-
PDF
- Split View
-
Views
-
Cite
Cite
Corinne R Leach, Catherine M Alfano, Jessica Potts, Lisa Gallicchio, K Robin Yabroff, Kevin C Oeffinger, Erin E Hahn, Lawrence N Shulman, Shawna V Hudson, Personalized Cancer Follow-Up Care Pathways: A Delphi Consensus of Research Priorities, JNCI: Journal of the National Cancer Institute, Volume 112, Issue 12, December 2020, Pages 1183–1189, https://doi.org/10.1093/jnci/djaa053
Close - Share Icon Share
Abstract
Development of personalized, stratified follow-up care pathways where care intensity and setting vary with needs could improve cancer survivor outcomes and efficiency of health-care delivery. Advancing such an approach in the United States requires identification and prioritization of the most pressing research and data needed to create and implement personalized care pathway models. Cancer survivorship research and care experts (n = 39) participated in an in-person workshop on this topic in 2018. Using a modified Delphi technique—a structured, validated system for identifying consensus—an expert panel identified critical research questions related to operationalizing personalized, stratified follow-up care pathways for individuals diagnosed with cancer. Consensus for the top priority research questions was achieved iteratively through 3 rounds: item generation, item consolidation, and selection of the final list of priority research questions. From the 28 research questions that were generated, 11 research priority questions were identified. The questions were categorized into 4 priority themes: determining outcome measures for new care pathways, developing and evaluating new care pathways, incentivizing new care pathway delivery, and providing technology and infrastructure to support self-management. Existing data sources to begin answering questions were also identified. Although existing data sources, including cancer registry, electronic medical record, and health insurance claims data, can be enhanced to begin addressing some questions, additional research resources are needed to address these priority questions.
The number of cancer survivors living in the United States is projected to increase from 16.9 million in 2019 to more than 22.1 million by 2030 (1). Many cancer survivors have complex medical needs including surveillance for recurrence, management of chronic effects of treatment, prevention or mitigation of late effects, and screening for subsequent cancers in addition to the management of their comorbid conditions and general health promotion (2–7). Cancer care is costly and projected to grow with the increasing prevalence of cancer survivors and expensive new treatments (8). Long-term survivorship care that appropriately addresses survivors’ unique health-care needs must encompass both oncology-specific care and general primary care. This care cannot be provided solely by medical oncology teams because of the shortage of oncologists and limited training and time for attention to noncancer-related care (9). Primary care providers cannot provide this care alone because many have limited training in the specific needs of cancer survivors (10,11). Additionally, cancer survivors do not all have the same follow-up care needs; some report multiple, substantial health problems and needs, whereas others have relatively few (12–15). Type of cancer diagnosis, age at diagnosis, types of treatment(s), treatment duration, genetic factors, lifestyle behaviors, and comorbidities all play a role in determining the long-term and late effects that cancer survivors experience.
To meet the needs of the diverse population of cancer survivors, some health services researchers have suggested building personalized cancer follow-up care pathways that take into account these differing patient needs and vary the intensity, setting, and types of providers involved in care (10). These different care pathways may involve comanagement of patient needs, which requires multiple health-care providers to collaborate with a focus on helping engage and support patients in self-management of their health. This type of approach has been shown to optimize both patient outcomes and efficient use of the health-care system in other countries, such as Canada (16) and the United Kingdom (17,18).
Based on the need for a more strategic, national approach to address this issue, the American Cancer Society (ACS)–American Society of Clinical Oncology (ASCO) Summit on Implementing Personalized Stratified Cancer Survivorship Care was held in 2018 and identified 4 key strategies to move this field forward in the United States. One of the 4 key strategies was to identify and prioritize gaps for which research is needed to most efficiently develop and implement personalized survivorship care pathways (19). To support this research agenda setting process, ACS convened multidisciplinary survivorship research and care experts for a follow-up in-person meeting in the fall of 2018. This group identified research priority questions during the meeting that were then refined and further prioritized using a Delphi consensus process conducted through iterative online surveys. This commentary continues the work initiated by ACS and ASCO, as described above, to develop personalized stratified models of care in the United States by presenting a prioritized list of research questions needed to inform care pathways.
Delphi Process and Analysis
A modified Delphi method, an iterative survey process that builds consensus on a specific topic among a panel of experts using a series of questionnaires, was used (20). Responses are anonymous, and importantly, one individual’s voice does not carry more weight than others. This method allows participants to reassess their initial rankings or judgments during each round of the process based on how the group as a whole ranks the items (20). Data were collected using Research Electronic Data Capture, a software package commonly used in clinical and translational research (21). The Delphi process was reviewed by Emory University’s institutional review board and was deemed to be exempt from review because all responses were anonymous. Figure 1 presents a flowchart of the methods used.
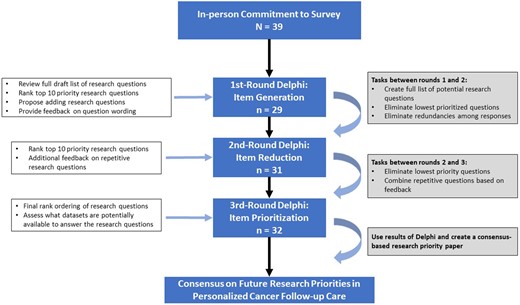
All participants in the Delphi had attended the ACS–ASCO Summit on Implementing Personalized Stratified Cancer Survivorship Care and/or the ACS State of the Science and Research Prioritization on Implementing Risk Stratified Survivorship Care in the United States. Individuals were invited to these meetings based on their research or clinical backgrounds and expertise in cancer survivorship care. Detailed demographic and employment characteristics of participants by round are shown in Table 1. Most respondents to the final survey identified as researchers (59.4%) or clinicians (53.1%). Respondents’ clinical and/or research focus was predominantly medical oncology (28.1%), health services research (21.9%), primary care (18.8%), epidemiology or biostatistics (15.6%), behavioral science or social science (15.6%), or a combination of categories. On average, participants in the final round of the Delphi survey had engaged in cancer care or survivorship research for 16.4 years.
