-
PDF
- Split View
-
Views
-
Cite
Cite
You-Lin Qiao, Ting Wu, Rong-Cheng Li, Yue-Mei Hu, Li-Hui Wei, Chang-Gui Li, Wen Chen, Shou-Jie Huang, Fang-Hui Zhao, Ming-Qiang Li, Qin-Jing Pan, Xun Zhang, Qing Li, Ying Hong, Chao Zhao, Wen-Hua Zhang, Yan-Ping Li, Kai Chu, Mei Li, Yun-Fei Jiang, Juan Li, Hui Zhao, Zhi-Jie Lin, Xue-Lian Cui, Wen-Yu Liu, Cai-Hong Li, Dong-Ping Guo, Li-Dong Ke, Xin Wu, Jie Tang, Guo-Qi Gao, Ba-Yi Li, Bin Zhao, Feng-Xian Zheng, Cui-Hong Dai, Meng Guo, Jun Zhao, Ying-Ying Su, Jun-Zhi Wang, Feng-Cai Zhu, Shao-Wei Li, Hui-Rong Pan, Yi-Min Li, Jun Zhang, Ning-Shao Xia, Efficacy, Safety, and Immunogenicity of an Escherichia coli-Produced Bivalent Human Papillomavirus Vaccine: An Interim Analysis of a Randomized Clinical Trial, JNCI: Journal of the National Cancer Institute, Volume 112, Issue 2, February 2020, Pages 145–153, https://doi.org/10.1093/jnci/djz074
Close - Share Icon Share
Abstract
The high cost and insufficient supply of human papillomavirus (HPV) vaccines have slowed the pace of controlling cervical cancer. A phase III clinical trial was conducted to evaluate the efficacy, safety, and immunogenicity of a novel Escherichia coli-produced bivalent HPV-16/18 vaccine.
A multicenter, randomized, double-blind trial started on November 22, 2012 in China. In total, 7372 eligible women aged 18–45 years were age-stratified and randomly assigned to receive three doses of the test or control (hepatitis E) vaccine at months 0, 1, and 6. Co-primary endpoints included high-grade genital lesions and persistent infection (over 6 months) associated with HPV-16/18. The primary analysis was performed on a per-protocol susceptible population of individuals who were negative for relevant HPV type-specific neutralizing antibodies (at day 0) and DNA (at day 0 through month 7) and who received three doses of the vaccine. This report presents data from a prespecified interim analysis used for regulatory submission.
In the per-protocol cohort, the efficacies against high-grade genital lesions and persistent infection were 100.0% (95% confidence interval = 55.6% to 100.0%, 0 of 3306 in the vaccine group vs 10 of 3296 in the control group) and 97.8% (95% confidence interval = 87.1% to 99.9%, 1 of 3240 vs 45 of 3246), respectively. The side effects were mild. No vaccine-related serious adverse events were noted. Robust antibody responses for both types were induced and persisted for at least 42 months.
The E coli-produced HPV-16/18 vaccine is well tolerated and highly efficacious against HPV-16/18–associated high-grade genital lesions and persistent infection in women.
Human papillomavirus (HPV) is the most common viral infection of the genital tract (1). Persistent infection with carcinogenic types may lead to precancerous lesions with the possibility of progressing to cancer (2). Globally, HPV types 16 and 18 are responsible for approximately 70% of cervical cancer cases (3). A large majority (>85%) of cervical cancer cases (445 000 annually) occurs in developing regions with limited resources, where cervical cancer accounts for approximately 12% of all cancers in women (4). Three highly efficacious HPV vaccines are currently commercialized and are listed in national immunization programs in more than 70 countries (4). These efforts have led to notable accomplishments, including a continuing reduction in the prevalence of HPV-16/18 and related precancerous lesions (5–7). Unfortunately, the high cost and insufficient supply of the current vaccines have greatly slowed the pace of their implementation in lower -income countries for the World Health Organization call to action to eliminate cervical cancer (8,9).
The currently marketed HPV vaccines, HPV-6/11/16/18 quadrivalent vaccine (10), HPV-6/11/16/18/31/33/45/52/58 9-valent vaccine (11), and HPV-16/18 bivalent vaccine (12), are all composed of L1 virus-like particles (VLPs) produced in eukaryotic cells as the immunogens. Notably, an Escherichia coli-produced bivalent HPV-16/18 L1 VLP vaccine candidate was shown to be safe and immunogenic in women (13,14). The aim of the present phase III trial was to assess the efficacy, safety, and immunogenicity of this HPV vaccine in women.
Methods
Study Design and Participants
This study is a multicenter, randomized, double-blind, controlled (hepatitis E vaccine) clinical trial in adult women. Independent ethics committee approvals were obtained, and the study was conducted in accordance with Good Clinical Practice.
Participants were recruited at five study sites in China. Women were eligible to participate in the study if they were healthy, nonpregnant, and aged 18–45 years (Supplementary Table 1, available online). Written informed consent was obtained from all participants (ClinicalTrials.gov, NCT01735006). At each site, the participants were age-stratified (into two groups: 18–26 and 27–45 years) and randomly assigned (1:1) to receive either the HPV-16/18 vaccine or the control vaccine, which were identical in appearance, according to a permuted-block randomization list (with 12 codes per block) provided by an independent statistician.
The co-primary efficacy endpoints included the following: histopathologically confirmed HPV-16– and/or HPV-18–related grade II or higher cervical intraepithelial neoplasia (CIN), vulvar intraepithelial neoplasia (VIN), and/or vaginal intraepithelial neoplasia (VaIN) (the pathological endpoint) and persistent HPV-16 and/or HPV-18 infection (over 6 months, >150 days) (the persistent infection endpoint).
Vaccines
The bivalent HPV test vaccine was a mixture of two aluminum hydroxide adjuvant-absorbed recombinant L1 VLPs of HPV-16 and HPV-18 expressed in E coli (13–15). The vaccine formulation contained 40 μg of HPV-16 and 20 μg of HPV-18 L1 VLP, suspended in 0.5 mL of buffered saline and 208 μg of aluminum adjuvant. The control vaccine was a commercialized recombinant hepatitis E vaccine (HepE) (Xiamen Innovax, Xiamen, China) (16,17).
Vaccination and Follow-up Visits
Three doses of the HPV vaccine or control vaccine were administered intramuscularly at day 0, month 1 (28–60 days), and month 6 (150–240 days). All the participants were trained to record all adverse events (AEs) occurring within 1 month after each injection in diary cards. Throughout the trial, all serious adverse events (SAEs) and pregnancy outcomes were requested to be reported. Serum samples were collected at day 0 and at month 7 for all the participants. Clinical follow-up visits included a gynecologic examination with collection of endocervical swab samples for Papanicolaou testing and HPV DNA testing. The visits were scheduled at day 0 and months 7, 12, 18, 24, 30, 42, 54, and 66 for all participants. At the time of the current interim analysis, data were available up to month 42.
Efficacy and Immunogenicity Endpoint Assessments
Cytology, histopathology, and HPV DNA testing were performed in a central laboratory at the Cancer Institute of the Chinese Academy of Medical Sciences. Cytology results were reported according to the Bethesda system-2001 (18,19). Histopathological analysis was performed blindly by an independent pathology panel at the Cancer Institute of the Chinese Academy of Medical Sciences. Details of the colposcopy referral and biopsy algorithm are described in Supplementary Figure 1 (available online). The final judgments of endpoint events were made by the gynecologist and the pathologist in the independent data and safety monitoring board (DSMB), who blindly reviewed the data for each case proposed by the principal investigator (Supplementary Methods, available online).
HPV DNA testing was first performed using the HPV DNA enzyme immunoassay method (Labo Biomedical Products, the Netherlands). Samples with positive or borderline (defined per the manual of the kit) findings were further typed by a reverse hybridization line probe assay (Labo Biomedical Products, the Netherlands, based on licensed Innogenetics LiPA technology) and by HPV-16– and HPV-18–specific polymerase chain reactions (HPV TS16/18, Labo Biomedical Products, the Netherlands) (Supplementary Figure 2, available online). Detection of the high-risk type of HPV in paraffin-embedded tissue specimens was considered to be associated with lesions. If more than one high-risk type of HPV was found in the paraffin section, causality was confirmed if the same HPV type was detected in the closest preceding exfoliated cell samples. Otherwise, all high-risk HPV types presenting in the lesion were considered to be associated with that lesion.
Immunogenicity was analyzed in the per-protocol subset for immunogenicity (PPS-I), which included all participants who complied with the protocol, were negative for immunoglobulin G (IgG) antibody against the relevant types of HPV at entry, were negative for the relevant types of HPV DNA at day 0 through month 7, and had IgG antibody results at month 7. The immune persistence of the vaccine was assessed in a subcohort of PPS-I (PPS-P), containing participants from one selected study site who had IgG antibody data available at any of the follow-up visits after month 7. IgG antibodies against HPV-16 and HPV-18 at day 0 and month 7 were tested by an enzyme-linked immunosorbent assay using E coli-expressed L1 VLPs of either type as coating antigens (20). The positive samples were further quantified using references traceable to the World Health Organization international standards for antibodies against HPV-16 (NIBSC code 05/134) or HPV-18 (NIBSC code 10/140), expressed in international units (IUs). The lower detection limits of the assays were 3.1 IU/mL for HPV-16 antibodies and 2.0 IU/mL for HPV-18 antibodies. For calculating the geometric mean concentration (GMC), antibody titers below the lower detection limit of the assay were given an arbitrary value of half the cutoff value. To determine the previous or prevalent infection at entry, neutralizing antibodies against HPV-16 or HPV-18 on day 0 were tested using the pseudovirion-based neutralization assay (20,21), which would preserve the power of the study and avoid overexcluding participants from the PPS cohort.
Statistical Analysis
The study was designed based on preventive efficacy against the pathological and persistent infection endpoints related to HPV-16 and/or -18 (HPV-16/18). Efficacy and confidence intervals (CIs) were calculated based on the observed difference in event rates using accrued person time as the denominator. An exact conditional procedure was used to evaluate efficacy under the assumption that the numbers of cases with endpoint events in the vaccine and control groups were independent Poisson random variables (22).
Efficacy analyses were mainly based on the PPS (PPS-E for pathological endpoints and PPS-PI for persistent infection endpoints). The PPS cohort contained participants who were susceptible to infection by a relevant type of HPV (susceptible participants, ie, negative for the corresponding type of neutralizing antibody on day 0 and negative for the corresponding type of HPV DNA from day 0 through month 7); had received three doses of the test or control vaccine; had at least one effective endpoint follow-up visit after month 7; and had no severe protocol violation. To estimate vaccine efficacy in a population with less perfect compliance, a modified intention-to-treat analysis (mITT, mITT for pathological endpoint, and mITT for persistent infection endpoint) was conducted on unrestricted susceptible participants who received at least one dose of the vaccine and were susceptible to infection by a relevant type of HPV. Case counting for the PPS and mITT analyses started from month 7. ITT analysis included those who received at least one dose, and baseline cases (at entry) were excluded.
The null hypotheses for the pathological endpoint and persistent infection, with an overall two-tailed alpha of 0.05, are that the lower limit of the 95% CI of efficacy is not higher than 0% and not higher than 25%, respectively. Two planned interim efficacy analyses were triggered on the basis of a fixed number of primary endpoint events detected in the PPS cohort. For the persistent infection endpoint, the interim efficacy analysis with a two-tailed alpha of 0.025 was triggered when at least 29 cases of persistent HPV-16/18 infection were identified. For the pathological endpoint, the calculated nominal alpha value was used to control the probability of type I error below 0.05 (two-tailed) based on the O’Brien-Fleming alpha dissipation function (23). The interim analysis with a two-tailed alpha value of 0.004 was performed when at least nine CIN2+ cases associated with HPV-16/18 infection were detected.
To ensure adequate power for the interim analysis (a power of 80% with a two-sided alpha of 0.004) and the final analysis (a power of at least 90% with a two-sided alpha of 0.049) for a true vaccine efficacy of 85% to 90%, with an anticipated annual event rate of 0.2% for pathological endpoint, a total of 4327 per-protocol participants was required for the study. To compensate for dropouts, a total sample size of more than 6000 was needed.
