-
PDF
- Split View
-
Views
-
Cite
Cite
Simron Singh, Thomas A Hope, Emily B Bergsland, Lisa Bodei, David L Bushnell, Jennifer A Chan, Beth R Chasen, Aman Chauhan, Satya Das, Arvind Dasari, Jaydira Del Rivero, Ghassan El-Haddad, Karyn A Goodman, Daniel M Halperin, Mark A Lewis, O Wolf Lindwasser, Sten Myrehaug, Nitya P Raj, Diane L Reidy-Lagunes, Heloisa P Soares, Jonathan R Strosberg, Elise C Kohn, Pamela L Kunz, NET CTPM participants , Consensus report of the 2021 National Cancer Institute neuroendocrine tumor clinical trials planning meeting, JNCI: Journal of the National Cancer Institute, Volume 115, Issue 9, September 2023, Pages 1001–1010, https://doi.org/10.1093/jnci/djad096
Close - Share Icon Share
Abstract
Important progress has been made over the last decade in the classification, imaging, and treatment of neuroendocrine neoplasm (NENs), with several new agents approved for use. Although the treatment options available for patients with well-differentiated neuroendocrine tumors (NETs) have greatly expanded, the rapidly changing landscape has presented several unanswered questions about how best to optimize, sequence, and individualize therapy. Perhaps the most important development over the last decade has been the approval of 177Lu-DOTATATE for treatment of gastroenteropancreatic-NETs, raising questions around optimal sequencing of peptide receptor radionuclide therapy (PRRT) relative to other therapeutic options, the role of re-treatment with PRRT, and whether PRRT can be further optimized through use of dosimetry among other approaches. The NET Task Force of the National Cancer Institute GI Steering Committee convened a clinical trial planning meeting in 2021 with multidisciplinary experts from academia, the federal government, industry, and patient advocates to develop NET clinical trials in the era of PRRT. Key clinical trial recommendations for development included 1) PRRT re-treatment, 2) PRRT and immunotherapy combinations, 3) PRRT and DNA damage repair inhibitor combinations, 4) treatment for liver-dominant disease, 5) treatment for PRRT-resistant disease, and 6) dosimetry-modified PRRT.
Neuroendocrine neoplasms (NENs) comprise a highly diverse group of tumors that are classified by the World Health Organization (WHO) based on the site of origin, pathologic grade, and degree of differentiation. WHO grade is determined by the proliferative indices of Ki-67 and mitotic index (MI): grade 1 (Ki-67 < 3%, MI < 2/2 mm2), grade 2 (Ki-67 3%-20%, MI 2-20/2 mm2), and grade 3 (Ki-67 > 20%, MI > 20/2 mm2). Degree of differentiation is subdivided into well-differentiated neuroendocrine tumors (NETs) or poorly differentiated neuroendocrine carcinomas (NECs) (1). Notably, the 2019 WHO Classification for gastroenteropancreatic (GEP)-NETs further subdivided the grade 3 classification into 1) well-differentiated NET grade 3, and 2) poorly differentiated NEC (2). The most common sites of origin are the lung, pancreas, and small bowel. NETs from the gastrointestinal tract and pancreas are commonly referred to as gastroenteropancreatic or GEP-NETs. Many NETs can release neuropeptides and hormones such as serotonin, leading to symptoms of hormone hypersecretion.
In GEP-NET patients who are not upfront surgical candidates, systemic treatment typically begins with somatostatin analogs (SSAs) (3,4), which target somatostatin receptors (SSTR) found on the surface of most NET cells. SSAs are currently the foundation of most GEP-NET treatment because they are used to both treat hormone-related symptoms as well as delay progression of disease with favorable outcomes. In pancreatic NETs (PNETs), second-line treatment frequently consists of chemotherapies such as capecitabine and temozolomide or other targeted agents, including sunitinib and everolimus (5,6). Everolimus can also be used for gastrointestinal and lung NETs (7).
Perhaps the most important therapeutic development for NETs in the last decade is the approval of 177Lu-DOTATATE therapy for treatment of GEP-NETs. 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) is a form of radioligand therapy where an SSA is labeled with a beta-emitting radionuclide, 177Lutetium. This form of systemic radiation has led to an important shift in the treatment paradigm for NETs. After over 20 years of single-arm studies, mainly in Europe and Australia, most recently, 177Lu-DOTATATE was studied in the pivotal phase III NETTER-1 study and displayed superiority in progression-free survival compared with high-dose SSAs in patients with progressive, well-differentiated, low- and intermediate-grade small bowel NETs. At the time of the initial publication, the median progression-free survival (PFS) was not reached in the 177Lu-DOTATATE arm and was 8.4 months in the control arm (hazard ratio = 0.21, 95% confidence interval = 0.13 to 0.33, P < .001). Response rate (RR) was 18% in the 177Lu-DOTATATE arm and 3% in the control arm (8). In an updated analysis, there was no statistical difference in overall survival: 48 months in the 177Lu-DOTATATE arm and 36.3 months in the control arm. In addition, there were no new safety signals; notably, 2% of patients in the 177Lu-DOTATATE arm developed myelodysplastic syndrome. The US Food and Drug Administration (FDA) approval for 177Lu-DOTATATE included all GEP-NETs based on single-arm registries in Europe in addition to NETTER-1 (9,10). Although the treatment options available for patients with well-differentiated NETs have greatly expanded, this new wealth of options creates its own challenge because lines of therapy must be optimally sequenced and considered in the context of any individual patient’s longitudinal treatment course.