| Characteristics . | R1 . | R2 . | R3 . |
|---|---|---|---|
| (n = 29) . | (n = 31) . | (n = 32) . | |
| Age, mean (range), y | 50.5 (34-72) | 50.7 (34-72) | 53.7 (35-73) |
| aCurrent role, No. | |||
| Clinician | 12 | 14 | 17 |
| Researcher | 19 | 22 | 19 |
| Funder or program officer | 7 | 4 | 5 |
| Patient/Policy advocate or survivor | 3 | 4 | 5 |
| Administrator | —b | 4 | 4 |
| aClinical/Research focus, No. | |||
| Behavioral or social science | 5 | 5 | 5 |
| Epidemiology or biostatistics | 5 | 5 | 5 |
| Health services research | 10 | 10 | 7 |
| Implementation science | —b | 2 | 4 |
| Medical oncology | 5 | 8 | 9 |
| Oncology nursing | 5 | 5 | 4 |
| Primary care | 3 | 3 | 6 |
| Years engaged with cancer care and/or survivorship research or work, mean (range) | 15.1 (3-40) | 16.9 (2-43) | 16.4 (5-28) |
| Characteristics . | R1 . | R2 . | R3 . |
|---|---|---|---|
| (n = 29) . | (n = 31) . | (n = 32) . | |
| Age, mean (range), y | 50.5 (34-72) | 50.7 (34-72) | 53.7 (35-73) |
| aCurrent role, No. | |||
| Clinician | 12 | 14 | 17 |
| Researcher | 19 | 22 | 19 |
| Funder or program officer | 7 | 4 | 5 |
| Patient/Policy advocate or survivor | 3 | 4 | 5 |
| Administrator | —b | 4 | 4 |
| aClinical/Research focus, No. | |||
| Behavioral or social science | 5 | 5 | 5 |
| Epidemiology or biostatistics | 5 | 5 | 5 |
| Health services research | 10 | 10 | 7 |
| Implementation science | —b | 2 | 4 |
| Medical oncology | 5 | 8 | 9 |
| Oncology nursing | 5 | 5 | 4 |
| Primary care | 3 | 3 | 6 |
| Years engaged with cancer care and/or survivorship research or work, mean (range) | 15.1 (3-40) | 16.9 (2-43) | 16.4 (5-28) |
Respondents could select multiple roles, and as a result, percentages were not provided. R = round.
Not assessed in R1.
| Characteristics . | R1 . | R2 . | R3 . |
|---|---|---|---|
| (n = 29) . | (n = 31) . | (n = 32) . | |
| Age, mean (range), y | 50.5 (34-72) | 50.7 (34-72) | 53.7 (35-73) |
| aCurrent role, No. | |||
| Clinician | 12 | 14 | 17 |
| Researcher | 19 | 22 | 19 |
| Funder or program officer | 7 | 4 | 5 |
| Patient/Policy advocate or survivor | 3 | 4 | 5 |
| Administrator | —b | 4 | 4 |
| aClinical/Research focus, No. | |||
| Behavioral or social science | 5 | 5 | 5 |
| Epidemiology or biostatistics | 5 | 5 | 5 |
| Health services research | 10 | 10 | 7 |
| Implementation science | —b | 2 | 4 |
| Medical oncology | 5 | 8 | 9 |
| Oncology nursing | 5 | 5 | 4 |
| Primary care | 3 | 3 | 6 |
| Years engaged with cancer care and/or survivorship research or work, mean (range) | 15.1 (3-40) | 16.9 (2-43) | 16.4 (5-28) |
| Characteristics . | R1 . | R2 . | R3 . |
|---|---|---|---|
| (n = 29) . | (n = 31) . | (n = 32) . | |
| Age, mean (range), y | 50.5 (34-72) | 50.7 (34-72) | 53.7 (35-73) |
| aCurrent role, No. | |||
| Clinician | 12 | 14 | 17 |
| Researcher | 19 | 22 | 19 |
| Funder or program officer | 7 | 4 | 5 |
| Patient/Policy advocate or survivor | 3 | 4 | 5 |
| Administrator | —b | 4 | 4 |
| aClinical/Research focus, No. | |||
| Behavioral or social science | 5 | 5 | 5 |
| Epidemiology or biostatistics | 5 | 5 | 5 |
| Health services research | 10 | 10 | 7 |
| Implementation science | —b | 2 | 4 |
| Medical oncology | 5 | 8 | 9 |
| Oncology nursing | 5 | 5 | 4 |
| Primary care | 3 | 3 | 6 |
| Years engaged with cancer care and/or survivorship research or work, mean (range) | 15.1 (3-40) | 16.9 (2-43) | 16.4 (5-28) |
Respondents could select multiple roles, and as a result, percentages were not provided. R = round.
Not assessed in R1.
For each survey round, up to 2 emails were sent to meeting participants with a link to the Research Electronic Data Capture survey. Round 1 began on November 5, 2018, and Round 3 closed on January 15, 2019. Response rates over the 3 rounds were 74.4%, 79.5%, and 82.1%, respectively (see Figure 1). Table 2 provides details regarding how questions were modified, combined, or dropped between rounds based on participant rankings and feedback. The table also specifies wording changes or the combining of questions based on participant feedback in each round.
