (7.3%) | 36 (4.9%) | |
| Not reported | 6 (1.0%) | 0 (0.0%) | |
| Tumor stage (AJCC 8) | .020 | ||
| IIIA | 144 (22.7%) | 119 (16.1%) | |
| IIIB | 121 (19.1%) | 173 (23.5%) | |
| IIIC | 325 (51.3%) | 398 (54.0%) | |
| IIID | 21 (3.3%) | 20 (2.7%) | |
| Not reported | 23 (3.6%) | 27 (3.7%) |
AJCC = American Joint Committee on Cancer; IQR = interquartile range.
Baseline characteristics of stage III melanoma cases diagnosed before or after July 2018, based on the timepoint when adjuvant treatment was introduced in Swedena
| . | Precohort, diagnosis . | Postcohort, diagnosis . | P . |
|---|---|---|---|
| . | Jan. 2016-June 2018 . | July 2018-Dec. 2020 . | . |
| Characteristics . | n = 634 . | n = 737 . | . |
| Sex | .114 | ||
| Male | 394 (62.1%) | 426 (57.8%) | |
| Female | 240 (37.9%) | 311 (42.2%) | |
| Age, median (IQR), y | 66.0 (51.2-75.0) | 66.0 (54.0-74.0) | .502 |
| Age, y | .958 | ||
| 0-74 | 474 (74.8%) | 553 (75.0%) | |
| 75+ | 160 (25.2%) | 184 (25.0%) | |
| Site of primary melanoma | .556 | ||
| Extremities | 267 (42.1%) | 305 (41.4%) | |
| Head or neck | 65 (10.3%) | 64 (8.7%) | |
| Trunk | 276 (43.5%) | 343 (46.5%) | |
| Palm or subungual | 26 (4.1%) | 25 (3.4%) | |
| Subtype of primary melanoma | .834 | ||
| Superficial spreading melanoma | 292 (46.1%) | 348 (47.2%) | |
| Lentigo maligna melanoma | 7 (1.1%) | 10 (1.4%) | |
| Nodular melanoma | 211 (33.3%) | 248 (33.6%) | |
| Acral lentiginous melanoma | 23 (3.6%) | 27 (3.7%) | |
| Other | 61 (9.6%) | 70 (9.5%) | |
| Not reported | 40 (6.31%) | 34 (4.6%) | |
| Breslow thickness, median (IQR), mm | 3.00 (1.70-5.00) | 3.20 (1.90-5.20) | .154 |
| Breslow thickness | .002 | ||
| 0.1-0.7 | 23 (3.6%) | 10 (1.4%) | |
| 0.8-1.0 | 22 (3.4%) | 13 (1.8%) | |
| 1.1-2.0 | 166 (26.2%) | 179 (24.3%) | |
| 2.1-4.0 | 171 (27.0%) | 255 (34.6%) | |
| >4.0 | 239 (37.7%) | 264 (35.8%) | |
| Not reported | 13 (2.1%) | 16 (2.2%) | |
| Tumor ulceration | .336 | ||
| No | 304 (47.9%) | 330 (44.8%) | |
| Yes | 314 (49.5%) | 381 (51.7%) | |
| Not reported | 16 (2.52%) | 26 (3.53%) | |
| Node (N) category | .621 | ||
| N1 | 434 (68.5%) | 522 (70.8%) | |
| N2 | 146 (23.0%) | 155 (21.0%) | |
| N3 | 54 (8.5%) | 60 (8.1%) | |
| Type of locoregional spread | .011 | ||
| Occult lymph node (LN) metastasis | 498 (78.5%) | 602 (81.7%) | |
| Clinically detected LN metastasis | 84 (13.2%) | 99 (13.4%) | |
| In transit or satellite metastasis | 46 (7.3%) | 36 (4.9%) | |
| Not reported | 6 (1.0%) | 0 (0.0%) | |