Clinical trial planning meeting (CTPM) background
The National Cancer Institute (NCI) National Clinical Trials Network (NCTN) is a cooperative network comprising 5 clinical trial groups across the United States and Canada (Alliance, Canadian Cancer Trials Group [CCTG], Eastern Cooperative Oncology Group-American College of Radiology Imaging Network [ECOG-ACRIN], NRG, and Southwest Oncology Group [SWOG]). Numerous practice-changing trials have emerged from the NCI and NCTN task forces and trial mechanisms, including the recently concluded E2211 trial (a phase II study of capecitabine and temozolomide vs temozolomide alone in patients with advanced PNET) (11). In the setting of a rapidly changing therapeutic landscape, the NCI steering committees may call for a dedicated CTPM to prioritize and develop the next generation of trials to move the field forward and improve patient outcomes.
In 2009, the NET Task Force of the NCI GI Steering Committee (GISC) convened a CTPM, which made many key recommendations: evaluate primary sites and low- vs high-grade disease separately, evaluate novel therapies for carcinoid syndrome, develop prospective studies of liver directed therapies, examine PRRT in a phase III clinical trial, and use progression-free survival as the most relevant primary end point for phase II and III studies (12). This CTPM led to the development and conduct of key NCTN clinical trials: CALGB80701 (everolimus vs everolimus + bevacizumab in metastatic pancreatic NET) (13), S0518 (octreotide + interferon alpha vs octreotide + bevacizumab in poor-prognosis carcinoid patients) (14), A021202 (pazopanib vs placebo in progressive carcinoid) (15), E2211 (temozolomide ± capectitabine in pancreatic NET) (16), EA2142 (platinum chemotherapy with etoposide vs temozolomide + capecitabine in NEC) (NCT02595424), and EA2161 (sapanisertib in everolimus refractory pancreatic NETs) (17). In addition, the 2009 CTPM provided guidance for future clinical trial development in NENs. In the intervening 10 years, the field has changed considerably, with advances in pathologic classification, molecular understanding, diagnostic imaging, and treatment. The last decade has seen an unprecedented number of FDA approvals in the treatment of NETs, including everolimus for GEP-NET (6,18) and lung NETs (7), lanreotide for GEP-NETs (4), sunitinib for PNETs (5), telotristat for carcinoid syndrome diarrhea (19), and 177Lu-DOTATATE for GEP-NETs (20).
With such transformative changes, the NCI GISC decided to convene a new dedicated NET CTPM to address “Treatment in the Era of PRRT.” Originally scheduled for 2020, the NET CTPM took place in March of 2021 due to disruptions from the COVID-19 pandemic. Two years of pre-CTPM discussions and workshops were conducted with NET leadership, including members of the GISC members, NET Task Force, early career investigators, researchers, NCI staff, patient advocates, international NET experts (from Australia and Germany), and invited industry representatives (with limited participation sessions).
The NET CTPM was specifically designed to address questions around optimal sequencing of PRRT relative to other therapeutic options, the role of PRRT re-treatment, enhancing efficacy by using PRRT combination therapies and/or an individualized approach to dosing, and minimizing toxicity to PRRT. There was consensus that answering these questions via clinical trials must be considered in the context of the life cycle of the disease and by disease characteristics such as stage, grade, degree of differentiation, and primary site (Figure 1). Of note, immediately following the March 2021 CTPM, the concept, A022001, a phase II randomized, prospective trial of 177Lu-DOTATATE vs capecitabine and temozolomide in well-differentiated PNETs, was approved by the GISC. This information of approval was not available at the CTPM, and discussions did not include this new concept; however, it will have an impact on the landscape of NEN trials and future trial priorities. In addition, we did not include alpha emitters in the clinical trial concepts due to issues of feasibility given that 212Pb and 225Ac agents are in the very early stages of development and are not part of the CTEP portfolio.