Details on research question rankings, modifications to, and combinations of questions over the 3 Delphi rounds
| Research question . | R3 points . | R2 points . | R1 points . |
|---|---|---|---|
| What are appropriate outcome measures of “success” for risk-based follow-up care? | 243 | 196 | 145 |
| What important variables should be considered in analyses of appropriate intensity of care? | 177 | 109 | 88 |
| What types of system/policy changes might incentivize health systems to shift care for low-risk patients to PCPs or APPs? | 155 | 142 | 85 |
| What types of system/policy changes might incentivize oncologists to shift care for low-risk patients to PCPs or APPs? | 144 | 140 | 90 |
| How well do current risk assessment tools work in cancer populations or in specific subpopulations of survivors? | 130 | 82 | 84 |
| What policies, practices, and infrastructure are needed to facilitate self-management at the health system and practice levels? | 121 | 88 | 80 |
| What are the low-value and/or noncost-effective or harmful survivorship care components that need deimplementation? | 117 | 101 | 70 |
| How can clinical decision support tools facilitate triage of patients into risk-based care pathways? | 113 | 135 | 103 |
| How can technology and digital tools (eg, patient portals) be used to engage patients in their survivorship care and self-management outside of the exam room?a | 110 | 75 | — |
| How can patient-facing technology be used to reach and assist patients in self-management outside of clinical settings in ways that improve management of late and long-term effects, care quality, efficiency, and costs?a | — | 68 | — |
| How should technology be used to reach and assist patients in self-management outside of clinical settings while providing quality and efficient care?a | — | — | 75 |
| How can patient-facing self-management technology be used to enhance quality and efficient care?a | — | — | 62 |
| How can technology and digital tools be used to engage patients in their survivorship care outside the exam room?a | — | — | 58 |
| How can we adapt online patient portals to be more interactive and engaging around survivorship care?a | — | — | 25 |
| How should follow-up care clinicians (eg, PCP/APP-led follow-up clinicians) be engaged in/aware of care to facilitate provision of follow-up care?b | 103 | 75 | — |
| How should we engage PCPs in cancer care to facilitate eventual provision of follow-up care?b | — | — | 79 |
| What program changes in medical or nursing training need to occur to facilitate risk-based survivorship care in primary care settings or APP-led clinics?b | — | 31 | 35 |
| How can APPs best be used in risk-based follow-up care delivery?b | — | — | 35 |
| How can mathematic models incorporate clinical judgment, patient preferences, level of patient activation, and cost to identify appropriate pathways?c | 103 | 97 | — |
| How can mathematic models incorporate clinical judgment, patient preferences, and cost to identify appropriate pathways?c | — | — | 75 |
| What are the best practices for introducing risk-based follow-up care to manage expectations and fears of patients?d | 83 | 51 | — |
| How should risk-based follow-up care be introduced to manage expectations and fears of patients?d | — | — | 13 |
| What are appropriate payment models for risk-stratified follow-up care? | 80 | 57 | 33 |
| Which aspects of survivorship care are prime candidates for survivors to manage on their own using self-management training provided by their oncologist?e | 76 | — | — |
| Which aspects of survivorship care are prime candidates for self-management?e | — | 75 | 43 |
| How can self-management be used to reduce unnecessary clinic visits?e | — | — | 20 |
| What is the appropriate care pathway for patients with nononcological comorbidities and/or patients at high risk of other noncancer-related morbidity or mortality?f | 68 | 69 | — |
| What is the appropriate care pathway for patients at high risk of nononcologic-associated or other cause-related mortality?f | — | — | 52 |
| What process(es) ensures that patients with current or high risk of comorbidities see appropriate specialists?f | — | — | 43 |
| What interventions are needed to aid patients in their self-management of late/long-term chronic disease associated with cancer treatment?f | — | — | 54 |
| How can we use NCI-designated cancer centers and/or integrated health-care settings to test efficiencies in transitioning survivors out of oncology?g | — | 50 | — |
| What can we learn from NCI-designated cancer centers engaged in survivorship care?g | — | — | 36 |
| How can we use these and other integrated health-care settings to test efficiencies in transitioning survivors out of oncology?g | — | — | 65 |
| When in the cancer treatment time line should risk-based care be introduced to facilitate adoption? | — | 43 | 28 |
| What are methods for facilitating self-assessment of late effects and reporting to appropriate health-care professionals? | — | 44 | 11 |
| In what setting(s) is self-management most cost-effective? | — | — | 8 |
| Research question . | R3 points . | R2 points . | R1 points . |
|---|---|---|---|
| What are appropriate outcome measures of “success” for risk-based follow-up care? | 243 | 196 | 145 |
| What important variables should be considered in analyses of appropriate intensity of care? | 177 | 109 | 88 |
| What types of system/policy changes might incentivize health systems to shift care for low-risk patients to PCPs or APPs? | 155 | 142 | 85 |
| What types of system/policy changes might incentivize oncologists to shift care for low-risk patients to PCPs or APPs? | 144 | 140 | 90 |
| How well do current risk assessment tools work in cancer populations or in specific subpopulations of survivors? | 130 | 82 | 84 |
| What policies, practices, and infrastructure are needed to facilitate self-management at the health system and practice levels? | 121 | 88 | 80 |
| What are the low-value and/or noncost-effective or harmful survivorship care components that need deimplementation? | 117 | 101 | 70 |
| How can clinical decision support tools facilitate triage of patients into risk-based care pathways? | 113 | 135 | 103 |
| How can technology and digital tools (eg, patient portals) be used to engage patients in their survivorship care and self-management outside of the exam room?a | 110 | 75 | — |
| How can patient-facing technology be used to reach and assist patients in self-management outside of clinical settings in ways that improve management of late and long-term effects, care quality, efficiency, and costs?a | — | 68 | — |
| How should technology be used to reach and assist patients in self-management outside of clinical settings while providing quality and efficient care?a | — | — | 75 |
| How can patient-facing self-management technology be used to enhance quality and efficient care?a | — | — | 62 |