When 10 CIN2+ cases related to HPV-16/18 were identified in the per-protocol cohort through the visits by February 28, 2017, an interim analysis was performed by the DSMB independently. Based on the positive suggestions given by the DSMB, interim analyses for licensing purposes were conducted, and the major findings are reported here.
Analyses for immunogenicity were performed in the PPS-P and PPS-I cohorts. Safety analyses were performed for all the participants who received at least one injection.
Data from different sites involved in this trial were combined for analysis by using SAS software (version 9.1). All reported P values are two-sided with an alpha value of 0.05, except for those specifically indicated.
Results
In total, 8827 women attended the enrollment visit between November 22, 2012, and April 1, 2013. Of these women, 7372 met the eligibility requirements and were randomly assigned to receive the test vaccine (n = 3689) or the control vaccine (n = 3683) (Figure 1). A total 95.1% of women received all three doses of the assigned regimens (Supplementary Table 2, available online). Baseline characteristics were similarly distributed between groups (Table 1). A total of 81.4% and 89.4% of the participants were susceptible to HPV-16 and HPV-18 infections, respectively (ie, negative for type-specific neutralizing antibody on day 0 and negative for the relevant type of HPV DNA from day 0 through month 7).
Study profile. *By mistake, one participant in the vaccine group was given the control vaccine (the commercialized recombinant hepatitis E vaccine containing 30 μg of recombinant hepatitis E virus capsid protein absorbed to aluminum adjuvant) for dose 1, and two participants in the control group were given the test vaccine for dose 3. According to the protocol, these participants were retained in the randomly assigned group for efficacy analysis but were classified into the vaccine group for safety analysis. †Susceptible to infection by a relevant type of human papillomavirus (HPV) is defined as negative for the corresponding type of neutralizing antibody on day 0 and negative for the corresponding type of HPV DNA from day 0 through month 7. PPS-E = per-protocol set for the pathological endpoint; PPS-I = per-protocol set for the immune endpoint; PPS-P = per-protocol set for the immune persistence endpoint; PPS-PI = per-protocol set for the persistent infection endpoint.
| Characteristic . | Vaccine group No. (%) (n = 3689) . | Control group No. (%) (n = 3683) . |
|---|---|---|
| General | ||
| Mean age (SD), y | 30.0 (7.40) | 29.9 (7.33) |
| Younger subgroup, age 18–26 y | 1861 (50.4) | 1862 (50.6) |
| Senior subgroup, age 27–45 y | 1828 (49.6) | 1821 (49.4) |
| Baseline HPV-associated findings | ||
| HPV-16 | ||
| Detectable virus DNA from day 0 to month 7 | 141 (3.8) | 138 (3.7) |
| Detectable neutralizing antibodies on day 0 | 467 (12.7) | 459 (12.5) |
| Susceptible to infection* | 2985 (80.9) | 3013 (81.8) |
| HPV-18 | ||
| Detectable virus DNA from day 0 to month 7 | 53 (1.4) | 60 (1.6) |
| Detectable neutralizing antibodies on day 0 | 186 (5.0) | 180 (4.9) |
| Susceptible to infection* | 3300 (89.5) | 3292 (89.4) |
| Cytological findings at day 0 | ||
| NILM | 3341 (90.6) | 3354 (91.1) |
| ASC-US | 195 (5.3) | 192 (5.2) |
| Negative on Hybrid Capture-2 test | 109 (3.0) | 109 (3.0) |
| Positive on Hybrid Capture-2 test | 86 (2.3) | 83 (2.3) |
| LSIL | 108 (2.9) | 96 (2.6) |
| HSIL | 31 (0.8) | 26 (0.7) |
| ASC-H | 4 (0.1) | 10 (0.3) |
| AIS | 1 (0.03) | 0 (0.0) |
| AGC | 7 (0.2) | 2 (0.05) |
| Histopathological findings at day 0† | ||
| CIN1 | 40 (1.1) | 44 (1.2) |
| VaIN1 | 3 (0.08) | 2 (0.05) |
| CIN2 | 29 (0.8) | 32 (0.9) |
| CIN3 | 9 (0.2) | 13 (0.4) |
| MIC | 0 (0.0) | 1 (0.03) |
| SCC | 1 (0.03) | 1 (0.03) |
| Characteristic . | Vaccine group No. (%) (n = 3689) . | Control group No. (%) (n = 3683) . |
|---|---|---|
| General | ||
| Mean age (SD), y | 30.0 (7.40) | 29.9 (7.33) |
| Younger subgroup, age 18–26 y | 1861 (50.4) | 1862 (50.6) |
| Senior subgroup, age 27–45 y | 1828 (49.6) | 1821 (49.4) |
| Baseline HPV-associated findings | ||
| HPV-16 | ||
| Detectable virus DNA from day 0 to month 7 | 141 (3.8) | 138 (3.7) |
| Detectable neutralizing antibodies on day 0 | 467 (12.7) | 459 (12.5) |
| Susceptible to infection* | 2985 (80.9) | 3013 (81.8) |
| HPV-18 | ||
| Detectable virus DNA from day 0 to month 7 | 53 (1.4) | 60 (1.6) |
| Detectable neutralizing antibodies on day 0 | 186 (5.0) | 180 (4.9) |
| Susceptible to infection* | 3300 (89.5) | 3292 (89.4) |
| Cytological findings at day 0 | ||
| NILM | 3341 (90.6) | 3354 (91.1) |
| ASC-US | 195 (5.3) | 192 (5.2) |
| Negative on Hybrid Capture-2 test | 109 (3.0) | 109 (3.0) |
| Positive on Hybrid Capture-2 test | 86 (2.3) | 83 (2.3) |
| LSIL | 108 (2.9) | 96 (2.6) |
| HSIL | 31 (0.8) | 26 (0.7) |
| ASC-H | 4 (0.1) | 10 (0.3) |
| AIS | 1 (0.03) | 0 (0.0) |
| AGC | 7 (0.2) | 2 (0.05) |
| Histopathological findings at day 0† | ||
| CIN1 | 40 (1.1) | 44 (1.2) |
| VaIN1 | 3 (0.08) | 2 (0.05) |
| CIN2 | 29 (0.8) | 32 (0.9) |
| CIN3 | 9 (0.2) | 13 (0.4) |
| MIC | 0 (0.0) | 1 (0.03) |
| SCC | 1 (0.03) | 1 (0.03) |
Susceptible to infection by a relevant type of human papillomavirus (HPV, ie, negative for type-specific neutralizing antibodies on day 0 and negative for the relevant type of HPV DNA from day 0 to month 7. Missing data on neutralizing antibodies or DNA were not deemed negative. AGC = atypical glandular cells; AIS = adenocarcinoma in situ; ASC-H = atypical squamous cells cannot exclude high-grade lesion; ASC-US = Atypical squamous cells of undetermined significance; CIN1 = cervical intraepithelial neoplasia grade 1; CIN2 = cervical intraepithelial neoplasia grade 2; CIN3 = cervical intraepithelial neoplasia grade 3; HSIL = high-grade squamous intraepithelial lesion; LSIL = low-grade squamous intraepithelial lesion; MIC = microinvasive carcinoma; NILM = negative for intraepithelial lesion or malignancy; SCC = squamous cell carcinoma; VaIN1 = vaginal intraepithelial neoplasia grade 1.
No cases of vaginal intraepithelial neoplasia grade 2 or higher (VaIN2+) or vulvar intraepithelial neoplasia (VIN) were diagnosed at entry.
| Characteristic . | Vaccine group No. (%) (n = 3689) . | Control group No. (%) (n = 3683) . |
|---|---|---|
| General | ||
| Mean age (SD), y | 30.0 (7.40) | 29.9 (7.33) |
| Younger subgroup, age 18–26 y | 1861 (50.4) | 1862 (50.6) |
| Senior subgroup, age 27–45 y | 1828 (49.6) | 1821 (49.4) |
| Baseline HPV-associated findings | ||
| HPV-16 | ||
| Detectable virus DNA from day 0 to month 7 | 141 (3.8) | 138 (3.7) |
| Detectable neutralizing antibodies on day 0 | 467 (12.7) | 459 (12.5) |
| Susceptible to infection* | 2985 (80.9) | 3013 (81.8) |
| HPV-18 | ||
| Detectable virus DNA from day 0 to month 7 | 53 (1.4) | 60 (1.6) |
| Detectable neutralizing antibodies on day 0 | 186 (5.0) | 180 (4.9) |
| Susceptible to infection* | 3300 (89.5) | 3292 (89.4) |
| Cytological findings at day 0 | ||
| NILM | 3341 (90.6) | 3354 (91.1) |
| ASC-US | 195 (5.3) | 192 (5.2) |
| Negative on Hybrid Capture-2 test | 109 (3.0) | 109 (3.0) |
| Positive on Hybrid Capture-2 test | 86 (2.3) | 83 (2.3) |
| LSIL | 108 (2.9) | 96 (2.6) |
| HSIL | 31 (0.8) | 26 (0.7) |
| ASC-H | 4 (0.1) | 10 (0.3) |
| AIS | 1 (0.03) | 0 (0.0) |
| AGC | 7 (0.2) | 2 (0.05) |
| Histopathological findings at day 0† | ||
| CIN1 | 40 (1.1) | 44 (1.2) |
| VaIN1 | 3 (0.08) | 2 (0.05) |
| CIN2 | 29 (0.8) | 32 (0.9) |
| CIN3 | 9 (0.2) | 13 (0.4) |
| MIC | 0 (0.0) | 1 (0.03) |
| SCC | 1 (0.03) | 1 (0.03) |
| Characteristic . | Vaccine group No. (%) (n = 3689) . | Control group No. (%) (n = 3683) . |
|---|---|---|
| General | ||
| Mean age (SD), y | 30.0 (7.40) | 29.9 (7.33) |
| Younger subgroup, age 18–26 y | 1861 (50.4) | 1862 (50.6) |
| Senior subgroup, age 27–45 y | 1828 (49.6) | 1821 (49.4) |
| Baseline HPV-associated findings | ||
| HPV-16 | ||
| Detectable virus DNA from day 0 to month 7 | 141 (3.8) | 138 (3.7) |
| Detectable neutralizing antibodies on day 0 | 467 (12.7) | 459 (12.5) |
| Susceptible to infection* | 2985 (80.9) | 3013 (81.8) |
| HPV-18 | ||
| Detectable virus DNA from day 0 to month 7 | 53 (1.4) | 60 (1.6) |
| Detectable neutralizing antibodies on day 0 | 186 (5.0) | 180 (4.9) |
| Susceptible to infection* | 3300 (89.5) | 3292 (89.4) |
| Cytological findings at day 0 | ||
| NILM | 3341 (90.6) | 3354 (91.1) |
| ASC-US | 195 (5.3) | 192 (5.2) |
| Negative on Hybrid Capture-2 test | 109 (3.0) | 109 (3.0) |
| Positive on Hybrid Capture-2 test | 86 (2.3) | 83 (2.3) |
| LSIL | 108 (2.9) | 96 (2.6) |
| HSIL | 31 (0.8) | 26 (0.7) |
| ASC-H | 4 (0.1) | 10 (0.3) |
| AIS | 1 (0.03) | 0 (0.0) |
| AGC | 7 (0.2) | 2 (0.05) |
| Histopathological findings at day 0† | ||
| CIN1 | 40 (1.1) | 44 (1.2) |
| VaIN1 | 3 (0.08) | 2 (0.05) |
| CIN2 | 29 (0.8) | 32 (0.9) |
| CIN3 | 9 (0.2) | 13 (0.4) |
| MIC | 0 (0.0) | 1 (0.03) |
| SCC | 1 (0.03) | 1 (0.03) |
Susceptible to infection by a relevant type of human papillomavirus (HPV, ie, negative for type-specific neutralizing antibodies on day 0 and negative for the relevant type of HPV DNA from day 0 to month 7. Missing data on neutralizing antibodies or DNA were not deemed negative. AGC = atypical glandular cells; AIS = adenocarcinoma in situ; ASC-H = atypical squamous cells cannot exclude high-grade lesion; ASC-US = Atypical squamous cells of undetermined significance; CIN1 = cervical intraepithelial neoplasia grade 1; CIN2 = cervical intraepithelial neoplasia grade 2; CIN3 = cervical intraepithelial neoplasia grade 3; HSIL = high-grade squamous intraepithelial lesion; LSIL = low-grade squamous intraepithelial lesion; MIC = microinvasive carcinoma; NILM = negative for intraepithelial lesion or malignancy; SCC = squamous cell carcinoma; VaIN1 = vaginal intraepithelial neoplasia grade 1.