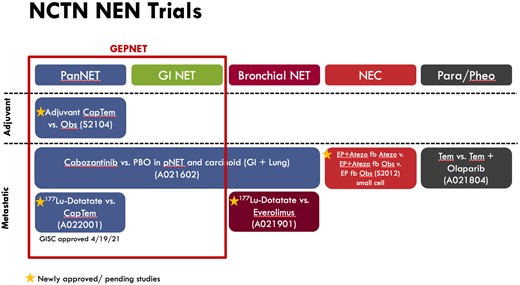
The lifecycle of NET clinical trials. Cap/Tem = capecitabine and temozolomide; EP = etoposide and platinum; fb = followed by; GEP-NET = gastroenteropancreatic neuroendocrine tumor; GI NET = gastrointestinal neuroendocrine tumor; NEC = neuroendocrine carcinoma; Obs = observation; PanNET = pancreatic neuroendocrine tumor; Para/Pheo = paraganglioma and pheochromocytoma; PBO = placebo; Tem = temozolomide.
The NET CTPM focused on helping to chart the path forward in an era of PRRT clinical trials, including a discussion of updated design principles as an important component. The major themes of the CTPM were 1) principles of clinical trial design in the modern era of NET classification and treatment landscape, 2) enhancing efficacy and increasing response to PRRT, 3) the role of subsequent therapy in both PRRT-resistant and PRRT-sensitive populations, and 4) issues related to optimal timing, sequencing, and dosimetry for PRRT. A full executive summary was written by the NCI Steering Committee (21).
Principles of clinical trial design and clinical trial considerations for NETs
Clinical trial design can be challenging in NETs given the indolent natural history, disease heterogeneity with differences in tumor biology, and response to treatment according to primary tumor location, grade, differentiation, and somatostatin receptor expression (22-24). Clinical trials should be designed to account for these factors (12). Accordingly, part of the CTPM meeting was devoted to refining guiding principles of NET clinical trial design that can be used to guide development of new NET trials in the years to come. A summary of considerations for clinical trial design in advanced NETs is detailed in Box 1.
Considerations for clinical trial design in advanced neuroendocrine neoplasmsa
Study design
Clinical trials should be conducted separately for bronchial, midgut and pancreatic NETs and if not feasible, patients should be: a) stratified according to site of origin, and/or b) be enrolled into cohorts of pancreatic and extrapancreatic NET.
Clinical trials should be conducted separately for well-differentiated and poorly differentiated neuroendocrine neoplasms.
Clinical trials including well-differentiated NETs should be stratified according to grade or enroll participants into cohorts based on grade (eg, G1/2 vs G3)
Trials should account for number of prior lines or types of therapy.
For randomized trials using a placebo control, cross-over at the time of disease progression for participants receiving placebo should be considered.
Imaging considerations
Protocols of trials evaluating SSTR targeted therapies should clearly address how SSTR positive disease is defined and how to account for variability in SSTR expression in patients.
Endpoints
PFS remains the recommended primary endpoint for phase III studies in well-differentiated NET.
OS may be an appropriate endpoint for phase II and phase III trials for poorly differentiated NEC.
Inclusion of correlative studies to identify potential biomarkers of efficacy is encouraged.
Trials should include patient-reported outcome measures to provide additional assessment of the impact of treatment for patients.
aNEC = neuroendocrine carcinoma; NET = neuroendocrine tumor; OS = overall survival; SSTR = somatostatin receptor.
Current issues in NETs and trial concepts
The extensive pre-CTPM subcommittee work identified 6 key areas for clinical trial development: 1) PRRT re-treatment, 2) PRRT and immunotherapy combinations, 3) PRRT and DNA damage repair combinations, 4) treatment for liver-dominant disease, 5) treatment for PRRT-resistant disease, and 6) dosimetry-modified PRRT. The following section outlines clinical development in these 6 areas.
PRRT re-treatment
A standard course of 177Lu-DOTATATE consists of one 200-mCi dose given every 8 weeks for a total of 4 doses. Most patients with NETs who benefit from an initial course of PRRT maintain their SSTR positivity at progression, and a SSTR-targeted radioligand therapy theoretically should have an anticancer effect upon rechallenge. Despite routine off-label use of PRRT re-treatment in progressive NETs globally, there are no prospective data investigating re-treatment or risk of therapy-related myeloid neoplasms (tMN) such as acute myeloid leukemia or myelodysplastic syndrome. Several single-center retrospective cohort studies have reported both safety and efficacy with repeat PRRT. In 1 meta-analysis, the median PFS in 414 patients was 12.5 months (95% confidence interval = 9.8 to 15.2 months) (25). Of note, grade 3 and 4 adverse events were reported in only 5% of patients, with no reported cases of tMN (25). A retrospective analysis of 26 patients re-treated with 177Lu-DOTATATE observed a median PFS of 22 months (26). Another retrospective study reported a median PFS of 14.6 months with PRRT re-treatment in 168 GEP-NETs and lung NETs (27). Limitations of the evidence for PRRT re-treatment include small sample sizes, the flaws associated with retrospective studies, and diverse combinations of initial treatments, including 90Y- and 177Lu-based therapies, although re-treatment was always with 177Lu-based therapies. In addition, the concept of PRRT retreatment may evolve with the introduction of alpha emitter therapy (28).