| How can technology and digital tools be used to engage patients in their survivorship care outside the exam room?a | — | — | 58 |
| How can we adapt online patient portals to be more interactive and engaging around survivorship care?a | — | — | 25 |
| How should follow-up care clinicians (eg, PCP/APP-led follow-up clinicians) be engaged in/aware of care to facilitate provision of follow-up care?b | 103 | 75 | — |
| How should we engage PCPs in cancer care to facilitate eventual provision of follow-up care?b | — | — | 79 |
| What program changes in medical or nursing training need to occur to facilitate risk-based survivorship care in primary care settings or APP-led clinics?b | — | 31 | 35 |
| How can APPs best be used in risk-based follow-up care delivery?b | — | — | 35 |
| How can mathematic models incorporate clinical judgment, patient preferences, level of patient activation, and cost to identify appropriate pathways?c | 103 | 97 | — |
| How can mathematic models incorporate clinical judgment, patient preferences, and cost to identify appropriate pathways?c | — | — | 75 |
| What are the best practices for introducing risk-based follow-up care to manage expectations and fears of patients?d | 83 | 51 | — |
| How should risk-based follow-up care be introduced to manage expectations and fears of patients?d | — | — | 13 |
| What are appropriate payment models for risk-stratified follow-up care? | 80 | 57 | 33 |
| Which aspects of survivorship care are prime candidates for survivors to manage on their own using self-management training provided by their oncologist?e | 76 | — | — |
| Which aspects of survivorship care are prime candidates for self-management?e | — | 75 | 43 |
| How can self-management be used to reduce unnecessary clinic visits?e | — | — | 20 |
| What is the appropriate care pathway for patients with nononcological comorbidities and/or patients at high risk of other noncancer-related morbidity or mortality?f | 68 | 69 | — |
| What is the appropriate care pathway for patients at high risk of nononcologic-associated or other cause-related mortality?f | — | — | 52 |
| What process(es) ensures that patients with current or high risk of comorbidities see appropriate specialists?f | — | — | 43 |
| What interventions are needed to aid patients in their self-management of late/long-term chronic disease associated with cancer treatment?f | — | — | 54 |
| How can we use NCI-designated cancer centers and/or integrated health-care settings to test efficiencies in transitioning survivors out of oncology?g | — | 50 | — |
| What can we learn from NCI-designated cancer centers engaged in survivorship care?g | — | — | 36 |
| How can we use these and other integrated health-care settings to test efficiencies in transitioning survivors out of oncology?g | — | — | 65 |
| When in the cancer treatment time line should risk-based care be introduced to facilitate adoption? | — | 43 | 28 |
| What are methods for facilitating self-assessment of late effects and reporting to appropriate health-care professionals? | — | 44 | 11 |
| In what setting(s) is self-management most cost-effective? | — | — | 8 |
Combined questions between rounds. Dashes indicate that the value was not included in the survey round. APP = advanced practice provider; NCI = National Cancer Institute; PCP = primary care provider; R = round.
Combined questions between rounds.
Revised question wording after R1.
Revised question wording after R1 based on participant qualitative feedback.
Combined questions and revised wording based on participant qualitative feedback.
Combined questions after R1.
Combined questions after R1.
Details on research question rankings, modifications to, and combinations of questions over the 3 Delphi rounds
| Research question . | R3 points . | R2 points . | R1 points . |
|---|---|---|---|
| What are appropriate outcome measures of “success” for risk-based follow-up care? | 243 | 196 | 145 |
| What important variables should be considered in analyses of appropriate intensity of care? | 177 | 109 | 88 |
| What types of system/policy changes might incentivize health systems to shift care for low-risk patients to PCPs or APPs? | 155 | 142 | 85 |
| What types of system/policy changes might incentivize oncologists to shift care for low-risk patients to PCPs or APPs? | 144 | 140 | 90 |
| How well do current risk assessment tools work in cancer populations or in specific subpopulations of survivors? | 130 | 82 | 84 |
| What policies, practices, and infrastructure are needed to facilitate self-management at the health system and practice levels? | 121 | 88 | 80 |
| What are the low-value and/or noncost-effective or harmful survivorship care components that need deimplementation? | 117 | 101 | 70 |
| How can clinical decision support tools facilitate triage of patients into risk-based care pathways? | 113 | 135 | 103 |
| How can technology and digital tools (eg, patient portals) be used to engage patients in their survivorship care and self-management outside of the exam room?a | 110 | 75 | — |
| How can patient-facing technology be used to reach and assist patients in self-management outside of clinical settings in ways that improve management of late and long-term effects, care quality, efficiency, and costs?a | — | 68 | — |
| How should technology be used to reach and assist patients in self-management outside of clinical settings while providing quality and efficient care?a | — | — | 75 |
| How can patient-facing self-management technology be used to enhance quality and efficient care?a | — | — | 62 |
| How can technology and digital tools be used to engage patients in their survivorship care outside the exam room?a | — | — | 58 |
| How can we adapt online patient portals to be more interactive and engaging around survivorship care?a | — | — | 25 |
| How should follow-up care clinicians (eg, PCP/APP-led follow-up clinicians) be engaged in/aware of care to facilitate provision of follow-up care?b | 103 | 75 | — |
| How should we engage PCPs in cancer care to facilitate eventual provision of follow-up care?b | — | — | 79 |
| What program changes in medical or nursing training need to occur to facilitate risk-based survivorship care in primary care settings or APP-led clinics?b | — | 31 | 35 |
| How can APPs best be used in risk-based follow-up care delivery?b | — | — | 35 |
| How can mathematic models incorporate clinical judgment, patient preferences, level of patient activation, and cost to identify appropriate pathways?c | 103 | 97 | — |
| How can mathematic models incorporate clinical judgment, patient preferences, and cost to identify appropriate pathways?c | — | — | 75 |
| What are the best practices for introducing risk-based follow-up care to manage expectations and fears of patients?d | 83 | 51 | — |
| How should risk-based follow-up care be introduced to manage expectations and fears of patients?d | — | — | 13 |
| What are appropriate payment models for risk-stratified follow-up care? | 80 | 57 | 33 |