No cases of vaginal intraepithelial neoplasia grade 2 or higher (VaIN2+) or vulvar intraepithelial neoplasia (VIN) were diagnosed at entry.
The participants were followed for an average of 3.3 years. In the PPS cohort for the pathological endpoint (PPS-E), which included 6602 of 7372 women who underwent randomization (89.6%), 10 women in total, all in the control group, developed high-grade genital lesions associated with HPV-16/18 (seven CIN2 cases and three CIN3 cases). The HPV vaccine prevented 100.0% (95% CI = 55.6% to 100.0%, 0 of 3306 in the vaccine group vs 10 of 3296 in the control group) of HPV-16/18–related high-grade genital lesions in this population. Furthermore, the vaccine provided an efficacy of 97.8% (95% CI = 87.1% to 99.9%, 1 of 3240 vs 45 of 3246) against persistent infection (over 6 months) associated with HPV-16/18 in the per-protocol cohorts (PPS-PI) (Table 2). The robustness of the vaccine efficacies against both co-primary endpoints were further confirmed in the Kaplan-Meier plots (Figure 2). The percentage of women completing all three vaccinations was very high, so the per-protocol and mITT analyses are similar (Table 2).
![Time until the development of high-grade genital lesions or persistent infection associated with human papillomavirus (HPV)-16/18 in the susceptible population. A) The accumulation of cases with high-grade genital lesions related to HPV-16/18 in the per-protocol set (PPS-E). B) The accumulation of cases with persistent infection related to HPV-16/18 in the per-protocol set (per-protocol set for the persistent infection endpoint [PPS-PI]). Error bars represent 95% confidence intervals. The bars for the vaccine group were artificially moved slightly to the left to allow better discrimination from those for the control group. Of note, due to the closing calendar date for this interim analysis, a small number of participants were at risk at months 42 and 48.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/112/2/10.1093_jnci_djz074/1/m_djz074f2.jpeg?Expires=1750200985&Signature=nFhXGp3vPv~YgkUrAbZLiuvdHHyBEf3LtljFbPp8SmVh8tCJhj18JV8Tsj0Y55Ee5Mlha2xUeDplDdXIS7s4ZQmmMAbl8GBcPQ~R13Y6w~f1QbBebtm~GgXYxWOTRs1D9Rh5s76Rt8U2Us4UI7fiFCxtZRq7DHzszbYDtJ5-sWipLzH9A7ZovVoATNffYXvjBa5Cb23iUhjrLuGqh~PFoBSoofgmPofJlRe73LScTQ5PYNTPXV2JmTAQEKlIZEFymDmllmSehd64q7yZ82v-AVgz2d7nfOByKfQ5~uPLZ6GtLlwAc5-8MK06LccmzOZwsksOJA0OH5TlDp2iPMHUVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Time until the development of high-grade genital lesions or persistent infection associated with human papillomavirus (HPV)-16/18 in the susceptible population. A) The accumulation of cases with high-grade genital lesions related to HPV-16/18 in the per-protocol set (PPS-E). B) The accumulation of cases with persistent infection related to HPV-16/18 in the per-protocol set (per-protocol set for the persistent infection endpoint [PPS-PI]). Error bars represent 95% confidence intervals. The bars for the vaccine group were artificially moved slightly to the left to allow better discrimination from those for the control group. Of note, due to the closing calendar date for this interim analysis, a small number of participants were at risk at months 42 and 48.
Vaccine efficacy against genital lesions, persistent infection, or incident infection associated with human papillomavirus (HPV)-16 or HPV-18
| Endpoint . | Vaccine group . | Control group . | Vaccine efficacy, % (95% CI) . | P‡ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total participants . | Person-years at risk* . | No. of cases . | Rate† . | Total participants . | Person-years at risk* . | No. of cases . | Rate† . | |||
| Lesion of the cervix, vagina, and vulva related to HPV-16/18§ | ||||||||||
| Participants in the per-protocol susceptible population (PPS-E) | ||||||||||
| Lesion grade | ||||||||||
| High grade (CIN2+/VIN2+/VaIN2+) | 3306 | 9119.9 | 0 | 0 | 3296 | 9079.3 | 10 | 0.1 | 100.0 (55.6 to 100.0)‖ | .002 |
| Any grade (CIN1+/VIN1+/VaIN1+) | 3306 | 9119.9 | 0 | 0 | 3296 | 9075.7 | 14 | 0.2 | 100.0 (70.0 to 100.0) | <.001 |
| HPV type-specific high-grade lesion | ||||||||||
| HPV-16 | 2885 | 7977.1 | 0 | 0 | 2911 | 8022.7 | 9 | 0.1 | 100.0 (49.0 to 100.0) | .004 |
| HPV-18 | 3175 | 8765.7 | 0 | 0 | 3153 | 8694.4 | 1 | 0.01 | 100.0 (−3768.3 to 100.0) | 1.00 |
| Participants in the unrestricted susceptible population (mITT-E) | ||||||||||
| Lesion grade | ||||||||||
| High grade (CIN2+/VIN2+/VaIN2+) | 3386 | 9304.1 | 0 | 0 | 3386 | 9291.1 | 10 | 0.1 | 100.0 (55.4 to 100.0) | .002 |
| Any grade (CIN1+/VIN1+/VaIN1+) | 3386 | 9304.1 | 0 | 0 | 3386 | 9287.5 | 14 | 0.2 | 100.0 (69.9 to 100.0) | <.001 |
| HPV type-specific high-grade lesion | ||||||||||
| HPV-16 | 2943 | 8110.8 | 0 | 0 | 2972 | 8165.6 | 9 | 0.1 | 100.0 (49.0 to 100.0) | .004 |
| HPV-18 | 3252 | 8943.9 | 0 | 0 | 3239 | 8897.4 | 1 | 0.01 | 100.0 (−3779.7 to 100.0) | 1.00 |
| Persistent infection ≥6-month duration | ||||||||||
| Participants in the per-protocol susceptible population (PPS-PI) | ||||||||||
| Related to HPV-16 or -18 | 3240 | 9026.5 | 1 | 0.01 | 3246 | 8904.9 | 45 | 0.5 | 97.8 (87.1 to 99.9) | <.001 |
| Related to HPV-16 | 2833 | 7887.0 | 1 | 0.01 | 2863 | 7886.9 | 28 | 0.4 | 96.4 (78.4 to 99.9) | <.001 |
| Related to HPV-18 | 3112 | 8687.7 | 0 | 0 | 3110 | 8595.7 | 19 | 0.2 | 100.0 (78.8 to 100.0) | <.001 |
| Participants in the unrestricted susceptible population (mITT-PI) | ||||||||||
| Related to HPV-16 or -18 | 3313 | 9219.9 | 1 | 0.01 | 3330 | 9113.4 | 48 | 0.5 | 97.9 (88.0 to 99.9) | <.001 |
| Related to HPV-16 | 2884 | 8020.5 | 1 | 0.01 | 2920 | 8026.7 | 30 | 0.4 | 96.7 (79.9 to 99.9) | <.001 |
| Related to HPV-18 | 3182 | 8872.6 | 0 | 0 | 3190 | 8797.2 | 20 | 0.2 | 100.0 (79.9 to 100.0) | <.001 |
| Incident infection | ||||||||||
| Participants in the per-protocol susceptible population (PPS-II) | ||||||||||
| Related to HPV-16 or -18 | 3307 | 9059.1 | 44 | 0.5 | 3300 | 8952.0 | 144 | 1.6 | 69.8 (57.4 to 79.0) | <.001 |
| Related to HPV-16 | 2886 | 7920.5 | 31 | 0.4 | 2914 | 7955.0 | 87 | 1.1 | 64.2 (45.5 to 77.1) | <.001 |
| Related to HPV-18 | 3176 | 8729.6 | 14 | 0.2 | 3157 | 8628.8 | 66 | 0.8 | 79.0 (62.3 to 89.1) | <.001 |
| Participants in the unrestricted susceptible population (mITT-II) | ||||||||||
| Related to HPV-16 or -18 | 3388 | 9263.0 | 45 | 0.5 | 3391 | 9161.6 | 153 | 1.7 | 70.9 (59.2 to 79.6) | <.001 |
| Related to HPV-16 | 2945 | 8064.6 | 32 | 0.4 | 2975 | 8096.4 | 91 | 1.1 | 64.7 (46.7 to 77.2) | <.001 |
| Related to HPV-18 | 3253 | 8924.4 | 14 | 0.2 | 3244 | 8832.9 | 71 | 0.8 | 80.5 (65.1 to 89.8) | <.001 |
| Endpoint . | Vaccine group . | Control group . | Vaccine efficacy, % (95% CI) . | P‡ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total participants . | Person-years at risk* . | No. of cases . | Rate† . | Total participants . | Person-years at risk* . | No. of cases . | Rate† . | |||
| Lesion of the cervix, vagina, and vulva related to HPV-16/18§ | ||||||||||
| Participants in the per-protocol susceptible population (PPS-E) | ||||||||||
| Lesion grade | ||||||||||
| High grade (CIN2+/VIN2+/VaIN2+) | 3306 | 9119.9 | 0 | 0 | 3296 | 9079.3 | 10 | 0.1 | 100.0 (55.6 to 100.0)‖ | .002 |
| Any grade (CIN1+/VIN1+/VaIN1+) | 3306 | 9119.9 | 0 | 0 | 3296 | 9075.7 | 14 | 0.2 | 100.0 (70.0 to 100.0) | <.001 |
| HPV type-specific high-grade lesion | ||||||||||
| HPV-16 | 2885 | 7977.1 | 0 | 0 | 2911 | 8022.7 | 9 | 0.1 | 100.0 (49.0 to 100.0) | .004 |
| HPV-18 | 3175 | 8765.7 | 0 | 0 | 3153 | 8694.4 | 1 | 0.01 | 100.0 (−3768.3 to 100.0) | 1.00 |
| Participants in the unrestricted susceptible population (mITT-E) | ||||||||||
| Lesion grade | ||||||||||
| High grade (CIN2+/VIN2+/VaIN2+) | 3386 | 9304.1 | 0 | 0 | 3386 | 9291.1 | 10 | 0.1 | 100.0 (55.4 to 100.0) | .002 |
| Any grade (CIN1+/VIN1+/VaIN1+) | 3386 | 9304.1 | 0 | 0 | 3386 | 9287.5 | 14 | 0.2 | 100.0 (69.9 to 100.0) | <.001 |
| HPV type-specific high-grade lesion | ||||||||||
| HPV-16 | 2943 | 8110.8 | 0 | 0 | 2972 | 8165.6 | 9 | 0.1 | 100.0 (49.0 to 100.0) | .004 |
| HPV-18 | 3252 | 8943.9 | 0 | 0 | 3239 | 8897.4 | 1 | 0.01 | 100.0 (−3779.7 to 100.0) | 1.00 |
| Persistent infection ≥6-month duration | ||||||||||
| Participants in the per-protocol susceptible population (PPS-PI) | ||||||||||
| Related to HPV-16 or -18 | 3240 | 9026.5 | 1 | 0.01 | 3246 | 8904.9 | 45 | 0.5 | 97.8 (87.1 to 99.9) | <.001 |
| Related to HPV-16 | 2833 | 7887.0 | 1 | 0.01 | 2863 | 7886.9 | 28 | 0.4 | 96.4 (78.4 to 99.9) | <.001 |
| Related to HPV-18 | 3112 | 8687.7 | 0 | 0 | 3110 | 8595.7 | 19 | 0.2 | 100.0 (78.8 to 100.0) | <.001 |
| Participants in the unrestricted susceptible population (mITT-PI) | ||||||||||
| Related to HPV-16 or -18 | 3313 | 9219.9 | 1 | 0.01 | 3330 | 9113.4 | 48 | 0.5 | 97.9 (88.0 to 99.9) | <.001 |
| Related to HPV-16 | 2884 | 8020.5 | 1 | 0.01 | 2920 | 8026.7 | 30 | 0.4 | 96.7 (79.9 to 99.9) | <.001 |
| Related to HPV-18 | 3182 | 8872.6 | 0 | 0 | 3190 | 8797.2 | 20 | 0.2 | 100.0 (79.9 to 100.0) | <.001 |
| Incident infection | ||||||||||
| Participants in the per-protocol susceptible population (PPS-II) | ||||||||||
| Related to HPV-16 or -18 | 3307 | 9059.1 | 44 | 0.5 | 3300 | 8952.0 | 144 | 1.6 | 69.8 (57.4 to 79.0) | <.001 |
| Related to HPV-16 | 2886 | 7920.5 | 31 | 0.4 | 2914 | 7955.0 | 87 | 1.1 | 64.2 (45.5 to 77.1) | <.001 |
| Related to HPV-18 | 3176 | 8729.6 | 14 | 0.2 | 3157 | 8628.8 | 66 | 0.8 | 79.0 (62.3 to 89.1) | <.001 |
| Participants in the unrestricted susceptible population (mITT-II) | ||||||||||
| Related to HPV-16 or -18 | 3388 | 9263.0 | 45 | 0.5 | 3391 | 9161.6 | 153 | 1.7 | 70.9 (59.2 to 79.6) | <.001 |
| Related to HPV-16 | 2945 | 8064.6 | 32 | 0.4 | 2975 | 8096.4 | 91 | 1.1 | 64.7 (46.7 to 77.2) | <.001 |
| Related to HPV-18 | 3253 | 8924.4 | 14 | 0.2 | 3244 | 8832.9 | 71 | 0.8 | 80.5 (65.1 to 89.8) | <.001 |
Person-years at risk was defined as the cumulative follow-up years of the at-risk participants at the indicated time point. CI = confidence interval. CIN1+ = cervical intraepithelial neoplasia grade 1 and above; CIN2+ = cervical intraepithelial neoplasia grade 2 and above; mITT-E = modified intention-to-treat analysis for the pathological endpoint; mITT-II = modified intention-to-treat analysis for the incident infection endpoint; mITT-PI = modified intention-to-treat analysis for the persistent infection endpoint; PPS-E = per-protocol set for the pathological endpoint; PPS-II = per-protocol set for the incident infection endpoint; PPS-PI = per-protocol set for the persistent infection endpoint; VaIN1+ = vaginal intraepithelial neoplasia grade 1 and above; VaIN2+ = vaginal intraepithelial neoplasia grade 2 and above; VIN1+ = vulvar intraepithelial neoplasia grade 1 and above; VIN2+ = vulvar intraepithelial neoplasia grade 2 and above.