The NET CTPM recognized the need for a PRRT re-treatment trial, which led to the development of a joint CCTG-SWOG randomized phase II trial in well-differentiated, progressive small bowel NETs. This trial tests PRRT re-treatment (defined as 2 doses) vs everolimus as the control arm (NET RETREAT, CCTG NE-1). Eligible patients must have had stable disease for at least 12 months from completion of initial PRRT with no intervening therapies. Re-treatment will be limited to 2 doses to minimize risk of long-term hematologic toxicities. The NET RETREAT study will represent the first prospective study to evaluate PRRT re-treatment efficacy and safety.
PRRT and immunotherapy combinations
To date, single-agent immune checkpoint inhibitors (CPIs) have demonstrated variable activity in the treatment of well-differentiated NETs and extra pulmonary NECs (29-33). This is likely due, at least in part, to the low mutational burden seen in NETs compared with other tumor types that respond to immunotherapy. However, combination immunotherapy with ipilimumab and nivolumab leads to higher response rates for patients with well-differentiated grade 3 NETs as seen in the Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) studies (25%-44%), leading to the recent inclusion of this combination in the NCCN NET guidelines for progressive disease (34,35).
Studies of CPIs in the treatment of small-cell lung cancer, which shares some similarities with poorly differentiated NEC, have demonstrated promising results, and CPIs are now used to treat small-cell lung cancer in the first-line setting with platinum and etoposide chemotherapy and in the progressive setting (36-39). Select studies suggest clinical activity of CPIs in the “higher-grade NENs,” although it remains uncertain if these tumors were NECs, high-grade well-differentiated NETs, or a combination of the two (34,40,41).
It is hypothesized that the irradiation of SSTR-avid disease by 177Lu-DOTATATE causes DNA damage, potentially releasing neoantigens (42). Addition of CPI to 177Lu-DOTATATE could induce immune activation toward these neoantigens and augment the benefit of 177Lu-DOTATATE. Activity of the combination of CPI with external beam radiation has been demonstrated in some, but not all, cancers, with additional trials ongoing (43-46). Four active clinical studies of 177Lu-radiolabeled therapies and CPIs evaluate safety and preliminary activity: prostate (NCT03805594 and NCT03658447), merkel cell carcinoma (NCT04261855), and NET (NCT03457948). Early data from the 2 studies conducted in patients with prostate cancer have demonstrated safety of the combination of 177Lu-PSMA and pembrolizumab; in both studies, promising clinical activity was also noted (47,48). A randomized study of 177Lu-DOTATATE with or without CPI in patients with metastatic and unfavorable biology, SSTR-positive, high-grade well-differentiated NETs was proposed during the NET CTPM. This study would include any line of treatment, and enrolled patients would be stratified by site of tumor origin and line of therapy and is still in development.
PRRT and DNA damage repair combinations
177Lu-DOTATATE is presumed to elicit cell death through creating single-strand DNA breaks that devolve into double-strand breaks (49). The clinical activity of single-agent 177Lu-DOTATATE is meaningful; however, an important subset of patients with NETs do not experience prolonged PFS (10,20). Therefore, there is an opportunity to develop combination approaches to enhance efficacy and treat refractory disease. One class of agents that carries this potential is the DNA damage repair inhibitors. These inhibitors augment injury by allowing devolution into DNA double-strand breaks that either kill the cell through augmentation of replication stress (50) or propagate injurious changes that lead to apoptosis (51-53). Several phase I studies in patients with GEP-NETs are ongoing or soon commencing, combining 177Lu-DOTATATE with the ribonucleotide reductase inhibitor triapine (NCT04234568), the DNA-dependent protein kinase inhibitor peposertib (NCT04750954), and the poly-ADP ribose polymerase (PARP) inhibitor olaparib (NCT04086485). In considering combination therapies with PARP inhibitors, it will also be important to assess the long-term risks of tMNs. The rate of MDS following 177Lu-DOTATATE is 2%; however, rates have been reported as high as 8% when PRRT is given combination or sequence with alkylating agents (54).
Once the recommended phase II doses of each of these combinations are established, it may be difficult to select which combination should move forward in a comparative study against 177Lu-DOTATATE monotherapy. To answer this question, a randomized, multi-arm phase II/III study of each of these combinations vs 177Lu-DOTATATE may be considered in the future. A design of this type could use a pick-the-winner approach whereby the winning arm (defined by the primary endpoint) in phase II could carry forward to phase III.