| Which aspects of survivorship care are prime candidates for survivors to manage on their own using self-management training provided by their oncologist?e | 76 | — | — |
| Which aspects of survivorship care are prime candidates for self-management?e | — | 75 | 43 |
| How can self-management be used to reduce unnecessary clinic visits?e | — | — | 20 |
| What is the appropriate care pathway for patients with nononcological comorbidities and/or patients at high risk of other noncancer-related morbidity or mortality?f | 68 | 69 | — |
| What is the appropriate care pathway for patients at high risk of nononcologic-associated or other cause-related mortality?f | — | — | 52 |
| What process(es) ensures that patients with current or high risk of comorbidities see appropriate specialists?f | — | — | 43 |
| What interventions are needed to aid patients in their self-management of late/long-term chronic disease associated with cancer treatment?f | — | — | 54 |
| How can we use NCI-designated cancer centers and/or integrated health-care settings to test efficiencies in transitioning survivors out of oncology?g | — | 50 | — |
| What can we learn from NCI-designated cancer centers engaged in survivorship care?g | — | — | 36 |
| How can we use these and other integrated health-care settings to test efficiencies in transitioning survivors out of oncology?g | — | — | 65 |
| When in the cancer treatment time line should risk-based care be introduced to facilitate adoption? | — | 43 | 28 |
| What are methods for facilitating self-assessment of late effects and reporting to appropriate health-care professionals? | — | 44 | 11 |
| In what setting(s) is self-management most cost-effective? | — | — | 8 |
| Research question . | R3 points . | R2 points . | R1 points . |
|---|---|---|---|
| What are appropriate outcome measures of “success” for risk-based follow-up care? | 243 | 196 | 145 |
| What important variables should be considered in analyses of appropriate intensity of care? | 177 | 109 | 88 |
| What types of system/policy changes might incentivize health systems to shift care for low-risk patients to PCPs or APPs? | 155 | 142 | 85 |
| What types of system/policy changes might incentivize oncologists to shift care for low-risk patients to PCPs or APPs? | 144 | 140 | 90 |
| How well do current risk assessment tools work in cancer populations or in specific subpopulations of survivors? | 130 | 82 | 84 |
| What policies, practices, and infrastructure are needed to facilitate self-management at the health system and practice levels? | 121 | 88 | 80 |
| What are the low-value and/or noncost-effective or harmful survivorship care components that need deimplementation? | 117 | 101 | 70 |
| How can clinical decision support tools facilitate triage of patients into risk-based care pathways? | 113 | 135 | 103 |
| How can technology and digital tools (eg, patient portals) be used to engage patients in their survivorship care and self-management outside of the exam room?a | 110 | 75 | — |
| How can patient-facing technology be used to reach and assist patients in self-management outside of clinical settings in ways that improve management of late and long-term effects, care quality, efficiency, and costs?a | — | 68 | — |
| How should technology be used to reach and assist patients in self-management outside of clinical settings while providing quality and efficient care?a | — | — | 75 |
| How can patient-facing self-management technology be used to enhance quality and efficient care?a | — | — | 62 |
| How can technology and digital tools be used to engage patients in their survivorship care outside the exam room?a | — | — | 58 |
| How can we adapt online patient portals to be more interactive and engaging around survivorship care?a | — | — | 25 |
| How should follow-up care clinicians (eg, PCP/APP-led follow-up clinicians) be engaged in/aware of care to facilitate provision of follow-up care?b | 103 | 75 | — |
| How should we engage PCPs in cancer care to facilitate eventual provision of follow-up care?b | — | — | 79 |
| What program changes in medical or nursing training need to occur to facilitate risk-based survivorship care in primary care settings or APP-led clinics?b | — | 31 | 35 |
| How can APPs best be used in risk-based follow-up care delivery?b | — | — | 35 |
| How can mathematic models incorporate clinical judgment, patient preferences, level of patient activation, and cost to identify appropriate pathways?c | 103 | 97 | — |
| How can mathematic models incorporate clinical judgment, patient preferences, and cost to identify appropriate pathways?c | — | — | 75 |
| What are the best practices for introducing risk-based follow-up care to manage expectations and fears of patients?d | 83 | 51 | — |
| How should risk-based follow-up care be introduced to manage expectations and fears of patients?d | — | — | 13 |
| What are appropriate payment models for risk-stratified follow-up care? | 80 | 57 | 33 |
| Which aspects of survivorship care are prime candidates for survivors to manage on their own using self-management training provided by their oncologist?e | 76 | — | — |
| Which aspects of survivorship care are prime candidates for self-management?e | — | 75 | 43 |
| How can self-management be used to reduce unnecessary clinic visits?e | — | — | 20 |
| What is the appropriate care pathway for patients with nononcological comorbidities and/or patients at high risk of other noncancer-related morbidity or mortality?f | 68 | 69 | — |
| What is the appropriate care pathway for patients at high risk of nononcologic-associated or other cause-related mortality?f | — | — | 52 |
| What process(es) ensures that patients with current or high risk of comorbidities see appropriate specialists?f | — | — | 43 |
| What interventions are needed to aid patients in their self-management of late/long-term chronic disease associated with cancer treatment?f | — | — | 54 |
| How can we use NCI-designated cancer centers and/or integrated health-care settings to test efficiencies in transitioning survivors out of oncology?g | — | 50 | — |
| What can we learn from NCI-designated cancer centers engaged in survivorship care?g | — | — | 36 |
| How can we use these and other integrated health-care settings to test efficiencies in transitioning survivors out of oncology?g | — | — | 65 |
| When in the cancer treatment time line should risk-based care be introduced to facilitate adoption? | — | 43 | 28 |
| What are methods for facilitating self-assessment of late effects and reporting to appropriate health-care professionals? | — | 44 | 11 |
| In what setting(s) is self-management most cost-effective? | — | — | 8 |
Combined questions between rounds. Dashes indicate that the value was not included in the survey round. APP = advanced practice provider; NCI = National Cancer Institute; PCP = primary care provider; R = round.
Combined questions between rounds.
Revised question wording after R1.