The rate is the number of participants with the endpoint per 100 person-years at risk.
Fisher’s exact test was used to calculate the P values with two-sided test.
HPV-16/18 associated adenocarcinoma in situ and cervical cancer were defined as the high-grade lesion endpoint.
The 99.6% CI of the efficacy against HPV-16/18–related CIN2+/VIN2+/VaIN2+ in the PPS-E cohort is 14.2% to 100.0%.
Vaccine efficacy against genital lesions, persistent infection, or incident infection associated with human papillomavirus (HPV)-16 or HPV-18
| Endpoint . | Vaccine group . | Control group . | Vaccine efficacy, % (95% CI) . | P‡ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total participants . | Person-years at risk* . | No. of cases . | Rate† . | Total participants . | Person-years at risk* . | No. of cases . | Rate† . | |||
| Lesion of the cervix, vagina, and vulva related to HPV-16/18§ | ||||||||||
| Participants in the per-protocol susceptible population (PPS-E) | ||||||||||
| Lesion grade | ||||||||||
| High grade (CIN2+/VIN2+/VaIN2+) | 3306 | 9119.9 | 0 | 0 | 3296 | 9079.3 | 10 | 0.1 | 100.0 (55.6 to 100.0)‖ | .002 |
| Any grade (CIN1+/VIN1+/VaIN1+) | 3306 | 9119.9 | 0 | 0 | 3296 | 9075.7 | 14 | 0.2 | 100.0 (70.0 to 100.0) | <.001 |
| HPV type-specific high-grade lesion | ||||||||||
| HPV-16 | 2885 | 7977.1 | 0 | 0 | 2911 | 8022.7 | 9 | 0.1 | 100.0 (49.0 to 100.0) | .004 |
| HPV-18 | 3175 | 8765.7 | 0 | 0 | 3153 | 8694.4 | 1 | 0.01 | 100.0 (−3768.3 to 100.0) | 1.00 |
| Participants in the unrestricted susceptible population (mITT-E) | ||||||||||
| Lesion grade | ||||||||||
| High grade (CIN2+/VIN2+/VaIN2+) | 3386 | 9304.1 | 0 | 0 | 3386 | 9291.1 | 10 | 0.1 | 100.0 (55.4 to 100.0) | .002 |
| Any grade (CIN1+/VIN1+/VaIN1+) | 3386 | 9304.1 | 0 | 0 | 3386 | 9287.5 | 14 | 0.2 | 100.0 (69.9 to 100.0) | <.001 |
| HPV type-specific high-grade lesion | ||||||||||
| HPV-16 | 2943 | 8110.8 | 0 | 0 | 2972 | 8165.6 | 9 | 0.1 | 100.0 (49.0 to 100.0) | .004 |
| HPV-18 | 3252 | 8943.9 | 0 | 0 | 3239 | 8897.4 | 1 | 0.01 | 100.0 (−3779.7 to 100.0) | 1.00 |
| Persistent infection ≥6-month duration | ||||||||||
| Participants in the per-protocol susceptible population (PPS-PI) | ||||||||||
| Related to HPV-16 or -18 | 3240 | 9026.5 | 1 | 0.01 | 3246 | 8904.9 | 45 | 0.5 | 97.8 (87.1 to 99.9) | <.001 |
| Related to HPV-16 | 2833 | 7887.0 | 1 | 0.01 | 2863 | 7886.9 | 28 | 0.4 | 96.4 (78.4 to 99.9) | <.001 |
| Related to HPV-18 | 3112 | 8687.7 | 0 | 0 | 3110 | 8595.7 | 19 | 0.2 | 100.0 (78.8 to 100.0) | <.001 |
| Participants in the unrestricted susceptible population (mITT-PI) | ||||||||||
| Related to HPV-16 or -18 | 3313 | 9219.9 | 1 | 0.01 | 3330 | 9113.4 | 48 | 0.5 | 97.9 (88.0 to 99.9) | <.001 |
| Related to HPV-16 | 2884 | 8020.5 | 1 | 0.01 | 2920 | 8026.7 | 30 | 0.4 | 96.7 (79.9 to 99.9) | <.001 |
| Related to HPV-18 | 3182 | 8872.6 | 0 | 0 | 3190 | 8797.2 | 20 | 0.2 | 100.0 (79.9 to 100.0) | <.001 |
| Incident infection | ||||||||||
| Participants in the per-protocol susceptible population (PPS-II) | ||||||||||
| Related to HPV-16 or -18 | 3307 | 9059.1 | 44 | 0.5 | 3300 | 8952.0 | 144 | 1.6 | 69.8 (57.4 to 79.0) | <.001 |
| Related to HPV-16 | 2886 | 7920.5 | 31 | 0.4 | 2914 | 7955.0 | 87 | 1.1 | 64.2 (45.5 to 77.1) | <.001 |
| Related to HPV-18 | 3176 | 8729.6 | 14 | 0.2 | 3157 | 8628.8 | 66 | 0.8 | 79.0 (62.3 to 89.1) | <.001 |
| Participants in the unrestricted susceptible population (mITT-II) | ||||||||||
| Related to HPV-16 or -18 | 3388 | 9263.0 | 45 | 0.5 | 3391 | 9161.6 | 153 | 1.7 | 70.9 (59.2 to 79.6) | <.001 |
| Related to HPV-16 | 2945 | 8064.6 | 32 | 0.4 | 2975 | 8096.4 | 91 | 1.1 | 64.7 (46.7 to 77.2) | <.001 |
| Related to HPV-18 | 3253 | 8924.4 | 14 | 0.2 | 3244 | 8832.9 | 71 | 0.8 | 80.5 (65.1 to 89.8) | <.001 |
| Endpoint . | Vaccine group . | Control group . | Vaccine efficacy, % (95% CI) . | P‡ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total participants . | Person-years at risk* . | No. of cases . | Rate† . | Total participants . | Person-years at risk* . | No. of cases . | Rate† . | |||
| Lesion of the cervix, vagina, and vulva related to HPV-16/18§ | ||||||||||
| Participants in the per-protocol susceptible population (PPS-E) | ||||||||||
| Lesion grade | ||||||||||
| High grade (CIN2+/VIN2+/VaIN2+) | 3306 | 9119.9 | 0 | 0 | 3296 | 9079.3 | 10 | 0.1 | 100.0 (55.6 to 100.0)‖ | .002 |
| Any grade (CIN1+/VIN1+/VaIN1+) | 3306 | 9119.9 | 0 | 0 | 3296 | 9075.7 | 14 | 0.2 | 100.0 (70.0 to 100.0) | <.001 |
| HPV type-specific high-grade lesion | ||||||||||
| HPV-16 | 2885 | 7977.1 | 0 | 0 | 2911 | 8022.7 | 9 | 0.1 | 100.0 (49.0 to 100.0) | .004 |
| HPV-18 | 3175 | 8765.7 | 0 | 0 | 3153 | 8694.4 | 1 | 0.01 | 100.0 (−3768.3 to 100.0) | 1.00 |
| Participants in the unrestricted susceptible population (mITT-E) | ||||||||||
| Lesion grade | ||||||||||
| High grade (CIN2+/VIN2+/VaIN2+) | 3386 | 9304.1 | 0 | 0 | 3386 | 9291.1 | 10 | 0.1 | 100.0 (55.4 to 100.0) | .002 |
| Any grade (CIN1+/VIN1+/VaIN1+) | 3386 | 9304.1 | 0 | 0 | 3386 | 9287.5 | 14 | 0.2 | 100.0 (69.9 to 100.0) | <.001 |
| HPV type-specific high-grade lesion | ||||||||||
| HPV-16 | 2943 | 8110.8 | 0 | 0 | 2972 | 8165.6 | 9 | 0.1 | 100.0 (49.0 to 100.0) | .004 |
| HPV-18 | 3252 | 8943.9 | 0 | 0 | 3239 | 8897.4 | 1 | 0.01 | 100.0 (−3779.7 to 100.0) | 1.00 |
| Persistent infection ≥6-month duration | ||||||||||
| Participants in the per-protocol susceptible population (PPS-PI) | ||||||||||
| Related to HPV-16 or -18 | 3240 | 9026.5 | 1 | 0.01 | 3246 | 8904.9 | 45 | 0.5 | 97.8 (87.1 to 99.9) | <.001 |
| Related to HPV-16 | 2833 | 7887.0 | 1 | 0.01 | 2863 | 7886.9 | 28 | 0.4 | 96.4 (78.4 to 99.9) | <.001 |
| Related to HPV-18 | 3112 | 8687.7 | 0 | 0 | 3110 | 8595.7 | 19 | 0.2 | 100.0 (78.8 to 100.0) | <.001 |
| Participants in the unrestricted susceptible population (mITT-PI) | ||||||||||
| Related to HPV-16 or -18 | 3313 | 9219.9 | 1 | 0.01 | 3330 | 9113.4 | 48 | 0.5 | 97.9 (88.0 to 99.9) | <.001 |
| Related to HPV-16 | 2884 | 8020.5 | 1 | 0.01 | 2920 | 8026.7 | 30 | 0.4 | 96.7 (79.9 to 99.9) | <.001 |
| Related to HPV-18 | 3182 | 8872.6 | 0 | 0 | 3190 | 8797.2 | 20 | 0.2 | 100.0 (79.9 to 100.0) | <.001 |
| Incident infection | ||||||||||
| Participants in the per-protocol susceptible population (PPS-II) | ||||||||||
| Related to HPV-16 or -18 | 3307 | 9059.1 | 44 | 0.5 | 3300 | 8952.0 | 144 | 1.6 | 69.8 (57.4 to 79.0) | <.001 |
| Related to HPV-16 | 2886 | 7920.5 | 31 | 0.4 | 2914 | 7955.0 | 87 | 1.1 | 64.2 (45.5 to 77.1) | <.001 |
| Related to HPV-18 | 3176 | 8729.6 | 14 | 0.2 | 3157 | 8628.8 | 66 | 0.8 | 79.0 (62.3 to 89.1) | <.001 |
| Participants in the unrestricted susceptible population (mITT-II) | ||||||||||
| Related to HPV-16 or -18 | 3388 | 9263.0 | 45 | 0.5 | 3391 | 9161.6 | 153 | 1.7 | 70.9 (59.2 to 79.6) | <.001 |
| Related to HPV-16 | 2945 | 8064.6 | 32 | 0.4 | 2975 | 8096.4 | 91 | 1.1 | 64.7 (46.7 to 77.2) | <.001 |
| Related to HPV-18 | 3253 | 8924.4 | 14 | 0.2 | 3244 | 8832.9 | 71 | 0.8 | 80.5 (65.1 to 89.8) | <.001 |
Person-years at risk was defined as the cumulative follow-up years of the at-risk participants at the indicated time point. CI = confidence interval. CIN1+ = cervical intraepithelial neoplasia grade 1 and above; CIN2+ = cervical intraepithelial neoplasia grade 2 and above; mITT-E = modified intention-to-treat analysis for the pathological endpoint; mITT-II = modified intention-to-treat analysis for the incident infection endpoint; mITT-PI = modified intention-to-treat analysis for the persistent infection endpoint; PPS-E = per-protocol set for the pathological endpoint; PPS-II = per-protocol set for the incident infection endpoint; PPS-PI = per-protocol set for the persistent infection endpoint; VaIN1+ = vaginal intraepithelial neoplasia grade 1 and above; VaIN2+ = vaginal intraepithelial neoplasia grade 2 and above; VIN1+ = vulvar intraepithelial neoplasia grade 1 and above; VIN2+ = vulvar intraepithelial neoplasia grade 2 and above.