Dosimetry-modified PRRT
One unique feature of 177Lu-DOTATATE is its gamma emission from the 177Lu atom, thereby allowing imaging with Single Photon Emission Computed Tomography (SPECT)/Computed Tomography (CT). This enables quantitative measurement of deposited energy to the tumor and normal organs, which is termed dosimetry. Currently PRRT is administered in a “one dose fits all” model: every patient receives the standard 200 mCi administered every 8 weeks for 4 total doses. PRRT activity occurs through targeted deposit of its radiation dose to tumors, and tumor response has been correlated to dose (55,56). It is postulated that modulating dose to a target lesional absorbed dose (LAD) may improve upon the current standard “one dose fits all” model.
There are 2 main approaches to implementing dosimetry-modified PRRT. The first approach is to increase the administered activity until dose limits to target organs are reached. The kidney has traditionally been considered to be a dose-limiting organ, transposing prior experiences with 90Y and the dose threshold from external radiotherapy (57,58). In addition, it has recently become clear that the bone marrow may also be a major dose-limiting organ with 177Lu-DOTATATE and that the thresholds used for external radiotherapy cannot be applied to PRRT (59,60). The second approach is to target an optimal dose to defined tumors measured by target LAD. This approach may better mirror the strategy of external beam radiation therapy, whereby treatment planning dosimetry is used to deliver precise doses to the tumors. To date, this latter approach has not been tested, although LAD appears to be important in treatment of PNETs (55). A consensus of the appropriate target doses for the LAD method is needed, which may be difficult given the heterogeneity of NETs. Appropriate tumor dose targets may need to be adjusted for factors such as primary sites (61).
One of the main limitations for the implementation of dosimetry-modified PRRT is the heterogeneity of the measurement methods. Before the virtual CTPM, we convened a meeting with interested companies who develop software packages to measure PRRT dose to discuss the underlying variability and encourage development of a more harmonized approach. It is uncertain whether treatment should be modulated based on radiation dose to the lesion with the highest absorbed dose or based on average absorbed dose across lesions or targeted to at-risk organ limits. Another question is how to optimize dosimetry across doses and time to get the most accurate data with the least burden on the patient. Recent results demonstrate that a single–time point approach is feasible (62). The CTPM prioritized development of a randomized trial comparing standard dosing of 177Lu-DOTATATE to a dosimetry-informed dosing strategy, targeting cumulative dose to target lesions of 80-120 Gray.
Treatment for liver-dominant disease
Liver-directed therapies include surgery, ablation, and hepatic arterial embolization. The latter is particularly appropriate for patients with extensive, unresectable liver metastases (49,50). There are several embolization modalities, including bland embolization, chemoembolization, and radioembolization using 90Yttrium spheres. Data supporting the use of embolization in NETs derive almost exclusively from small institutional retrospective series, although several small prospective studies have also validated the use of this technique (63-66). Many studies indicate response rates of approximately 50%, with median time- to progression in the range of roughly 12 to 18 months. In addition, most patients with hormonal syndromes are reported to experience symptomatic responses. The RETNET phase II clinical trial is poised to provide important prospective data comparing bland embolization with chemoembolization in metastatic NET (NCT02724540). Of note, a third arm of this study, using drug eluting beads (drug-eluting bead doxorubicin), was discontinued for toxicity.
One of the most common real-world questions in the treatment of patients with liver-dominant NETs is selecting between liver-directed and systemic therapy such as PRRT. The underlying rationale is that PRRT appears to be more effective in treating smaller tumors (67,68); therefore, cytoreduction of disease before PRRT is hypothesized to improve the efficacy of PRRT. Follow-up analysis of the NETTER-1 trial demonstrated that patients with large liver lesions, those greater than 3 cm, had a worse outcome with PRRT than those with less than 3-cm lesions. This leads to the question of whether LDT should precede PRRT (67). It is proposed that LDT may debulk large liver lesions decreasing a tumor sink and may result in increased dose available to remaining tumors and potentially increasing the efficacy of subsequent PRRT. Multiple trials evaluating the timing of LDT with PRRT were discussed; however, trial development is challenging due to several factors, including choice of primary endpoint (hepatic PFS or overall/whole-body PFS) and patient selection related to volume of extra-hepatic disease.
Data are currently limited regarding additive toxicity of PRRT and liver-directed therapy. One retrospective institutional study suggested that patients who were heavily pretreated with liver-directed therapy experienced more hepatotoxicity with subsequent PRRT (69). The risks and benefits of combination of PRRT with radioembolization are of particular interest. A retrospective series evaluating 27 patients who received both 90Yttrium and PRRT suggested that outcomes were superior in patients receiving both treatments (70). A prospective phase II study of 30 evaluable patients examined radioembolization using 166Holmium following 4 cycles of PRRT with 177Lu-DOTATATE reported an objective response rate in the treated volume of 43% (71). Although most patients in both these studies tolerated the combination of radioembolization and PRRT, it is important to note that 1 patient in each experienced fatal hepatotoxicity. It is likely that patients already showing signs of postradioembolization liver disease are at particularly high risk of hepatotoxicity from PRRT if they have extensive liver tumor burden.