Revised question wording after R1 based on participant qualitative feedback.
Combined questions and revised wording based on participant qualitative feedback.
Combined questions after R1.
Combined questions after R1.
Round 1 (Item Generation)
Respondents were asked to provide demographic information and feedback on a list of 28 research questions generated from the meeting. Participants were asked to add missing key research questions and to modify any potentially problematic wording of the existing research questions. Next, participants selected their top 10 research questions from the list, ranking them from 1 (high) to 10 (low).
Descriptive statistics were calculated to identify question rankings for the group of respondents. For each participant, a first-priority score yielded 10 points for that specific question, a second-priority score yielded 9 points, and so on. Each individual question score was summed based on all participant responses, and a summary score and respective ranking was developed. Based on participant feedback, specific research questions were added, modified, or combined to reduce redundancies and to improve clarity. Questions were combined based on consensus among 4 study team coders and using open-ended feedback from participants. The lowest scoring items were dropped to create a list of 20 priority research questions.
Round 2 (Item Reduction)
The top 20 questions identified by respondents from round 1 were presented to all participants, including those who did not respond to the first survey. Participants again selected the 10 highest priority questions ranking them from 1 to 10 and were asked to provide suggestions on research question wording and to identify potential questions that could be combined based on redundancies.
After round 2, additional combining and revising of research questions occurred, based on participant feedback. The consolidation process yielded 15 research questions, or 75% of questions from round 2, for consideration in round 3.
Round 3 (Item Prioritization)
In the final round, participants rank-ordered their top 10 priority research questions from the presented list of 15 questions. Each participant was also asked to provide suggestions of potential data sources currently available to answer each of these research questions.
Selection of Final Top 10 List
The original goal was to identify 10 top priority questions; however, the 10th and 11th questions received the same consensus ranking score. Therefore, the Delphi led to the identification and prioritization of 11 research questions, shown in Table 3. Based on participant feedback on the third survey, a final consensus-based priority research question list was developed, and research priority thematic areas were identified.
| Final question ranking . | Theme . | Research question . |
|---|---|---|
| 1 | 1: Determining outcome measures for new care pathways | What are appropriate outcome measures of “success” for personalized cancer follow-up care pathways? |
| 2 | 2: Developing and evaluating new care pathways | What important variables should be considered in determining the appropriate intensity of care? |
| 3 | 3: Incentivizing new care pathway delivery | What types of system/policy changes might incentivize health systems to shift care for low-risk patients to primary care or specialty follow-up clinics? |
| 4 | 3: Incentivizing new care pathway delivery | What types of system/policy changes might incentivize oncologists to shift care for low-risk patients to follow-up care clinicians outside the original treatment team? |
| 5 | 2: Developing and evaluating new care pathways | How well do current risk assessment tools work in cancer populations or in specific subpopulations of survivors? |
| 6 | 4: Technology and infrastructure to support self-management | What policies, practices, and infrastructure are needed to facilitate self-management at the health system and practice level? |
| 7 | 5: Incentivizing new care pathway delivery | What are the low-value and/or noncost-effective or harmful survivorship care components that need deimplementation? |
| 8 | 2: Developing and evaluating new care pathways | How can clinical decision support tools facilitate triage of patients into personalize cancer follow-up care pathways? |
| 9 | 4: Technology and infrastructure to support self-management | How can technology and digital tools (eg, patient portals) be used to engage patients in their survivorship care and self-management outside of the exam room? |
| 10a | 5: Policy and health systems changes | How should follow-up care clinicians (eg, PCP/APP-led follow-up clinicians) be engaged in/aware of cancer care to facilitate provision of follow-up care? |
| 10b | 2: Developing and evaluating new care pathways | How can mathematic models incorporate clinical judgment, patient preferences, level of patient activation, and cost to identify appropriate pathways? |
| Final question ranking . | Theme . | Research question . |
|---|---|---|
| 1 | 1: Determining outcome measures for new care pathways | What are appropriate outcome measures of “success” for personalized cancer follow-up care pathways? |
| 2 | 2: Developing and evaluating new care pathways | What important variables should be considered in determining the appropriate intensity of care? |
| 3 | 3: Incentivizing new care pathway delivery | What types of system/policy changes might incentivize health systems to shift care for low-risk patients to primary care or specialty follow-up clinics? |
| 4 | 3: Incentivizing new care pathway delivery | What types of system/policy changes might incentivize oncologists to shift care for low-risk patients to follow-up care clinicians outside the original treatment team? |
| 5 | 2: Developing and evaluating new care pathways | How well do current risk assessment tools work in cancer populations or in specific subpopulations of survivors? |
| 6 | 4: Technology and infrastructure to support self-management | What policies, practices, and infrastructure are needed to facilitate self-management at the health system and practice level? |
| 7 | 5: Incentivizing new care pathway delivery | What are the low-value and/or noncost-effective or harmful survivorship care components that need deimplementation? |
| 8 | 2: Developing and evaluating new care pathways | How can clinical decision support tools facilitate triage of patients into personalize cancer follow-up care pathways? |
| 9 | 4: Technology and infrastructure to support self-management | How can technology and digital tools (eg, patient portals) be used to engage patients in their survivorship care and self-management outside of the exam room? |
| 10a | 5: Policy and health systems changes | How should follow-up care clinicians (eg, PCP/APP-led follow-up clinicians) be engaged in/aware of cancer care to facilitate provision of follow-up care? |
| 10b | 2: Developing and evaluating new care pathways | How can mathematic models incorporate clinical judgment, patient preferences, level of patient activation, and cost to identify appropriate pathways? |
APP = advanced practice provider; PCP = primary care provider.