The rate is the number of participants with the endpoint per 100 person-years at risk.
Fisher’s exact test was used to calculate the P values with two-sided test.
HPV-16/18 associated adenocarcinoma in situ and cervical cancer were defined as the high-grade lesion endpoint.
The 99.6% CI of the efficacy against HPV-16/18–related CIN2+/VIN2+/VaIN2+ in the PPS-E cohort is 14.2% to 100.0%.
Additionally, the vaccine statistically significantly lowered the risk of incident infection (Table 2) and cytological abnormalities of the cervix related to HPV-16/18 (Supplementary Table 4, available online). For the nonvaccine types HPV-31/33/45, the vaccine decreased persistent infections related to these types as a combined group (47.2%, 95% CI = 0.0% to 73.1%; Table 3), although this difference did not reach statistical significance (P = .05). The vaccine showed no effects on reducing genital lesions or clearing the prevalent infection for women with detectable HPV DNA for the vaccine types at baseline (Supplementary Table 5, available online). Among those who received a single dose in the mITT cohort, only one persistent infection and two incident infection events related to HPV-16/18 occurred, and all of them were in the control group.
Vaccine cross-protection against carcinogenic human papillomavirus (HPV) persistent infection (6 m) other than HPV-16/18 in the per-protocol cohort*
| HPV type . | Vaccine group . | Control group . | Vaccine efficacy % (95% CI) . | P§ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants . | No. of events . | Person-years at risk† . | Rate‡ . | No. of participants . | No. of events . | Person-years at risk† . | Rate‡ . | |||
| HPV-31 | 3278 | 8 | 9151.3 | 0.1 | 3280 | 13 | 9092.0 | 0.1 | 38.9 (−59.1 to 78.0) | .38 |
| HPV-33 | 3282 | 7 | 9159.7 | 0.1 | 3274 | 15 | 9072.3 | 0.2 | 53.8 (−20.4 to 84.1) | .13 |
| HPV-35 | 3289 | 8 | 9180.8 | 0.1 | 3286 | 15 | 9116.2 | 0.2 | 47.0 (−33.1 to 80.6) | .21 |
| HPV-39 | 3248 | 33 | 9011.2 | 0.4 | 3243 | 31 | 8952.0 | 0.3 | −5.8 (−78.5 to 37.2) | .90 |
| HPV-45 | 3299 | 1 | 9228.3 | 0.01 | 3298 | 5 | 9167.4 | 0.1 | 80.1 (−77.6 to 99.6) | .22 |
| HPV-51 | 3225 | 41 | 8916.4 | 0.5 | 3218 | 28 | 8878.6 | 0.3 | −45.8 (−144.8 to 12.0) | .15 |
| HPV-52 | 3075 | 67 | 8455.1 | 0.8 | 3108 | 52 | 8521.9 | 0.6 | −29.9 (−90.3 to 10.9) | .20 |
| HPV-56 | 3257 | 31 | 9041.7 | 0.3 | 3266 | 29 | 9016.6 | 0.3 | −6.6 (−83.3 to 37.8) | .90 |
| HPV-58 | 3258 | 23 | 9059.5 | 0.3 | 3248 | 28 | 8978.3 | 0.3 | 18.6 (−46.6 to 55.2) | .58 |
| HPV-59 | 3283 | 15 | 9157.8 | 0.2 | 3289 | 12 | 9125.8 | 0.1 | −24.6 (−191.4 to 45.6) | .70 |
| HPV-66 | 3261 | 22 | 9065.8 | 0.2 | 3263 | 26 | 9024.0 | 0.3 | 15.8 (−54.6 to 54.5) | .67 |
| HPV-68 | 3257 | 25 | 9059.8 | 0.3 | 3257 | 20 | 9005.2 | 0.2 | −24.2 (−135.9 to 33.7) | .55 |
| HPV-31/33/45 | 3314 | 16 | 9223.4 | 0.2 | 3312 | 30 | 9135.8 | 0.3 | 47.2 (0.0 to 73.1) | .05 |
| HPV type . | Vaccine group . | Control group . | Vaccine efficacy % (95% CI) . | P§ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants . | No. of events . | Person-years at risk† . | Rate‡ . | No. of participants . | No. of events . | Person-years at risk† . | Rate‡ . | |||
| HPV-31 | 3278 | 8 | 9151.3 | 0.1 | 3280 | 13 | 9092.0 | 0.1 | 38.9 (−59.1 to 78.0) | .38 |
| HPV-33 | 3282 | 7 | 9159.7 | 0.1 | 3274 | 15 | 9072.3 | 0.2 | 53.8 (−20.4 to 84.1) | .13 |
| HPV-35 | 3289 | 8 | 9180.8 | 0.1 | 3286 | 15 | 9116.2 | 0.2 | 47.0 (−33.1 to 80.6) | .21 |
| HPV-39 | 3248 | 33 | 9011.2 | 0.4 | 3243 | 31 | 8952.0 | 0.3 | −5.8 (−78.5 to 37.2) | .90 |
| HPV-45 | 3299 | 1 | 9228.3 | 0.01 | 3298 | 5 | 9167.4 | 0.1 | 80.1 (−77.6 to 99.6) | .22 |
| HPV-51 | 3225 | 41 | 8916.4 | 0.5 | 3218 | 28 | 8878.6 | 0.3 | −45.8 (−144.8 to 12.0) | .15 |
| HPV-52 | 3075 | 67 | 8455.1 | 0.8 | 3108 | 52 | 8521.9 | 0.6 | −29.9 (−90.3 to 10.9) | .20 |
| HPV-56 | 3257 | 31 | 9041.7 | 0.3 | 3266 | 29 | 9016.6 | 0.3 | −6.6 (−83.3 to 37.8) | .90 |
| HPV-58 | 3258 | 23 | 9059.5 | 0.3 | 3248 | 28 | 8978.3 | 0.3 | 18.6 (−46.6 to 55.2) | .58 |
| HPV-59 | 3283 | 15 | 9157.8 | 0.2 | 3289 | 12 | 9125.8 | 0.1 | −24.6 (−191.4 to 45.6) | .70 |
| HPV-66 | 3261 | 22 | 9065.8 | 0.2 | 3263 | 26 | 9024.0 | 0.3 | 15.8 (−54.6 to 54.5) | .67 |
| HPV-68 | 3257 | 25 | 9059.8 | 0.3 | 3257 | 20 | 9005.2 | 0.2 | −24.2 (−135.9 to 33.7) | .55 |
| HPV-31/33/45 | 3314 | 16 | 9223.4 | 0.2 | 3312 | 30 | 9135.8 | 0.3 | 47.2 (0.0 to 73.1) | .05 |
Per-protocol cohort included those who were susceptible to infection by a relevant type of carcinogenic HPV other than HPV-16/18 (negative for the corresponding type of HPV DNA from day 0 through month 7), had received three doses of the test or control vaccine, had an effective endpoint follow-up visit after month 7, and had no severe protocol violation. CI = confidence interval; HPV = human papillomavirus.
Person-years at risk was defined as the cumulative follow-up years of the at-risk participants at the indicated time point.
The rate is the number of cases per 100 person-years at risk.
Fisher exact test was used to calculate the P values with two-sided test.
Vaccine cross-protection against carcinogenic human papillomavirus (HPV) persistent infection (6 m) other than HPV-16/18 in the per-protocol cohort*
| HPV type . | Vaccine group . | Control group . | Vaccine efficacy % (95% CI) . | P§ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants . | No. of events . | Person-years at risk† . | Rate‡ . | No. of participants . | No. of events . | Person-years at risk† . | Rate‡ . | |||
| HPV-31 | 3278 | 8 | 9151.3 | 0.1 | 3280 | 13 | 9092.0 | 0.1 | 38.9 (−59.1 to 78.0) | .38 |
| HPV-33 | 3282 | 7 | 9159.7 | 0.1 | 3274 | 15 | 9072.3 | 0.2 | 53.8 (−20.4 to 84.1) | .13 |
| HPV-35 | 3289 | 8 | 9180.8 | 0.1 | 3286 | 15 | 9116.2 | 0.2 | 47.0 (−33.1 to 80.6) | .21 |
| HPV-39 | 3248 | 33 | 9011.2 | 0.4 | 3243 | 31 | 8952.0 | 0.3 | −5.8 (−78.5 to 37.2) | .90 |
| HPV-45 | 3299 | 1 | 9228.3 | 0.01 | 3298 | 5 | 9167.4 | 0.1 | 80.1 (−77.6 to 99.6) | .22 |
| HPV-51 | 3225 | 41 | 8916.4 | 0.5 | 3218 | 28 | 8878.6 | 0.3 | −45.8 (−144.8 to 12.0) | .15 |
| HPV-52 | 3075 | 67 | 8455.1 | 0.8 | 3108 | 52 | 8521.9 | 0.6 | −29.9 (−90.3 to 10.9) | .20 |
| HPV-56 | 3257 | 31 | 9041.7 | 0.3 | 3266 | 29 | 9016.6 | 0.3 | −6.6 (−83.3 to 37.8) | .90 |
| HPV-58 | 3258 | 23 | 9059.5 | 0.3 | 3248 | 28 | 8978.3 | 0.3 | 18.6 (−46.6 to 55.2) | .58 |
| HPV-59 | 3283 | 15 | 9157.8 | 0.2 | 3289 | 12 | 9125.8 | 0.1 | −24.6 (−191.4 to 45.6) | .70 |
| HPV-66 | 3261 | 22 | 9065.8 | 0.2 | 3263 | 26 | 9024.0 | 0.3 | 15.8 (−54.6 to 54.5) | .67 |
| HPV-68 | 3257 | 25 | 9059.8 | 0.3 | 3257 | 20 | 9005.2 | 0.2 | −24.2 (−135.9 to 33.7) | .55 |
| HPV-31/33/45 | 3314 | 16 | 9223.4 | 0.2 | 3312 | 30 | 9135.8 | 0.3 | 47.2 (0.0 to 73.1) | .05 |
| HPV type . | Vaccine group . | Control group . | Vaccine efficacy % (95% CI) . | P§ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants . | No. of events . | Person-years at risk† . | Rate‡ . | No. of participants . | No. of events . | Person-years at risk† . | Rate‡ . | |||
| HPV-31 | 3278 | 8 | 9151.3 | 0.1 | 3280 | 13 | 9092.0 | 0.1 | 38.9 (−59.1 to 78.0) | .38 |
| HPV-33 | 3282 | 7 | 9159.7 | 0.1 | 3274 | 15 | 9072.3 | 0.2 | 53.8 (−20.4 to 84.1) | .13 |
| HPV-35 | 3289 | 8 | 9180.8 | 0.1 | 3286 | 15 | 9116.2 | 0.2 | 47.0 (−33.1 to 80.6) | .21 |
| HPV-39 | 3248 | 33 | 9011.2 | 0.4 | 3243 | 31 | 8952.0 | 0.3 | −5.8 (−78.5 to 37.2) | .90 |
| HPV-45 | 3299 | 1 | 9228.3 | 0.01 | 3298 | 5 | 9167.4 | 0.1 | 80.1 (−77.6 to 99.6) | .22 |
| HPV-51 | 3225 | 41 | 8916.4 | 0.5 | 3218 | 28 | 8878.6 | 0.3 | −45.8 (−144.8 to 12.0) | .15 |
| HPV-52 | 3075 | 67 | 8455.1 | 0.8 | 3108 | 52 | 8521.9 | 0.6 | −29.9 (−90.3 to 10.9) | .20 |
| HPV-56 | 3257 | 31 | 9041.7 | 0.3 | 3266 | 29 | 9016.6 | 0.3 | −6.6 (−83.3 to 37.8) | .90 |
| HPV-58 | 3258 | 23 | 9059.5 | 0.3 | 3248 | 28 | 8978.3 | 0.3 | 18.6 (−46.6 to 55.2) | .58 |
| HPV-59 | 3283 | 15 | 9157.8 | 0.2 | 3289 | 12 | 9125.8 | 0.1 | −24.6 (−191.4 to 45.6) | .70 |
| HPV-66 | 3261 | 22 | 9065.8 | 0.2 | 3263 | 26 | 9024.0 | 0.3 | 15.8 (−54.6 to 54.5) | .67 |
| HPV-68 | 3257 | 25 | 9059.8 | 0.3 | 3257 | 20 | 9005.2 | 0.2 | −24.2 (−135.9 to 33.7) | .55 |
| HPV-31/33/45 | 3314 | 16 | 9223.4 | 0.2 | 3312 | 30 | 9135.8 | 0.3 | 47.2 (0.0 to 73.1) | .05 |
Per-protocol cohort included those who were susceptible to infection by a relevant type of carcinogenic HPV other than HPV-16/18 (negative for the corresponding type of HPV DNA from day 0 through month 7), had received three doses of the test or control vaccine, had an effective endpoint follow-up visit after month 7, and had no severe protocol violation. CI = confidence interval; HPV = human papillomavirus.
