-
PDF
- Split View
-
Views
-
Cite
Cite
Susana Cedrés, Enriqueta Felip, Cristina Cruz, Ana Martinez de Castro, Nuria Pardo, Alejandro Navarro, Alex Martinez-Marti, Jordin Remon, Jorge Zeron-Medina, Judith Balmaña, Alba Llop-Guevara, Josep M Miquel, Irene Sansano, Paolo Nuciforo, Francesco Mancuso, Violeta Serra, Ana Vivancos, Activity of HSP90 Inhibiton in a Metastatic Lung Cancer Patient With a Germline BRCA1 Mutation, JNCI: Journal of the National Cancer Institute, Volume 110, Issue 8, August 2018, Pages 914–917, https://doi.org/10.1093/jnci/djy012
Close - Share Icon Share
Abstract
Heat shock proteins (HSPs) are molecular chaperones that maintain proteins in their correct conformation to ensure stability and protect carcinoma cells from apoptosis. HSP90 inhibitors (HSP90i) block multiple targets simultaneously, and despite responses in a selected population, no HSP90i have yet been approved. We present a patient with a lung tumor with an exceptional response to cisplatin/gemcitabine in combination with HSP90i, which nowadays continues with HSP90i maintenance after three years. Whole-exome sequencing of the lung tumor unveiled a BRCA1/2 deficiency mutational signature, and mutation analysis confirmed a germline BRCA1 mutation. The striking efficacy of HSP90i plus chemotherapy vs chemotherapy alone was reproduced in a patient-derived xenograft (PDX) model from a breast cancer patient with a BRCA1 mutation (mean tumor volume [SD], No. of tumors: vehicle 8.38 [7.07] mm3, n = 3; HSP90i 4.18 [1.93] mm3, n = 5; cisplatin plus gemcitabine 3.31 [1.95] mm3, n = 5; cisplatin plus gemcitabine plus HSP90i 0.065 [0.076] mm3, n = 6). This case and the PDX demonstrate the efficacy for therapeutic inhibition of HSP90 in a BRCA-mutated patient, opening a new potential avenue for better identifying patients who might benefit most from HSP90i.
BRCA1 is a nuclear tumor suppressor critical for the DNA repair of double-strand breaks (DSBs) and interstrand-crosslinks (ICL) by the homologous recombination repair (HRR) mechanism. Inherited mutations in a BRCA1 gene are associated with an increased risk of developing breast and ovarian cancer by age 70 years (1). Tumors with BRCA1 mutations are sensitive to agents that induce DNA-DSBs including platinum salts and PARP inhibitors, but despite initial responses, acquired resistance develops (2). The overall prevalence of germline BRCA1/2 mutations in cancer patients is not well known and has been reported in 12% to 15% of ovarian cancers, 5% to 10% of pancreatic cancers, 5% of breast cancers, 2% of lung cancers, and 2% of prostate cancers (3,10).
Here we present the case of a patient diagnosed with non–small cell lung cancer (NSCLC) carrying a germline BRCA1 (gBRCA1) mutation with an ongoing response to HSP90i maintenance after cisplatin/gemicitabine plus HSP90i for more than three years (Figure 1). A female patient age 36 years was diagnosed with a hormone receptor–positive invasive ductal breast carcinoma (IDC) with no evidence of disease after local and adjuvant therapy (Supplementary Figure 1, available online). Eight years later, the patient was diagnosed with squamous NSCLC T3N0M0. She underwent right pneumonectomy and lymphadenectomy with a pathologic result of 11.5 cm squamous lung carcinoma with negative hormonal receptors and lymph nodes. The patient presented a chylothorax with slow resolution failing to receive adjuvant treatment. Three months after the pneumonectomy, a computed tomography (CT) scan showed liver metastasis. A hepatic biopsy diagnosed metastasis with the same pattern as the lung tumor. The patient was enrolled in a clinical trial with cisplatin+gemcitabine+HSP90i (DEBIO0932, NCT01714037) (11). After two cycles, a CT scan showed partial response, with a 51.5% reduction of target lesions. After completing six cycles of chemotherapy+HSP90i, the tumor shrank by 66.5%. After 38 months of treatment, the patient presented an almost complete response (92.0% reduction) and still continues with HSP90i monotherapy today.