| Final question ranking . | Theme . | Research question . |
|---|---|---|
| 1 | 1: Determining outcome measures for new care pathways | What are appropriate outcome measures of “success” for personalized cancer follow-up care pathways? |
| 2 | 2: Developing and evaluating new care pathways | What important variables should be considered in determining the appropriate intensity of care? |
| 3 | 3: Incentivizing new care pathway delivery | What types of system/policy changes might incentivize health systems to shift care for low-risk patients to primary care or specialty follow-up clinics? |
| 4 | 3: Incentivizing new care pathway delivery | What types of system/policy changes might incentivize oncologists to shift care for low-risk patients to follow-up care clinicians outside the original treatment team? |
| 5 | 2: Developing and evaluating new care pathways | How well do current risk assessment tools work in cancer populations or in specific subpopulations of survivors? |
| 6 | 4: Technology and infrastructure to support self-management | What policies, practices, and infrastructure are needed to facilitate self-management at the health system and practice level? |
| 7 | 5: Incentivizing new care pathway delivery | What are the low-value and/or noncost-effective or harmful survivorship care components that need deimplementation? |
| 8 | 2: Developing and evaluating new care pathways | How can clinical decision support tools facilitate triage of patients into personalize cancer follow-up care pathways? |
| 9 | 4: Technology and infrastructure to support self-management | How can technology and digital tools (eg, patient portals) be used to engage patients in their survivorship care and self-management outside of the exam room? |
| 10a | 5: Policy and health systems changes | How should follow-up care clinicians (eg, PCP/APP-led follow-up clinicians) be engaged in/aware of cancer care to facilitate provision of follow-up care? |
| 10b | 2: Developing and evaluating new care pathways | How can mathematic models incorporate clinical judgment, patient preferences, level of patient activation, and cost to identify appropriate pathways? |
| Final question ranking . | Theme . | Research question . |
|---|---|---|
| 1 | 1: Determining outcome measures for new care pathways | What are appropriate outcome measures of “success” for personalized cancer follow-up care pathways? |
| 2 | 2: Developing and evaluating new care pathways | What important variables should be considered in determining the appropriate intensity of care? |
| 3 | 3: Incentivizing new care pathway delivery | What types of system/policy changes might incentivize health systems to shift care for low-risk patients to primary care or specialty follow-up clinics? |
| 4 | 3: Incentivizing new care pathway delivery | What types of system/policy changes might incentivize oncologists to shift care for low-risk patients to follow-up care clinicians outside the original treatment team? |
| 5 | 2: Developing and evaluating new care pathways | How well do current risk assessment tools work in cancer populations or in specific subpopulations of survivors? |
| 6 | 4: Technology and infrastructure to support self-management | What policies, practices, and infrastructure are needed to facilitate self-management at the health system and practice level? |
| 7 | 5: Incentivizing new care pathway delivery | What are the low-value and/or noncost-effective or harmful survivorship care components that need deimplementation? |
| 8 | 2: Developing and evaluating new care pathways | How can clinical decision support tools facilitate triage of patients into personalize cancer follow-up care pathways? |
| 9 | 4: Technology and infrastructure to support self-management | How can technology and digital tools (eg, patient portals) be used to engage patients in their survivorship care and self-management outside of the exam room? |
| 10a | 5: Policy and health systems changes | How should follow-up care clinicians (eg, PCP/APP-led follow-up clinicians) be engaged in/aware of cancer care to facilitate provision of follow-up care? |
| 10b | 2: Developing and evaluating new care pathways | How can mathematic models incorporate clinical judgment, patient preferences, level of patient activation, and cost to identify appropriate pathways? |
APP = advanced practice provider; PCP = primary care provider.
Eleven Priority Research Questions and Four Priority Themes
Overview of Priority Research Themes
Four priority research themes emerged within the top 11 research questions, as shown in Table 3: determining outcome measures for new care pathways (question 1), developing and evaluating new care pathways (questions 2, 5, 8, 10b), incentivizing new care pathway delivery (questions 3, 4, 7, 10a), and providing technology and infrastructure to support self-management (questions 6, 9).
Determining Outcome Measures for New Care Pathways
Results of the Delphi process indicate that the highest priority theme involves developing standard process and outcome measures that serve as metrics of success for new care delivery models. The National Cancer Institute has proposed a conceptual framework that outlines high-quality survivorship care (22). This framework is multilevel, taking a socioecological approach by incorporating individual, interpersonal, organizational, community, and policy factors (22). Key survivorship care quality measures identified in this framework include the processes and outcomes of emergency room visits and hospitalizations, health-related quality of life and functioning, patient out-of-pocket costs and costs to the health-care system, and mortality (22). Quality of care is an additional important domain to be considered. Research questions identified in this commentary map to a number of the outcomes described in this framework; however, measures of these outcomes are not standard and differ across data sources, clinical settings, and research programs. They vary in comprehensiveness based on the data resources available for assessment and tracking. This commentary points to a clear need for further research that focuses on developing quality metrics that can be incorporated into routine care processes that can also be standardized, delivered, and measured across clinical settings.
Developing and Evaluating New Care Pathways
The second priority theme involves developing and evaluating new care pathways across cancers and treatment types. This involves specifying the types of care patients with differing risk and/or need profiles require and using currently available data to refine these algorithms over time. Adverse health effects from cancer and its various treatments occur over time, often decades later, and the spectrum of risk from treatment is in constant evolution as new treatments with differing toxicity profiles emerge. Longitudinal studies of newly diagnosed and long-term survivors are needed to identify and understand adverse effects of treatment over time, by diagnosis and treatment exposures, to delineate emerging risk profiles. Prospective studies of patients undergoing new treatments such as immunotherapies will be needed to understand short- and long-term outcomes and to refine risk and health-care need profiles in the future.