| Tumor stage (AJCC 8) | .020 | ||
| IIIA | 144 (22.7%) | 119 (16.1%) | |
| IIIB | 121 (19.1%) | 173 (23.5%) | |
| IIIC | 325 (51.3%) | 398 (54.0%) | |
| IIID | 21 (3.3%) | 20 (2.7%) | |
| Not reported | 23 (3.6%) | 27 (3.7%) |
| . | Precohort, diagnosis . | Postcohort, diagnosis . | P . |
|---|---|---|---|
| . | Jan. 2016-June 2018 . | July 2018-Dec. 2020 . | . |
| Characteristics . | n = 634 . | n = 737 . | . |
| Sex | .114 | ||
| Male | 394 (62.1%) | 426 (57.8%) | |
| Female | 240 (37.9%) | 311 (42.2%) | |
| Age, median (IQR), y | 66.0 (51.2-75.0) | 66.0 (54.0-74.0) | .502 |
| Age, y | .958 | ||
| 0-74 | 474 (74.8%) | 553 (75.0%) | |
| 75+ | 160 (25.2%) | 184 (25.0%) | |
| Site of primary melanoma | .556 | ||
| Extremities | 267 (42.1%) | 305 (41.4%) | |
| Head or neck | 65 (10.3%) | 64 (8.7%) | |
| Trunk | 276 (43.5%) | 343 (46.5%) | |
| Palm or subungual | 26 (4.1%) | 25 (3.4%) | |
| Subtype of primary melanoma | .834 | ||
| Superficial spreading melanoma | 292 (46.1%) | 348 (47.2%) | |
| Lentigo maligna melanoma | 7 (1.1%) | 10 (1.4%) | |
| Nodular melanoma | 211 (33.3%) | 248 (33.6%) | |
| Acral lentiginous melanoma | 23 (3.6%) | 27 (3.7%) | |
| Other | 61 (9.6%) | 70 (9.5%) | |
| Not reported | 40 (6.31%) | 34 (4.6%) | |
| Breslow thickness, median (IQR), mm | 3.00 (1.70-5.00) | 3.20 (1.90-5.20) | .154 |
| Breslow thickness | .002 | ||
| 0.1-0.7 | 23 (3.6%) | 10 (1.4%) | |
| 0.8-1.0 | 22 (3.4%) | 13 (1.8%) | |
| 1.1-2.0 | 166 (26.2%) | 179 (24.3%) | |
| 2.1-4.0 | 171 (27.0%) | 255 (34.6%) | |
| >4.0 | 239 (37.7%) | 264 (35.8%) | |
| Not reported | 13 (2.1%) | 16 (2.2%) | |
| Tumor ulceration | .336 | ||
| No | 304 (47.9%) | 330 (44.8%) | |
| Yes | 314 (49.5%) | 381 (51.7%) | |
| Not reported | 16 (2.52%) | 26 (3.53%) | |
| Node (N) category | .621 | ||
| N1 | 434 (68.5%) | 522 (70.8%) | |
| N2 | 146 (23.0%) | 155 (21.0%) | |
| N3 | 54 (8.5%) | 60 (8.1%) | |
| Type of locoregional spread | .011 | ||
| Occult lymph node (LN) metastasis | 498 (78.5%) | 602 (81.7%) | |
| Clinically detected LN metastasis | 84 (13.2%) | 99 (13.4%) | |
| In transit or satellite metastasis | 46 (7.3%) | 36 (4.9%) | |
| Not reported | 6 (1.0%) | 0 (0.0%) | |
| Tumor stage (AJCC 8) | .020 | ||
| IIIA | 144 (22.7%) | 119 (16.1%) | |
| IIIB | 121 (19.1%) | 173 (23.5%) | |
| IIIC | 325 (51.3%) | 398 (54.0%) | |
| IIID | 21 (3.3%) | 20 (2.7%) | |
| Not reported | 23 (3.6%) | 27 (3.7%) |
AJCC = American Joint Committee on Cancer; IQR = interquartile range.