Person-years at risk was defined as the cumulative follow-up years of the at-risk participants at the indicated time point.
The rate is the number of cases per 100 person-years at risk.
Fisher exact test was used to calculate the P values with two-sided test.
One month after the third dose of the HPV vaccine, all 2302 baseline seronegative women seroconverted for anti-HPV-16 (100.0%); the mean IgG antibody level (GMC) was 790.4 IU/mL (95% CI = 767.5 to 813.9 IU/mL), which was over 100 times higher than the mean antibody level acquired from natural infection (7.1 IU/mL, calculated from the antibody level at day 0 of 367 seropositive participants at entry) (data not shown). For HPV-18, the seroconversion rate was 99.9% (2799 of 2802), with a GMC of 267.9 IU/mL (95% CI = 260.4 to 275.6 IU/mL), which was over 50 times higher than the mean antibody level acquired from natural infection (4.7 IU/mL, the GMC of day 0 from 149 seropositive participants at entry) (data not shown). The vaccine-induced IgG antibodies decreased approximately 8 times (anti-HPV-16) or approximately 10 times (anti-HPV-18) during the first year after the third vaccination and then remained relatively stable to month 42 at levels of approximately 100 IU/mL (anti-HPV-16) and approximately 25 IU/mL (anti-HPV-18) (Figure 3).
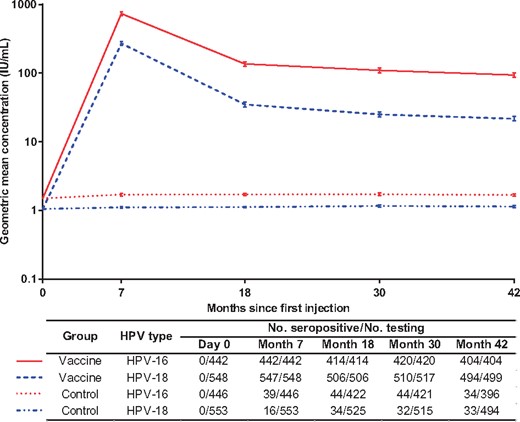
The persistence of vaccine-induced type-specific immunoglobulin G antibodies in women who were administered three doses of the assigned vaccine or control regimens. The immune persistence of the vaccine was assessed in a subcohort containing susceptible participants who were seronegative for the relevant types of human papillomavirus (HPV) at entry, who were DNA negative from day 0 to month 7, and who received three doses of the HPV vaccine or control HepE vaccine (per-protocol set for the immune persistence endpoint cohort). The positive samples were further quantified using references traceable to the World Health Organization international standards for antibodies against HPV-16 (NIBSC code 05/134) or HPV-18 (NIBSC code 10/140), expressed in international units (IUs). The lower detection limits of the Escherichia coli-produced virus-like particle-based enzyme-linked immunosorbent assays were 3.1 IU/mL for HPV-16 antibodies and 2.0 IU/mL for HPV-18 antibodies. For calculating the geometric mean concentration of the antibodies, antibody titers below the lower detection limit of the assay were given an arbitrary value of one-half the cutoff value. The bar showed the 95% confidence interval of the corresponding antibody level.
Total AEs, local or systemic reactions, and unsolicited events occurred at similar rates between groups (Table 4, Supplementary Table 6, available online). Pain at the injection site (34.0%) and fever (>37.0°C, 35.1%) were the most common reactions that occurred in the vaccine group. Most AEs were mild. The participants reporting SAEs were distributed similarly between groups, and none of the SAEs were considered to be related to vaccination (Supplementary Table 7, available online).
| AEs . | No. of AEs (rate, %) . | |
|---|---|---|
| Vaccine group . | Control group . | |
| No. of participants who received ≥1 injection | 3691† | 3681† |
| Solicited local AEs within 72 h after each vaccination | ||
| All local AEs | 1396 (37.8) | 1552 (42.2) |
| Local AEs ≥ grade 3 | 24 (0.7) | 68 (1.8) |
| All pain | 1256 (34.0) | 1328 (36.1) |
| Pain ≥ grade 3 | 0 (0) | 0 (0) |
| All induration | 261 (7.1) | 326 (8.9) |
| Induration ≥ grade 3 | 11 (0.3) | 25 (0.7) |
| All red | 162 (4.4) | 244 (6.6) |
| Red ≥ grade 3 | 6 (0.2) | 40 (1.1) |
| All swelling | 160 (4.3) | 282 (7.7) |
| Swelling ≥ grade 3 | 15 (0.4) | 52 (1.4) |
| All pruritus | 163 (4.4) | 377 (10.2) |
| Pruritus ≥ grade 3 | 0 (0) | 0 (0) |
| Solicited systemic AEs within 72 h after each vaccination | ||
| All systemic AEs | 1683 (45.6) | 1701 (46.2) |
| Systemic AEs ≥ grade 3 | 8 (0.2) | 5 (0.1) |
| All fever | 1296 (35.1) | 1253 (34.0) |
| Fever ≥ grade 3 | 7 (0.2) | 4 (0.1) |
| All fatigue and weakness | 275 (7.5) | 282 (7.7) |
| Fatigue and weakness ≥ grade 3 | 0 (0) | 1 (0.03) |
| All headache | 259 (7.0) | 274 (7.4) |
| Headache ≥ grade 3 | 0 (0) | 0 (0) |
| All cough | 189 (5.1) | 217 (5.9) |
| Cough ≥ grade 3 | 0 (0) | 0 (0) |
| All myalgia | 122 (3.3) | 164 (4.5) |
| Myalgia ≥ grade 3 | 0 (0) | 0 (0) |
| All nausea | 118 (3.2) | 125 (3.4) |
| Nausea ≥ grade 3 | 0 (0) | 0 (0) |
| All diarrhea | 102 (2.8) | 104 (2.8) |
| Diarrhea ≥ grade 3 | 1 (0.03) | 0 (0) |
| All allergic reaction | 40 (1.1) | 62 (1.7) |
| Allergic reaction ≥ grade 3 | 0 (0) | 0 (0) |
| All vomiting | 36 (1.0) | 44 (1.2) |
| Vomiting ≥ grade 3 | 0 (0) | 0 (0) |
| Unsolicited events within 30 days after each vaccination‡ | ||
| All | 1402 (38.0) | 1380 (37.5) |
| ≥Grade 3 | 19 (0.5) | 23 (0.6) |
| Serious AEs during the entire study§ | ||
| Within 30 days after each vaccination | ||
| All | 21 (0.6) | 22 (0.6) |
| Death‖ | 1(0.03) | 0 (0) |
| During the entire study | ||
| All | 205(5.6) | 244(6.6) |
| Death‖ | 3(0.08) | 3(0.08) |
| Pregnancy and pregnancy outcomes during the entire study | ||
| Women becoming pregnant | 977 (26.5) | 981 (26.7) |
| No. of pregnancy events¶ | 1187 | 1219 |
| Ongoing | 4 (0.3) | 7 (0.6) |
| Normal delivery | 554 (46.7) | 613 (50.3) |
| Spontaneous abortion | 68 (5.7) | 68 (5.6) |
| Stillbirth | 10 (0.8) | 14 (1.1) |
| Maternal complications | 11 (0.9) | 7 (0.6) |
| Elective termination | 540 (45.5) | 510 (41.8) |
| No. of infants# | 556 | 615 |
| Normal infant | 555 (99.8)# | 613 (99.7)# |
| Congenital anomaly | 0 (0) | 2 (0.3)** |
| Other complications or abnormality | 1 (0.2)†† | 0 (0) |
| AEs . | No. of AEs (rate, %) . | |
|---|---|---|
| Vaccine group . | Control group . | |
| No. of participants who received ≥1 injection | 3691† | 3681† |
| Solicited local AEs within 72 h after each vaccination | ||
| All local AEs | 1396 (37.8) | 1552 (42.2) |
| Local AEs ≥ grade 3 | 24 (0.7) | 68 (1.8) |
| All pain | 1256 (34.0) | 1328 (36.1) |
| Pain ≥ grade 3 | 0 (0) | 0 (0) |
| All induration | 261 (7.1) | 326 (8.9) |
| Induration ≥ grade 3 | 11 (0.3) | 25 (0.7) |
| All red | 162 (4.4) | 244 (6.6) |
| Red ≥ grade 3 | 6 (0.2) | 40 (1.1) |
| All swelling | 160 (4.3) | 282 (7.7) |
| Swelling ≥ grade 3 | 15 (0.4) | 52 (1.4) |
| All pruritus | 163 (4.4) | 377 (10.2) |
| Pruritus ≥ grade 3 | 0 (0) | 0 (0) |
| Solicited systemic AEs within 72 h after each vaccination | ||
| All systemic AEs | 1683 (45.6) | 1701 (46.2) |
| Systemic AEs ≥ grade 3 | 8 (0.2) | 5 (0.1) |
| All fever | 1296 (35.1) | 1253 (34.0) |
| Fever ≥ grade 3 | 7 (0.2) | 4 (0.1) |
| All fatigue and weakness | 275 (7.5) | 282 (7.7) |
| Fatigue and weakness ≥ grade 3 | 0 (0) | 1 (0.03) |
| All headache | 259 (7.0) | 274 (7.4) |
| Headache ≥ grade 3 | 0 (0) | 0 (0) |
| All cough | 189 (5.1) | 217 (5.9) |
| Cough ≥ grade 3 | 0 (0) | 0 (0) |
| All myalgia | 122 (3.3) | 164 (4.5) |
| Myalgia ≥ grade 3 | 0 (0) | 0 (0) |
| All nausea | 118 (3.2) | 125 (3.4) |
| Nausea ≥ grade 3 | 0 (0) | 0 (0) |
| All diarrhea | 102 (2.8) | 104 (2.8) |
| Diarrhea ≥ grade 3 | 1 (0.03) | 0 (0) |
| All allergic reaction | 40 (1.1) | 62 (1.7) |
| Allergic reaction ≥ grade 3 | 0 (0) | 0 (0) |
| All vomiting | 36 (1.0) | 44 (1.2) |
| Vomiting ≥ grade 3 | 0 (0) | 0 (0) |
| Unsolicited events within 30 days after each vaccination‡ | ||
| All | 1402 (38.0) | 1380 (37.5) |
| ≥Grade 3 | 19 (0.5) | 23 (0.6) |
| Serious AEs during the entire study§ | ||
| Within 30 days after each vaccination | ||
| All | 21 (0.6) | 22 (0.6) |
| Death‖ | 1(0.03) | 0 (0) |
| During the entire study | ||
| All | 205(5.6) | 244(6.6) |
| Death‖ | 3(0.08) | 3(0.08) |
| Pregnancy and pregnancy outcomes during the entire study | ||
| Women becoming pregnant | 977 (26.5) | 981 (26.7) |
| No. of pregnancy events¶ | 1187 | 1219 |
| Ongoing | 4 (0.3) | 7 (0.6) |
| Normal delivery | 554 (46.7) | 613 (50.3) |
| Spontaneous abortion | 68 (5.7) | 68 (5.6) |
| Stillbirth | 10 (0.8) | 14 (1.1) |
| Maternal complications | 11 (0.9) | 7 (0.6) |
| Elective termination | 540 (45.5) | 510 (41.8) |
| No. of infants# | 556 | 615 |
| Normal infant | 555 (99.8)# | 613 (99.7)# |
| Congenital anomaly | 0 (0) | 2 (0.3)** |
| Other complications or abnormality | 1 (0.2)†† | 0 (0) |
Symptoms with a frequency greater than 1% in any group are listed. Grade III pain was defined as influencing daily activities or requiring multiple uses of narcotic analgesics. Grade III induration, redness, and swelling were defined as having a diameter of more than 30 mm or limiting daily activities. Grade III pruritus was defined as having systemic pruritus. Grade I fever was defined as a temperature of more than 37.0°C, and grade III fever was defined as a temperature of more than 39.0°C. Grade III fatigue and weakness were defined as normal activities being weakened more than 50% with a strong influence on daily activities in addition to the prevention of working. Grade III headache was defined as serious influence on daily activities, with response to the initial anesthetic treatment. Grade III cough was defined as having a paroxysmal cough that could not be controlled via treatment. Grade III myalgia was defined as having severe muscular tenderness, with a strong influence on daily activities. Grade III nausea and vomiting were defined as having nausea or vomiting 6 times within 24 hours and no food intake, with intravenous infusion required. Grade III diarrhea was defined as having watery stools more than 6 times per day or bloody diarrhea, orthostatic hypotension, electrolyte imbalance, and more than 2 L intravenous infusion. A grade III allergic reaction was defined as extensive urticaria and angioedema. AE = adverse event.