Stereotactic ablative radiotherapy (SABR) has an emerging role in treating NET liver metastases (72,73). There is potential to combine SABR with PRRT for patients with more aggressive metastases with the goal of improved disease control. The CTPM reviewed a proposed study of high-dose SABR to 5 or less fluorodeoxyglucose (FDG)-avid metastases followed by PRRT with the hypothesis that SABR will improve PFS without additive toxicity. This concept will be further developed, with specific questions raised regarding the unknown dosimetric implication of combined external beam radiotherapy and PRRT and how it may be sequenced with other locally ablative modalities.
Treatment for PRRT-resistant disease
Patients with advanced NETs who have progressed on or rapidly following an initial course of PRRT need of novel treatment approaches. At least 10%-20% of patients with NETs receiving PRRT will progress within 18 months (10), although limited data regarding the defining biological underpinnings of PRRT-resistant disease are available (74).
The established progression rate of this patient population after PRRT may provide a unique opportunity for combination therapies to disease resistant to PRRT, such as radio-sensitization with PARP inhibitors (51,52), SSTR upregulation with HDAC inhibitors (75,76), radioimmunotherapy with checkpoint inhibitors, or chemoradiotherapy through combination with capecitabine (77,78). Additional safety and efficacy data from early-phase trials will be required before development of a randomized trial concept evaluating the optimal PRRT-enhancing strategy in this setting.
Summary
Unprecedented diagnostic and therapeutic advances have been made in the field of NETs in the last decade. This success was based largely on a framework established by the first NCI NET CTPM in 2009 that benchmarked key priorities for clinical trial development and design. These advances, most notably 177Lu-DOTATATE, have markedly changed the therapeutic landscape for NETs and generated key questions about enhancing the efficacy of PRRT, as well as its optimal timing, sequencing, and dosimetry. Key clinical trial considerations from the 2021 NET CTPM include 1) PRRT re-treatment, 2) PRRT and immunotherapy combinations, 3) PRRT and DNA damage repair inhibitor combinations, 4) treatment for liver-dominant disease, 5) treatment for PRRT-resistant disease, and 6) dosimetry-modified PRRT. In addition, the changing NET therapeutic landscape also led to key updates to NET clinical trial design elements including recommendations to develop separate clinical trials for well-differentiated and poorly differentiated NENs, use cross-over for randomized trials with placebo arms, incorporate clear definitions of SSTR positive disease, and include patient-reported outcome measures. We are hopeful that the 2021 NET CTPM will provide the foundation for the ensuing decade of NET clinical trials, a decade that is just as impactful as the last.
Data availability
There are no new data associated with this article therefore data sharing is not applicable.
Author contributions
Simron Singh, MD MPH (Conceptualization; Methodology; Supervision; Writing—original draft; Writing—review and editing), Jonathan R. Strosberg, MD (Writing—original draft; Writing—review and editing), Heloisa P. Soares, MD PhD (Conceptualization; Writing—original draft; Writing—review and editing), Diane L. Reidy-Lagunes, MD (Writing—original draft; Writing—review and editing), Nitya P. Raj, MD (Writing—original draft; Writing—review and editing), Sten Myrehaug, MD (Writing—original draft; Writing—review and editing), O. Wolf Lindwasser, PhD (Conceptualization; Writing—original draft; Writing—review and editing), Mark A. Lewis, MD (Conceptualization; Writing—original draft; Writing—review and editing), Daniel M. Halperin, MD (Writing—original draft; Writing—review and editing), Karyn A. Goodman, MD (Conceptualization; Writing—original draft; Writing—review and editing), Elise C. Kohn, MD (Conceptualization; Writing—original draft; Writing—review and editing), Ghassan El-Haddad, MD (Writing—original draft; Writing—review and editing), Arvind Dasari, MD (Conceptualization; Writing—original draft; Writing—review and editing), Satya Das, MD (Writing—original draft; Writing—review and editing), Aman Chauhan, MD (Writing—original draft; Writing—review and editing), Beth R. Chasen, MD (Writing—original draft; Writing—review and editing), Jennifer A. Chan, MD (Conceptualization; Writing—original draft; Writing—review and editing), David L. Bushnell, MD (Conceptualization; Writing—original draft; Writing—review and editing), Lisa Bodei, MD PhD (Writing—original draft; Writing—review and editing), Emily B. Bergsland, MD (Conceptualization; Writing—original draft; Writing—review and editing), Thomas A. Hope, MD (Conceptualization; Methodology; Supervision; Writing—original draft; Writing—review and editing), Jaydira Del Rivero, MD (Writing—original draft; Writing—review and editing), Pamela L. Kunz, MD (Conceptualization; Methodology; Project administration; Supervision; Writing—original draft; Writing—review and editing).
Funding
This work was supported by the National Cancer Institute.