The median time of follow-up in the precohort was 57 months (range = 42-72 months) and in the postcohort was 27 months (range = 12-42 months). The 2-year melanoma-specific survival rate was 87.0% (95% CI = 84.3% to 89.8%) and 88.9% (95% CI = 86.4% to 91.5%) in the precohort and the postcohort, respectively. The unadjusted HR was 0.92 (95% CI = 0.69 to 1.24, P = .60) (Figure 1, A). The 2-year overall survival rate was 84.3% (95% CI = 81.4% to 87.3%) and 86.1% (95% CI = 83.4% to 89.0%) in the precohort and postcohort, respectively, with an unadjusted HR of 0.93 (95% CI = 0.72 to 1.21, P = .60) (Figure 1, B). A post hoc power analysis based on an 85% survival rate in untreated stage III patients at 2 years indicated that with the sample size of the precohort (n = 634) and postcohort (n = 737), a survival gain of 5% or more should be detected in this study (80% power and an alpha of 5%).
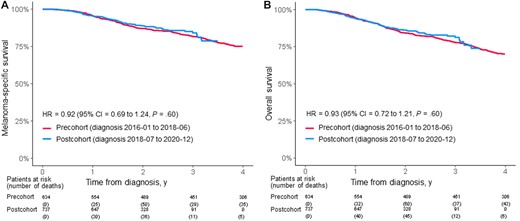
Kaplan-Meier estimate of melanoma-specific survival (A) and overall survival (B) in stage III melanoma patients diagnosed before (precohort) or after (postcohort) July 2018 (based on the timepoint when adjuvant treatment was introduced in Sweden).
The hazard ratio, adjusted for age (as a continuous variable), sex, and AJCC stage, in the precohort and postcohort was, for melanoma-specific survival, 0.92 (95% CI = 0.69 to 1.24, P = .59) and for overall survival, 0.91 (95% CI = 0.70 to 1.19, P = .51) (Figures 2 and 3). The forest plots further show hazard ratios for survival in the precohort and postcohort in different subgroups, grouped depending on age (adjusted for sex and AJCC stage), sex (adjusted for age and AJCC stage), or tumor staging characteristics (adjusted for age and sex). No statistically significant differences were noted for melanoma-specific (Figure 2) or overall survival (Figure 3) for any of the subgroups.
![Forest plot with melanoma-specific survival in subgroups. An adjusted Cox model was used to estimate the hazard ratios for the risk of death from melanoma in stage III melanoma patients diagnosed before (precohort) or after (postcohort) July 2018 (based on the timepoint when adjuvant treatment was introduced in Sweden). Patients were grouped depending on age (adjusted for sex and American Joint Committee on Cancer staging system, eighth edition [AJCC-8 stage], sex [adjusted for age and AJCC-8 stage] or tumor staging characteristics [adjusted for age and sex]). CI = confidence interval; HR = hazard ratio.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/115/9/10.1093_jnci_djad081/2/m_djad081f2.jpeg?Expires=1750210357&Signature=y7boKjyC6oc5ar0Z6skz7XnWLCNsuSIommU5gmb5ET2d-ujGGuU-yUopUdZ3YHaKddEBEem0krpFmEZcx~h7prMXRr4D3be0NCzqPOWU5tHYXsvcEtMD490Ii7-eheq06vFsdc1AAUuYvKBDSvG-e9sk9RETJr35rG-J8WCkfFhZvt9ONvbEEm0qb-mtDj4nVJb8eaQ7OkC7brNY2PiuoEv50kzSu2pvzF6zedKu92AruqDO99liqei199Bq646bejFSfJg241PATYVD9uJO-Cy5oviGwwxT6j0MAFuvdZXCoRTz5tWhTjIG-AocfVbh-BvVAZSEkBV6za5dX5vaLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Forest plot with melanoma-specific survival in subgroups. An adjusted Cox model was used to estimate the hazard ratios for the risk of death from melanoma in stage III melanoma patients diagnosed before (precohort) or after (postcohort) July 2018 (based on the timepoint when adjuvant treatment was introduced in Sweden). Patients were grouped depending on age (adjusted for sex and American Joint Committee on Cancer staging system, eighth edition [AJCC-8 stage], sex [adjusted for age and AJCC-8 stage] or tumor staging characteristics [adjusted for age and sex]). CI = confidence interval; HR = hazard ratio.