By mistake, one participant in the vaccine group was given the control vaccine for dose 1, and two participants in the control group were given the test vaccine for dose 3. These three participants were classified into the vaccine group for safety analysis, according to the protocol.
Unsolicited AEs included any AEs that occurred during the period from day 8 to day 30 after each vaccination and any AEs that occurred within 7 days after each vaccination but that had not been listed on the diary card for registering solicited AEs. A grade III unsolicited event was defined as a severe and noticeable limitation of daily activities that required help in daily life, medical treatment, or hospitalization.
The data and safety monitoring board did not consider any of the SAEs, death or not, to be related to vaccination. All SAEs, categorized by organ system and treatment group, are provided in Supplementary Table 7 (available online).
A total of six participants died during the entire study. Of them, one participant in the vaccine group committed suicide by consuming poison at 13 days post the first injection, and one participant with a history of type 2 diabetes (for >10 years) in the vaccine group died from diabetic ketoacidosis at 14 months post the third injection. The other four participants died from traffic accidents at 3 to 24 months after their last injections.
Some women became pregnant more than once.
There were four twins, two in the vaccine group and two in the control group.
One woman who received three doses of the HepE vaccine was diagnosed with 7 + 2 weeks of pregnancy at 72 days after the last injection, and the fetus was found to have hydronephrosis. Her baby girl was born after the normal period of gestation by caesarean section, and the baby was then diagnosed with congenital ureter stenosis of the right kidney one-half year later. A baby boy born to a participant who received three doses of the HepE vaccine was diagnosed with heritable thalassemia. The data and safety monitoring board did not consider these events to be related to vaccination.
One participant became pregnant at 36 months after her third injection with the test human papillomavirus vaccine. At 28 + 6 weeks of pregnancy, due to a complication of severe preeclampsia, a male infant with a body weight of 1.4 kg was delivered by Caesarean section. The newborn improved and was discharged after receiving 1 week of medical care and treatment. The data and safety monitoring board did not consider this event to be related to vaccination.
| AEs . | No. of AEs (rate, %) . | |
|---|---|---|
| Vaccine group . | Control group . | |
| No. of participants who received ≥1 injection | 3691† | 3681† |
| Solicited local AEs within 72 h after each vaccination | ||
| All local AEs | 1396 (37.8) | 1552 (42.2) |
| Local AEs ≥ grade 3 | 24 (0.7) | 68 (1.8) |
| All pain | 1256 (34.0) | 1328 (36.1) |
| Pain ≥ grade 3 | 0 (0) | 0 (0) |
| All induration | 261 (7.1) | 326 (8.9) |
| Induration ≥ grade 3 | 11 (0.3) | 25 (0.7) |
| All red | 162 (4.4) | 244 (6.6) |
| Red ≥ grade 3 | 6 (0.2) | 40 (1.1) |
| All swelling | 160 (4.3) | 282 (7.7) |
| Swelling ≥ grade 3 | 15 (0.4) | 52 (1.4) |
| All pruritus | 163 (4.4) | 377 (10.2) |
| Pruritus ≥ grade 3 | 0 (0) | 0 (0) |
| Solicited systemic AEs within 72 h after each vaccination | ||
| All systemic AEs | 1683 (45.6) | 1701 (46.2) |
| Systemic AEs ≥ grade 3 | 8 (0.2) | 5 (0.1) |
| All fever | 1296 (35.1) | 1253 (34.0) |
| Fever ≥ grade 3 | 7 (0.2) | 4 (0.1) |
| All fatigue and weakness | 275 (7.5) | 282 (7.7) |
| Fatigue and weakness ≥ grade 3 | 0 (0) | 1 (0.03) |
| All headache | 259 (7.0) | 274 (7.4) |
| Headache ≥ grade 3 | 0 (0) | 0 (0) |
| All cough | 189 (5.1) | 217 (5.9) |
| Cough ≥ grade 3 | 0 (0) | 0 (0) |
| All myalgia | 122 (3.3) | 164 (4.5) |
| Myalgia ≥ grade 3 | 0 (0) | 0 (0) |
| All nausea | 118 (3.2) | 125 (3.4) |
| Nausea ≥ grade 3 | 0 (0) | 0 (0) |
| All diarrhea | 102 (2.8) | 104 (2.8) |
| Diarrhea ≥ grade 3 | 1 (0.03) | 0 (0) |
| All allergic reaction | 40 (1.1) | 62 (1.7) |
| Allergic reaction ≥ grade 3 | 0 (0) | 0 (0) |
| All vomiting | 36 (1.0) | 44 (1.2) |
| Vomiting ≥ grade 3 | 0 (0) | 0 (0) |
| Unsolicited events within 30 days after each vaccination‡ | ||
| All | 1402 (38.0) | 1380 (37.5) |
| ≥Grade 3 | 19 (0.5) | 23 (0.6) |
| Serious AEs during the entire study§ | ||
| Within 30 days after each vaccination | ||
| All | 21 (0.6) | 22 (0.6) |
| Death‖ | 1(0.03) | 0 (0) |
| During the entire study | ||
| All | 205(5.6) | 244(6.6) |
| Death‖ | 3(0.08) | 3(0.08) |
| Pregnancy and pregnancy outcomes during the entire study | ||
| Women becoming pregnant | 977 (26.5) | 981 (26.7) |
| No. of pregnancy events¶ | 1187 | 1219 |
| Ongoing | 4 (0.3) | 7 (0.6) |
| Normal delivery | 554 (46.7) | 613 (50.3) |
| Spontaneous abortion | 68 (5.7) | 68 (5.6) |
| Stillbirth | 10 (0.8) | 14 (1.1) |
| Maternal complications | 11 (0.9) | 7 (0.6) |
| Elective termination | 540 (45.5) | 510 (41.8) |
| No. of infants# | 556 | 615 |
| Normal infant | 555 (99.8)# | 613 (99.7)# |
| Congenital anomaly | 0 (0) | 2 (0.3)** |
| Other complications or abnormality | 1 (0.2)†† | 0 (0) |
| AEs . | No. of AEs (rate, %) . | |
|---|---|---|
| Vaccine group . | Control group . | |
| No. of participants who received ≥1 injection | 3691† | 3681† |
| Solicited local AEs within 72 h after each vaccination | ||
| All local AEs | 1396 (37.8) | 1552 (42.2) |
| Local AEs ≥ grade 3 | 24 (0.7) | 68 (1.8) |
| All pain | 1256 (34.0) | 1328 (36.1) |
| Pain ≥ grade 3 | 0 (0) | 0 (0) |
| All induration | 261 (7.1) | 326 (8.9) |
| Induration ≥ grade 3 | 11 (0.3) | 25 (0.7) |
| All red | 162 (4.4) | 244 (6.6) |
| Red ≥ grade 3 | 6 (0.2) | 40 (1.1) |
| All swelling | 160 (4.3) | 282 (7.7) |
| Swelling ≥ grade 3 | 15 (0.4) | 52 (1.4) |
| All pruritus | 163 (4.4) | 377 (10.2) |
| Pruritus ≥ grade 3 | 0 (0) | 0 (0) |
| Solicited systemic AEs within 72 h after each vaccination | ||
| All systemic AEs | 1683 (45.6) | 1701 (46.2) |
| Systemic AEs ≥ grade 3 | 8 (0.2) | 5 (0.1) |
| All fever | 1296 (35.1) | 1253 (34.0) |
| Fever ≥ grade 3 | 7 (0.2) | 4 (0.1) |
| All fatigue and weakness | 275 (7.5) | 282 (7.7) |
| Fatigue and weakness ≥ grade 3 | 0 (0) | 1 (0.03) |
| All headache | 259 (7.0) | 274 (7.4) |
| Headache ≥ grade 3 | 0 (0) | 0 (0) |
| All cough | 189 (5.1) | 217 (5.9) |
| Cough ≥ grade 3 | 0 (0) | 0 (0) |
| All myalgia | 122 (3.3) | 164 (4.5) |
| Myalgia ≥ grade 3 | 0 (0) | 0 (0) |
| All nausea | 118 (3.2) | 125 (3.4) |
| Nausea ≥ grade 3 | 0 (0) | 0 (0) |
| All diarrhea | 102 (2.8) | 104 (2.8) |
| Diarrhea ≥ grade 3 | 1 (0.03) | 0 (0) |
| All allergic reaction | 40 (1.1) | 62 (1.7) |
| Allergic reaction ≥ grade 3 | 0 (0) | 0 (0) |
| All vomiting | 36 (1.0) | 44 (1.2) |
| Vomiting ≥ grade 3 | 0 (0) | 0 (0) |
| Unsolicited events within 30 days after each vaccination‡ | ||
| All | 1402 (38.0) | 1380 (37.5) |
| ≥Grade 3 | 19 (0.5) | 23 (0.6) |
| Serious AEs during the entire study§ | ||
| Within 30 days after each vaccination | ||
| All | 21 (0.6) | 22 (0.6) |
| Death‖ | 1(0.03) | 0 (0) |
| During the entire study | ||
| All | 205(5.6) | 244(6.6) |
| Death‖ | 3(0.08) | 3(0.08) |
| Pregnancy and pregnancy outcomes during the entire study | ||
| Women becoming pregnant | 977 (26.5) | 981 (26.7) |
| No. of pregnancy events¶ | 1187 | 1219 |
| Ongoing | 4 (0.3) | 7 (0.6) |
| Normal delivery | 554 (46.7) | 613 (50.3) |
| Spontaneous abortion | 68 (5.7) | 68 (5.6) |
| Stillbirth | 10 (0.8) | 14 (1.1) |
| Maternal complications | 11 (0.9) | 7 (0.6) |
| Elective termination | 540 (45.5) | 510 (41.8) |
| No. of infants# | 556 | 615 |
| Normal infant | 555 (99.8)# | 613 (99.7)# |
| Congenital anomaly | 0 (0) | 2 (0.3)** |
| Other complications or abnormality | 1 (0.2)†† | 0 (0) |
Symptoms with a frequency greater than 1% in any group are listed. Grade III pain was defined as influencing daily activities or requiring multiple uses of narcotic analgesics. Grade III induration, redness, and swelling were defined as having a diameter of more than 30 mm or limiting daily activities. Grade III pruritus was defined as having systemic pruritus. Grade I fever was defined as a temperature of more than 37.0°C, and grade III fever was defined as a temperature of more than 39.0°C. Grade III fatigue and weakness were defined as normal activities being weakened more than 50% with a strong influence on daily activities in addition to the prevention of working. Grade III headache was defined as serious influence on daily activities, with response to the initial anesthetic treatment. Grade III cough was defined as having a paroxysmal cough that could not be controlled via treatment. Grade III myalgia was defined as having severe muscular tenderness, with a strong influence on daily activities. Grade III nausea and vomiting were defined as having nausea or vomiting 6 times within 24 hours and no food intake, with intravenous infusion required. Grade III diarrhea was defined as having watery stools more than 6 times per day or bloody diarrhea, orthostatic hypotension, electrolyte imbalance, and more than 2 L intravenous infusion. A grade III allergic reaction was defined as extensive urticaria and angioedema. AE = adverse event.