Conflicts of interest
SS: Grants or contracts: AAA/Novartis; Consulting fees: AAA/Novartis, Ipsen; Participation on a Data Safety Monitoring Board or Advisory Board: AAA/Novartis, Ipsen;
TAH: Grants or contracts: GE Healthcare, Philips, Clovis Oncology, AAA/Novartis. Lantheus. Consulting fees: Curium, RayzeBio, ITM, Blue Earth Diagnostics. Participation on a Data Safety Monitoring Board or Advisory Board: Ipsen, Bayer. Leadership role: North American Neuroendocrine Tumor Society. Stock or stock options: RayzeBio.
EBB: Grants or contracts (to institution): Merck. Royalties or licenses: UpToDate. Leadership role: NCCN Neuroendocrine Tumor Panel (unpaid), North American Neuroendocrine Tumor Society (NANETS) Vice President, President, President Emeritus (unpaid).
LB: Grants or contracts: AAA/Novartis. Consulting (nonremunerated): AAA-Novartis, Ipsen, Clovis Oncology, MTTI. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: ITM, Great Point Partners, Iba. Support for attending meetings and/or travel: Great Point Partners. Participation on a Data Safety Monitoring Board: AAA/Novartis (Lu-PSMA-R2). Leadership role: NETRF, ABNM.
DLB: Grants or contracts: University of Iowa NET SPORE (NCI), NIH, RayzeBio.
JAC: Royalties or licenses: UpToDate. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Ipsen. Participation on a Data Safety Monitoring Board: AAA/Novartis, TerSera, Lexicon, Curium. Leadership role: North American Neuroendocrine Tumor Society (Board of Directors/Officer, unpaid). Stock or stock options: Merck.
BRC: None
AC: Grants or contracts (to institution): BMS, Clovis, Tersera; Consulting fees: Ipsen, Lexicon, ITM, Novartis/AAA, Seneca Therapeutics, Crinetics, Tersera, Curium; Receipt of equipment, materials, drugs, medical writing, gifts or other services: Nanopharmaceutics, EMD Serono.
SD: Consulting fees: Ipsen, Tersera, Novartis/AAA; Employment: AstraZeneca
AD: Grants or contracts (to institution): HutchMed, Eisai, Guardant Health, Natera, Enterome, Novartis, Xencor. Consulting fees: HutchMed, Novartis/AAA, Ipsen, Voluntis. Participation on a Data Safety Monitoring Board or Advisory Board: Oncobay.
JDR: None
GH: Consulting fees: Bayer HealthCare, Boston Scientific Corporation, Canon Medical Systems Corporation, Novartis Pharmaceuticals Corporation, Terumo Medical Corporation. Stock or stock options: Johnson and Johnson.
KAG: Participation on a Data Safety Monitoring Board or Advisory Board: DSMB for SMART Trial funded by Viewray, Advisory board for Phillips Medical. Leadership role: Co-chair of the NCI GI Steering Committee.
DMH: Grants or contracts: Tarveda, Genentech, AAA/Novartis, ITM, RayzeBio, Camurus; Consulting Fees: AAA/Novartis, ITM, Camurus, Amryt, TerSera, Crinetics, Chimeric Therapeutics; Participation on a Data Safety Monitoring Board or Advisory Board: AlphaMedix.
MAL: pending
OWL: None
SM: Grants or contracts (to institution): Ipsen, AAA/Novartis; Consulting fees: AAA/Novartis; Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events: AAA/Novartis
NPR: Grants or contracts (to institution): ITM, Corcept therapeutics, Xencor Inc; Support for attending meetings and/or travel advisory boards: Ipsen, HRA Pharma, Progenics pharmaceuticals, AAA/Novartis; Participation on a Data Safety Monitoring Board or Advisory Board: Safety monitoring committee for Crinetics Pharmaceuticals.
DLR: Grants or contracts (to institution): Merck, Novartis, Ipsen. Consulting fees: Chiasma, AAA/Novartis. Leadership role: NANETS.
HPS: Consulting fees: Novartis, TerSera, AstraZeneca, Ipsen, Pfizer; Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events: ITM Isotope Technologies Munich SE. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: NANETS Board of Directors, Healing NET Foundation Board of Directors.
JRS: Grants or contracts: Novartis, Merck, ITM. Consulting fees: Tersera. Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events: Ipsen, Tersera. Support for attending meetings and/or travel: Ipsen.
ECK: None
PLK: Grants or contracts (to institution): AAA/Novartis; Consulting fees: Amgen, Crinetics, Genentech, HutchMed, Ipsen, ITM, Natera, Novartis (Advanced Accelerator Applications), RayzeBio; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: NANETS President.