![Forest plot with overall survival in subgroups. An adjusted Cox model was used to estimate the hazard ratios for the risk of death from any cause in stage III melanoma patients diagnosed before (precohort) or after (postcohort) July 2018 (based on the timepoint when adjuvant treatment was introduced in Sweden). Patients were grouped depending on age (adjusted for sex and American Joint Committee on Cancer staging system, eighth edition [AJCC-8 stage], sex [adjusted for age and AJCC-8 stage] or tumor staging characteristics [adjusted for age and sex]). HR = hazard ratio.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/115/9/10.1093_jnci_djad081/2/m_djad081f3.jpeg?Expires=1750210358&Signature=zhR8kVsYIgfcXB2cIEse8o9zp53cCG6TxQEfFK6VCn7hQpakvCzHyAqzsJWh0gZWAGFWfv8zLpFiscD6WIYZimIbcNgBkPeYKypSPRmzh7OTi8xQaHrU48GO8rBe8iQ-W0srNklpCFEBRGC5tj8XvPrwkiJbB-B3I8wC~OXRr~ufUmnzy6f99OOrUSU2rIiAzXOLvy0IUOwcQT4YJX-OFIUSuCtAmcalOgwKANhNFXtbFBi2x0Gq2qcnsXNpmbq~GKx6GGyRAos43SYCLu-DVHi0cz6TnJCDrR6hNOZeYsnbZnVsTzFDXCbVnY5Vr~Qr69giMgymxgJhuleg-d4R2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Forest plot with overall survival in subgroups. An adjusted Cox model was used to estimate the hazard ratios for the risk of death from any cause in stage III melanoma patients diagnosed before (precohort) or after (postcohort) July 2018 (based on the timepoint when adjuvant treatment was introduced in Sweden). Patients were grouped depending on age (adjusted for sex and American Joint Committee on Cancer staging system, eighth edition [AJCC-8 stage], sex [adjusted for age and AJCC-8 stage] or tumor staging characteristics [adjusted for age and sex]). HR = hazard ratio.
Discussion
As a result of the EMA approvals for PD-1 inhibitor and BRAF+MEK inhibitor adjuvant treatments in stage III melanoma, the treatments were implemented for this indication in Sweden, a country with a tax-funded public health-care system, treatments freely available for all residents, and a high adherence to national treatment guidelines. The present study shows that there are still no apparent positive effects on the overall survival in our population after the introduction of adjuvant treatments. Based on the current prices in Sweden for the adjuvant agents used in stage III melanoma, we estimate that the annual cost for the drugs (not including hospital visits, tests, pharmacy handling, etc) at approximately 150 million SEK (13 million USD or 14 million EUR), which is 2% of the gross national annual budget for all oncological and hematological pharmaceuticals (7500 million SEK) (22). Certainly, there are other health- and economy-related factors to consider, including the considerable side effects associated with adjuvant therapy that can become life-threatening or chronic. The considerable benefit to patients and society from preventing relapses is also relevant. In both the COMBI-AD and EORTC 1325/KEYNOTE-054 studies, the distant metastasis-free survival difference was 17% at 2 years (23,24). Hence the adjuvant treatment also accordingly lowers the cost of treatment needed in the metastatic setting. The numbers needed to treat to prevent 1 recurrence has been estimated at 6 or 7 in the patients in the COMBI-AD and EORTC 1325/KEYNOTE-054 studies, respectively, and the number needed to harm (to cause a treatment related grade ≥3 event) has been estimated at 3 or 7 patients in these studies, respectively (25). Hence, most melanoma patients receiving the approved adjuvant treatments do not themselves have a benefit from the treatment. Moreover, the cost to prevent 1 relapse using PD-1 inhibitors has been estimated at close to 1 million USD (26). From a patient perspective, the importance of not having a relapse must be appreciated. There is, however, a clear need for better tools to identify patients that will have a survival benefit from adjuvant treatment than at present. Such methodologies are currently under clinical investigation in prospective trials, including the use of genetic profiling (Melagenix; NivoMela trial, NCT04309409) and liquid biopsies (ctDNA; DETECTION trial, NCT04901988). Results from these trials, together with the overall survival data COMBI-AD and EORTC 1325/KEYNOTE-054 studies, will be implemental, but results are not anticipated for several years. Other retrospective real-world studies have been carried out, assessing efficacy and tolerability in melanoma patients that have received adjuvant PD-1 or BRAF+MEK inhibitors at single or multiple centers (27-30). A common finding has been that the relapse-free survival rate and tolerability are similar as reported in the clinical trials. We have not identified any other study that, like the present study, was a nationwide, population-based registry study or a study comparing survival before or after the introduction of adjuvant treatment.