To begin this work with currently available data, assessment of risk and/or needs by disease and treatment exposures for survivors may be the best approach. Researchers can build on prior personalized care pathway models of care, such as those in the United States (7,23), England, and Northern Ireland (24–26). The compelling successes of these pathways in transitioning low-risk survivors to lower-intensity surveillance and follow-up care demonstrate the potential for developing and testing risk assessment measures. An initial strategy may be to develop a candidate algorithm for stratifying survivors into different levels of care for the most common cancers, which would cover a large percentage of survivors. As work progresses to develop new models of care, it will be essential to learn which current risk assessment tools work within respective subpopulations vs those that need improvement or different tools (question 5). Assessing degree of risk and which providers might be most appropriate to deliver ongoing care to specific groups are subjective issues (question 10a). Candidate algorithms for stratifying levels of care will also require calibration and validation. Needs will evolve with time as new treatments with yet unknown short- and long-term complications are introduced.
Incentivizing New Care Pathway Delivery
The third theme of priority research questions concerns the need to inform potential policy and systems changes that incentivize the adoption of a personalized care pathway approach to follow-up survivorship care. The current fee-for-service payment system used to reimburse most providers incentivizes health-care service quantity without consideration of the quality of care provision. As such, there are few financial incentives within fee-for-service payment for providers to adopt personalized care pathways. Other models of care delivery, including value-based payment models that place greater focus on quality of care and patient outcomes, will provide better incentives for the adoption of new care pathways. In addition to consensus on an approach to identify appropriate personalized survivorship care pathways, systems processes will need to be developed and introduced to begin shifting cancer care to this new model. Components currently included in survivorship care that have low value, are not cost-effective, or are harmful will require identification and then deimplementation as new systems are developed. Research should identify effective change-management procedures and processes and the appropriate levers that will help support or incentivize clinicians, patients, and health-care systems in changing follow-up care practice. Health-services research studies that identify return on investment from new care delivery models will inform payer interest in implementing these models and advocacy agendas to support the reimbursement of new care components (eg, self-management, telemedicine approaches, care coordination) that allow for personalized follow-up care pathway delivery. These systems processes can be developed in parallel to the development of personalized care pathways. Provider movement toward accepted models of care can be stimulated by evaluation and accreditation processes such as those performed by the Commission on Cancer and the standards that drive its accreditation process.
Technology and Infrastructure to Support Self-Management
The fourth priority theme focused on emerging technology, digital tools, and infrastructure needed to facilitate self-management. Providing this lower-touch level of care outside the exam room will require investment and testing of eHealth programs providing self-management support as well as patient portals that assess patient-reported outcomes (PROs) or use passive data collection over time for routine assessment of needs and functioning (27). Survivors at low risk for serious adverse outcomes may be ideal candidates for managing their cancer-related side effects and symptoms outside the clinical setting. However, evidence-based eHealth programs are needed to provide this type of support and to tailor programs to meet the needs of individuals with a cancer history in an equitable way and at every level of need.
Suggested Available Databases
Although “ideal” databases or research resources do not exist to definitively address these research questions, many participants provided data suggestions for beginning to answer parts of some of these questions. Several large, national publicly available datasets (eg, Medical Expenditure Panel Survey) were referenced because they can provide snapshots of the survivorship experience and parameter inputs for potential stratification algorithms. As well, participants suggested a number of potential data linkages between cancer registry data (eg, Surveillance, Epidemiology, and End Results; National Cancer Database; North American Association of Cancer Registries; National Program of Cancer Registries), electronic health records, health insurance claims data (eg, Medicare; Veterans Health Affairs), encounter data (eg, Kaiser Permanente), surveys (eg, Medicare Health Outcomes Survey; Consumer Assessment of Healthcare Providers and Systems), PROs, and/or digital health company data (eg, Carevive). Participants noted that linking cancer registry to other types of data and integrating longitudinal PROs with clinical, utilization, and cost data will be needed to definitively answer many of the priority questions identified.
Although it has been 15 years since the Institute of Medicine report on survivorship (28), there is still so much we do not know about cancer survivorship, in spite of research efforts. The heterogeneity of treatment effects for different cancers and different therapies is still poorly appreciated, and optimal models of survivorship care have yet to be elucidated. To some extent this may be the result of studies and interventions that were too general, rather than tailored to the needs of individual groups of patients. Being more precise in framing questions to be addressed may help, and the Delphi process described here is a good start.
The priorities identified here reflect the consensus of this group of national experts. Although an attempt was made to include them, there was limited participation in the meetings and Delphi survey by health-care payers, so their important perspectives are not included.
The US healthcare system is costly and stressed with shortages of primary care and oncology providers (9,29,30). It is essential that the provision of care is efficient and nonredundant while not compromising the quality of the care and patient outcomes. As the number of cancer survivors continues to increase, providing care for those with a history of cancer will be an increasing challenge. Further, not all cancer survivors are the same; they have disparate health-care needs and should not be approached in a one-size-fits-all manner. This commentary detailed how an expert panel generated consensus research priorities for advancing personalized survivorship care in the United States, 1 of the 4 key strategies from the 2018 ACS–ASCO Summit on Implementing Personalized Stratified Cancer Survivorship Care (19). The resulting top priority themes suggest the need for conducting research to determine outcome measures for new care pathways, developing and evaluating new care pathways, incentivizing new care pathway delivery, and building technology and infrastructure to support patient self-management. These key questions need to be answered to explore the potential of bringing personalized follow-up survivorship care to everyone with a history of cancer that is at an appropriate level based on need, is high quality and equitable, is efficient and sustainable for the health-care system, and leads to positive outcomes for all individuals with a history of cancer.
Funding
This work was funded by the American Cancer Society through the Intramural Research program.
Notes
Conflicts of Interest: There are no conflicts of interest.
Acknowledgments: The authors would like to acknowledge the contributions of the participants of the in-person meeting and the Delphi.
Disclaimer: The views expressed here are those of the authors only and do not represent any official position of the National Cancer Institute or National Institutes of Health.
References
Support MC. Evaluation of the Transforming Cancer Follow-up Programme in Northern Ireland Final Report.
Service NH. Stratified Pathways of Care – From Concept to Innovation. https://www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2017/11/Stratified-Pathways-of-Care.pdf.
Council IoMaNR.
Colleges AoAM.