Acknowledgements
We acknowledge the following CTPM participants for their contributions: Emily Bergsland, MD (University of California San Francisco, Alliance); Tom Beveridge, BSc, PhD (Ipsen Biopharmaceuticals); Lisa Bodei, MD, PhD (Memorial Sloan Kettering Cancer Center, Alliance); Anita Borek, MBA, BS (Genentech); Michelle Brockman, MBA (Genentech US Medical Affairs); David Bushnell, MD (University of Iowa, NET SPORE representative), Jacek Capala, PhD (National Cancer Institute); Jennifer Chan (Dana-Farber Cancer Institute, Alliance); Beth Chasen, MD (MD Anderson Cancer Center, SWOG); Aman Chauhan, MD (Current: University of Miami, SWOG, Previous: University of Kentucky); Satya (Nanu) Das, MD, MSCI (Current: AstraZeneca, Previous: Vanderbilt University Medical Center, ECOG-ACRIN); N. Arvind Dasari, MD, MS (MD Anderson Cancer Center, SWOG); Cynthia Davies-Venn, PhD (Emmes Corp); Jaydira Del Rivero, MD (National Cancer Institute, National Institutes of Health, Alliance); Sandra Demaria, MD (Weill Cornell Medical College); Martha Donoghue, MD (Food and Drug Administration); Jennifer Eads, MD (University of Pennsylvania, ECOG-ACRIN); Ghassan El-Haddad, MD (H Lee Moffitt Cancer Center and Research Institute, SWOG); Natalie Fielman (Leidos Biomedical Research, Inc); Lauren Fishbein, MD, PhD (University of Colorado); Germo Gericke, PhD (Advanced Accelerator Applications); Karyn Goodman, MD (Icahn School of Medicine at Mount Sinai, GI Steering Committee, Alliance, NRG); Daniel Halperin, MD (MD Anderson Cancer Center, SWOG); Andrew Hendifar, MD (Cedars-Sinai Medical Center, SWOG); Rodney Hicks, MB BS, MD (The Peter MacCallum Cancer Centre, Australia); Robert Hobbs, PhD, DABR (Johns Hopkins); Timothy Hobday, MD (Mayo Clinic, Alliance); Thomas Hope, MD (University of California San Francisco, Alliance); Renuka Iyer, MD (Roswell Park Comprehensive Cancer Center); Deborah Jaffe, PhD (National Cancer Institute); Andrew Kennedy, MD, FACRO (Sarah Cannon Research Institute); Elise Kohn, MD (National Cancer Institute); Matthew Kulke, MD (Boston University School of Medicine, Alliance); Charles Kunos MD PhD (National Cancer Institute); Pamela L. Kunz, MD (Yale School of Medicine, ECOG-ACRIN); Mark Lewis, MD (Intermountain Healthcare, SWOG); Frank Lin, MD (National Cancer Institute); Wolf Lindwasser, PhD (National Cancer Institute); Josh Mailman, MBA (NorCal CarciNET Community); Michael McDonald, MD, PhD (National Cancer Institute); Sandy McEwan, MSc, MD, FRCPC (Ipsen); Sten Myrehaug, MD (Odette Cancer Centre, University of Toronto, Canada, CCTG); Antonio Nakasato, MD (Novartis); Steve Nothwehr, PhD (National Cancer Institute); Fang-Shu Ou, PhD (Mayo Clinic, Alliance); Sukhmani Padda, MD; (Previous: Stanford University, ECOG-ACRIN); Current: Fox Chase Cancer Center/Temple University, ECOG-ACRIN; Marianne Pavel, MD (Friedrich-Alexander-University Erlangen- Nürnberg, Germany); Anthony Pilowa, BS (Exelixis, Inc); Nitya Raj, MD (Memorial Sloan Kettering Cancer Center; Alliance); Brian Ramnaraign, MD (University of Florida; NRG); Diane Reidy-Lagunes, MD MS (Memorial Sloan Kettering Cancer Center; Alliance); Larry Rubinstein, PhD (National Cancer Institute); Stephen Saletan, MD (Exelixis); Manisha Shah, MD (Ohio State University; ECOG-ACRIN); Simron Singh, MD, MPH (Odette Cancer Centre, University of Toronto, Canada, CCTG); Heloisa Soares, MD (Huntsman Cancer Institute, University of Utah; NRG); Michael Soulen, MD (University of Pennsylvania, ECOG-ACRIN); Jonathan Strosberg, MD (Moffitt Cancer Center; SWOG); Brian Untch, MD (Memorial Sloan Kettering Cancer Center; Alliance); Mona Wahba, MD, MSM (ITM); Rebecca Wong, MBChB MSc FRCP (Princess Margaret Cancer Center, Canada; CCTG); James Yao, MD (MD Anderson Cancer Center, SWOG).
The funder did not play a role in the content of the Clinical Trial Planning meeting; the writing of the manuscript; and the decision to submit the manuscript for publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
Author notes
Simron Singh and Thomas A. Hope contributed equally to this work.