The strength of the study is the population-based design covering a whole nation and the inclusive nature of the Swedish health-care system as well as high adherence to national guidelines. The main limit is the length of the follow-up, which, due to the nature of the study, is shorter in the postcohort (median = 27 months) than in the precohort (median = 57 months). In all the studies leading to approval of the agents, most relapses occurred during the first year, with an evident divergence of the relapse-free survival curves in favor of the adjuvant arm already at 6 months (7,8,23,31). In the EORTC 1325/KEYNOTE-054 study, the 2-year relapse-free survival was 68.3% and 47.1%, with a difference of more than 20% in favor of the patients receiving adjuvant treatment (23). A power calculation showed that at 2 years, our study is powered to detect survival differences of 5% or more. This indicates that if there is an overall survival benefit in patients receiving adjuvant treatment, it is probably substantially smaller than the relapse-free survival benefit. We plan to follow the defined precohort and postcohort, also for the next years to come, where additional events hopefully will give more insights. Another possible limitation is that there were some statistically significant differences in the tumor-specific characteristics, including thicker melanomas and less stage IIIA and in transit metastasis in the postcohort (possibly affected by the COVID-19 pandemic). Adjustments for these factors in the multivariable analyses did not, however, statistically significantly change the results. Throughout the pandemic, oncologic centers and treatment facilities in Sweden retained their previous capacities. There are no groups that we have identified that seem to have benefited. In fact, in the patient groups that would have been most likely to have received adjuvant treatment, there is no positive trend in the melanoma-specific survival, including the younger patients (HR = 1.09, 95% CI = 0.74 to 1.62), patients with ulcerated tumors (HR = 0.97, 95% CI = 0.69 to 1.37), and patients in stage IIIB (HR = 0.91, 95% CI = 0.42 to 2.00) or stage IIIC-D (0.95, 95% CI = 0.69 to 1.32).
The SweMR register is a comprehensive register of diagnosed melanomas and does not include oncologic treatments. The authors of this study have substantial insight into the oncological practices on a national level because we are melanoma specialists practicing in different regions of the country and have had prominent roles in the introduction of adjuvant melanoma treatment in Sweden. The authors are leaders and members of national steering committees, including The National Committees for Treatment Guidelines in Melanoma (Svenska Vårdprogramsgruppen för Melanom), The National Workgroup New Cancer Treatments (NAC), The Swedish Melanoma Study Group (SMSG), and SweMR as well as advisors to the Swedish Melanoma Patient Advocacy Group (Melanomföreningen). There are representatives in these groups from the melanoma oncology clinics in Sweden that are all in public hospitals that provide population-based health-care services in each of the 6 Swedish health-care regions (Region South, Region West, Region South-East, Region Stockholm, Region Mid-Sweden, and Region North). The National Clinical Cancer Care Guidelines are published and sustained by the Swedish Regional Cancer Center collaboration (32). After national approval, adjuvant treatment was implemented in all the melanoma oncology clinics in Sweden because these are obliged to follow the national guidelines. Owing to the study design, addressing the whole-population survival before or after the nationwide introduction in 2018, we have not analyzed individual data on received treatments. Due to the strict adherence to the national guidelines, patients did not receive adjuvant treatment before the national introduction. During the study period, there were 2 ongoing adjuvant studies in Sweden, including stage III melanoma, the placebo-controlled EORTC 1325/KEYNOTE-054 (open March to July, 2016), which on a national level included 4 patients, and the nonrandomized phase IIIb study COMBI-Aplus (open October 2018 to September 2019), with 9 patients included, all receiving dabrafenib and trametinib (4,33). Hence, we estimate that there were 2 patients (resulting from the 1:1 random assignment) that received adjuvant therapy (anti-PD-1) in the precohort, whereas all the patients receiving BRAF+MEK inhibitors in COMBI-Aplus were in the postcohort, that is, were treated after the approval of this treatment. In Sweden, adjuvant treatments with ipilimumab or IFNalpha-2b were never approved or implemented.