By mistake, one participant in the vaccine group was given the control vaccine for dose 1, and two participants in the control group were given the test vaccine for dose 3. These three participants were classified into the vaccine group for safety analysis, according to the protocol.
Unsolicited AEs included any AEs that occurred during the period from day 8 to day 30 after each vaccination and any AEs that occurred within 7 days after each vaccination but that had not been listed on the diary card for registering solicited AEs. A grade III unsolicited event was defined as a severe and noticeable limitation of daily activities that required help in daily life, medical treatment, or hospitalization.
The data and safety monitoring board did not consider any of the SAEs, death or not, to be related to vaccination. All SAEs, categorized by organ system and treatment group, are provided in Supplementary Table 7 (available online).
A total of six participants died during the entire study. Of them, one participant in the vaccine group committed suicide by consuming poison at 13 days post the first injection, and one participant with a history of type 2 diabetes (for >10 years) in the vaccine group died from diabetic ketoacidosis at 14 months post the third injection. The other four participants died from traffic accidents at 3 to 24 months after their last injections.
Some women became pregnant more than once.
There were four twins, two in the vaccine group and two in the control group.
One woman who received three doses of the HepE vaccine was diagnosed with 7 + 2 weeks of pregnancy at 72 days after the last injection, and the fetus was found to have hydronephrosis. Her baby girl was born after the normal period of gestation by caesarean section, and the baby was then diagnosed with congenital ureter stenosis of the right kidney one-half year later. A baby boy born to a participant who received three doses of the HepE vaccine was diagnosed with heritable thalassemia. The data and safety monitoring board did not consider these events to be related to vaccination.
One participant became pregnant at 36 months after her third injection with the test human papillomavirus vaccine. At 28 + 6 weeks of pregnancy, due to a complication of severe preeclampsia, a male infant with a body weight of 1.4 kg was delivered by Caesarean section. The newborn improved and was discharged after receiving 1 week of medical care and treatment. The data and safety monitoring board did not consider this event to be related to vaccination.
Pregnancy was reported in 977 women from the vaccine group (26.5%) and 981 women from the control group (26.7%) (Table 4). The proportions of women who became pregnant and their outcome profiles were similar for both groups. No congenital anomalies or pregnancy complications or abnormal events were associated with the vaccination in either group.
Discussion
Similar to the marketed HPV vaccines (10,12,24,25), the candidate E coli-produced HPV-16/18 vaccine almost entirely prevented high-grade genital lesions and persistent infection associated with the vaccine types of HPV in susceptible women. The vaccine was well tolerated; no SAEs related to vaccination were noted. Furthermore, robust vaccine-induced antibody responses for both types were detected in almost all participants who received three doses of the vaccine.
The test vaccine demonstrated consistent high prevention efficacy against HPV-16/18–associated CIN2+ lesions, CIN1+ lesions, and persistent infection in susceptible women. Additionally, the vaccine statistically significantly lowered the risk of incident infection as well as cytological abnormalities of the cervix related to HPV-16/18. Similar to the three licensed HPV vaccines, the test vaccine showed no effect on reducing genital lesions or clearing the prevalent infections for women with detectable HPV DNA of the vaccine types at baseline (11,26,27).
Limited by the lower rate and longer time needed to induce high-grade lesions for HPV-18 than HPV-16 (28–30), the vaccine efficacy against the type-specific CIN2+ endpoint was confirmed for HPV-16 but not HPV-18 in this interim analysis. Nevertheless, the test vaccine demonstrates consistent high efficacy for preventing HPV-18–associated persistent infection, incident infection, and cytological abnormalities. Thus, the E coli-produced HPV-16/18 vaccine is probably as effective at preventing high-grade lesions related to HPV-18 as it is at preventing high-grade lesions related to HPV-16.
The majority of the side effects of the test vaccine were mild. No SAEs were related to vaccination in either group. No meaningful clinical safety concerns regarding post-vaccination pregnancy or infant outcomes were raised. These findings confirm the well-tolerated profiles of HPV L1-VLP vaccines, which have been well established for the available HPV vaccines (31).
The test HPV vaccine induced robust and durable antibody responses to both types in the vaccine. The seroconversion rates approached or equaled 100%. Peak IgG GMCs at 1 month after the third dose were over 100-fold (for HPV-16) and 50-fold (for HPV-18) higher than those induced by natural infection, then declined approximately 10-fold 1 year later and were stable over the next 2 years. The remaining antibody titers at month 42 were 15-fold or 5-fold higher than those after natural infection for type 16 or 18, respectively. A similar serological pattern was also noted for Gardasil (32). The priority target population for HPV vaccination is young adolescents, a population for which a two-dose schedule was recommended. Immuno-bridging data for the test HPV vaccine in girls are available, which are encouraging (33).
Our study is not without limitations. Although encouraging cross-protection effects on infection caused by some non-vaccine types of HPV were observed, the study did not have enough power due to the small number of cases; more follow-up data are needed for the cross-protection analysis. Further studies such as head-to-head comparison studies with other HPV vaccines and bridging studies on girls of other races would be valuable for future implementation of this vaccine.
As a robust protein expression host, E coli has the characteristics of inexpensive culturing, high expression levels, short turnaround time, and easy scale-up if the protein can be correctly auto-folded (34). Hence, E coli has been widely used in the biopharmaceutical industry for producing recombinant proteins for therapeutic use. However, the formation of specific conformational structures is essential for ensuring the efficacy of VLP-based vaccines, which is not easily achieved in many cases. To date, only one E coli-expressed and VLP-based vaccine has been commercialized, which is well tolerated and highly efficacious against hepatitis E (16,17). It is estimated that millions of doses of the final HPV-16/18 VLP stock solution can be obtained from one 500-L fermentation through the current manufacturing process.
In conclusion, the E coli-produced bivalent HPV-16/18 vaccine is well tolerated and highly efficacious in preventing high-grade genital lesions and persistent infection associated with HPV-16/18. In addition to a potential new source of the cervical cancer preventive vaccine, the easy scale-up and low manufacturing cost of the E coli-based manufacturing process is a promising improvement with regard to accessibility for resource-limited countries.
Funding
This work was supported by grants from the Chinese National High-tech R&D Program (863 program, 2012AA02A408), the Chinese National Major Scientific and Technological Special Project for “Significant New Drug Development” (2018ZX09308010 and 2012ZX09101316), the National Natural Science Foundation of China (81673240 and U1705283), the Fujian Provincial Major Scientific and Technological Project (2015YZ0002), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS, 2017-I2M-B&R-03, and 2016-I2M-1–019), and Xiamen Innovax.
Notes
The study funder, Xiamen Innovax, prepared the test and control vaccines. The other funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. Investigators from the HPV-003 Study Group collected data and cared for the study participants.
Y-ML, H-RP, Z-JL, MG, X-HL, B-ZL, and H-HC report being either current or former employees of Xiamen Innovax. No other potential conflicts of interest relevant to this article were reported.
JZ, Y-LQ, N-SX, TW, R-CL, Y-MH, L-HW, C-GL, WC, S-JH, F-HZ, J-ZW, F-CZ, S-WL, H-RP, and Y-ML contributed to the study design, and JZ, Y-LQ, TW, S-JH, Y-YS, and MG were the core team for data analysis and manuscript preparation. All the authors had access to a summary of all the trial data and had final responsibility for the decision to submit for publication.
We thank the following investigators for their contributions to this HPV-003 study (listed in alphabetical order): other investigators from Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College: Bin Liu, Dao-Kuan Liu, Feng Chen, Hui-Fang Xu, Jian-Feng Cui, Li Zhang, and Yu-Qian Zhao; Liuzhou Maternity and Child Healthcare Hospital: Hong-Min Zhou and Hua Tao; Peking University People’s Hospital: Jing-Ran Li, Ming-Zhu Li, Shu-Hui Cui, and Yun Zhao; Liuzhou Center for Disease Control and Prevention: Hai-Bo Wang and Xiao-Wei Wang; Funing Center for Disease Control and Prevention: Qing-Xia Dai, Shang-Bo Yang, Tian-Jie Yue, and Ya-Chun Gao; Xinmi Maternal and Child Health Hospital: Hong-Juan Li and Jin-Hong Liu; Yangcheng Maternal and Child Health Hospital: Yuan-Ping Ma; Fengning Hospital of Traditional Chinese Medicine: Xian-Qing Bi and Yang Zhai; other investigators from Xiamen University: Chun-Lan Zhuang, Fang-Fang Hu, Fei-Xue Wei, Huan Yu, Lin Wang, Ling-Xian Qiu, Qiao-Qiao Song, Sheng-Xiang Ge, Shu Chen, Si-Jie Zhuang, Wei Sheng, Wen-Gang He, Xiao-Hui Liu, Xiao-Ping Min, Xin-Jing Ma, Xiao-Juan Yu, Xing-Mei Yao, Xun Fang, Ya-Fei Li, Ya Zheng, Yue Huang, and Zhao-Feng Bi; Xiamen Innovax: Bi-Zhen Lin and Huan-Huan Cao; Data and Safety Monitoring Board: Jie-Lai Xia (chair), Ai-Qiang Xu, Li Geng, Li Zhu, and Si-Yan Zhan; and Statistical and Programming Support: Qiong Du.
References
The Global Alliance for Vaccines and Immunisation, HPV Supply and Procurement Roadmap. https://www.gavi.org/library/gavi-documents/supply-procurement/hpv-supply-and-procurement--roadmap/. Accessed April 4,
WHO Director-General calls for all countries to take action to help end the suffering caused. WHO: sexual and reproductive health. http://www.who.int/reproductivehealth/call-to-action-elimination-cervical-cancer/en/. Accessed April 4,
The FUTURE II Study Group.
Author notes
You-Lin Qiao, TingWu, Rong-Cheng Li, Yue-Mei Hu, Li-Hui Wei, Chang-Gui Li and Wen Chen contributed equally to this work.
- cervical cancer
- human papillomavirus
- antibody formation
- china
- dna
- safety
- vaccines
- infections
- persistence
- genital lesions
- escherichia coli
- papillomavirus vaccine, bivalent
- neutralizing antibodies
- human papillomavirus 16
- per protocol analysis
- interim analysis
- surrogate endpoints
- adverse event
- immunogenicity