Studies on neoadjuvant immune checkpoint inhibitors in stage III-IV melanoma with resectable metastases have demonstrated superior efficiency when initiating the therapy with the tumor still in place (34-36). The benefit is believed to be related to the presence of loads of tumor antigens and tumor infiltrating lymphocytes reacting to immunotherapy. Translating the biological rational from the neoadjuvant treatments, it could be advantageous to have close follow-up in patients operated for high-risk melanomas (stage II or stage III with occult metastases) and treat only relapsing patients (37). To summarize the findings from Sweden, no improvement in survival can be detected after the introduction of adjuvant melanoma treatment in 2018. Although overall data from the EORTC 1325/KEYNOTE-054 study are still awaited, we believe that this finding is of potential interest to the melanoma community.
Data availability
Summary statistical data will be available from the corresponding author on reasonable request. Data including individual participant data from the Swedish Melanoma Registry (SweMR) are not available for sharing.
Author contributions
Hildur Helgadottir, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing), Lars Ny, MD, PhD (Investigation; Methodology; Writing—review & editing), Gustav J. Ullenhag, MD, PhD (Investigation; Methodology; Writing—review & editing), Johan Falkenius, MD, PhD (Conceptualization; Investigation; Methodology), Rasmus Mikiver, BSc (Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing—review & editing), Roger Olofsson Bagge, MD, PhD (Investigation; Methodology; Writing—review & editing), Karolin Isaksson, MD, PhD (Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing—review & editing).
Funding
This work was supported by Regional Cancer Centers (RCC) in Sweden and with grants from the Swedish Cancer society (20 0156 F and 21 1486 Pj), from Region Stockholm (grant number 20200638), the Cancer Research Funds of Radiumhemmet (grant numbers 194092 and 224023), and governmental funding for health-care research (ALF) (grant number 2021-YF0069).
Conflicts of interest
HH reports having received speaker honorarium from Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD), and Pierre Fabre and has served on advisory boards for MSD and Novartis. LN reports having received institutional research grants from Merck and Syndax Pharmaceuticals; speaker honoraria from BMS, Johnson&Johnson, MSD, and Novartis; has served on advisory boards for BMS, MSD, Novartis, Pierre Fabre, Sanofi Genzyme, and Zealth; and is a shareholder in SATMEG Ventures AB. GU reports having received honoraria for teaching activities by BMS, MSD, Pierre Fabre, and Novartis; and for consulting or advising by BMS, Novartis, MSD, Sanofi, Alligator Bioscience, SeqCure Immunology AB, and Ilya Pharma; and is a stockowner in ESSITY and Oncopeptides. ROB reports having received institutional research grants from BMS, Endomagnetics Ltd (Endomag), and SkyLineDx; speaker honorarium from Roche, Pfizer, and Pierre-Fabre; has served on advisory boards for Amgen, BMS, MSD, BD/BARD, Novartis, Roche, and Sanofi Genzyme; and is a shareholder in SATMEG Ventures AB. KI reports having received speaker honorarium from Pierre Fabre. JF and RM declared no conflicts of interest.
Acknowledgements
The authors thank the many clinicians and other personnel working with the reporting to and administration of the Swedish Melanoma Registry (SweMR) since the start of the registry in 1990 and the cause of death registry since the start of the registry in 1960. The funders had no involvement in study design, data collection, analysis or interpretation of data, writing the manuscript, or deciding to submit the manuscript.
References
Statistics Sweden (SCB). Summary of Population Statistics 1960–2022. https://www.scb.se/en/finding-statistics/statistics-by-subject-area/population/population-composition/population-statistics/#_Keyfigures. Accessed December 2,
International Agency for Research on Cancer (IARC) of the World Health Organization (WHO). Globocan