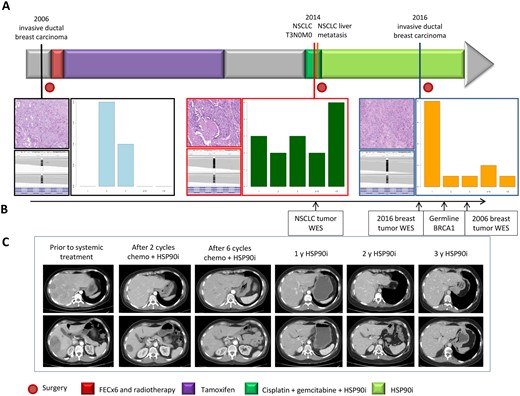
Tumor sites and therapies timelines. A) In the upper section, timelines of the different tumors and treatments received. In the lower part, morphological appearance of the first invasive ductal breast carcinoma, lung tumor, and second breast cancer (hematoxylin and eosin, 20×); allelic expression of BRCA1 and patterns of insertions and deletions of indel length distribution in the three tumors through exome sequencing. B) Timing of genotypic analysis performed in different samples along the evolution of disease. C) Tumor response represented with computed tomography scans of the liver metastasis of non–small cell lung cancer at the time points indicated: previous systemic treatment, after two cycles of chemotherapy plus HSP90i, at the end of six cycles of chemotherapy, after 12 months with HSP90i, after 24 months with HSP90, and at 36 months with HSP90i. The scale bars are presented on the left of images, and each small bar represents 2 cm. FEC = fluorouracil, epirubicin, cyclophosphamide; WES = whole-exome sequencing.
After 28 months of treatment with HSP90i, the patient was diagnosed with triple-negative breast cancer, and a right mastectomy with lymph axillar dissection was performed. The pathology exam revealed a 19 mm IDC grade 3 and negative lymph nodes. No adjuvant treatment was indicated to maintain the NSCLC therapy with HSP90i.
We sought to understand the unexpected and exquisite sensitivity to chemotherapy plus HSP90i followed by HSP90i maintenance in this patient. Detailed methods are presented in the Supplementary Methods (available online). Statistics were determined by one- or two-way analysis of variance (ANOVA) with Tukey’s post hoc test (two-sided). A P value of less than .05 was considered statistically significant. After institutional review board approval and informed consent for a translational study at our institution, we performed whole-exome sequencing (WES) of the lung tumor and germline DNA, identifying a BRCA1/2 deficiency mutational signature but no additional or actionable driver mutations (Supplementary Table 1, available online) (12). The patient was referred for germline genetic testing, and the analysis of BRCA1/2 in blood unveiled a pathogenic heterozygous variant BRCA1 c.527delC (p.176fs) exon 8. WES of the breast tumor performed at the same point revealed a genetically different tumor with some coincident mutations (Supplementary Table 1, available online).
To confirm the exceptional response of chemotherapy plus HSP90i, we tested whether the combination was more effective than chemotherapy alone in a platinum-naïve PDX from a gBRCA1 carrier who had developed breast cancer (PDX127) (13). Mice were maintained in accordance with local guidelines and therapeutic interventions approved by the Animal Care and Use Committees of Vall D’Hebron Institute of Oncology. Animal care procedures followed recommendations from appropriate committees. This tumor model does not exhibit BRCA1 foci but is able to recruit the HRR marker RAD51 to sites of DNA damage, and is therefore resistant to PARP inhibition.
Inhibition of HSP90 with AUY922 (n = 5) in PDX127 or administration of cisplatin/gemcitabine chemotherapy (n = 5) resulted in progressive disease (mean tumor volume ±SD = 4.18 ±1.93 mm3 and 3.31±1.95 mm3, P = .006 and P < .001, respectively). The control tumor (vehicle n = 3) grew to 8.38±7.07 mm3 (Figure 2). Remarkably, the triple combination resulted in a complete response (n = 6, 0.065 ±0.076 mm3, P < .001 with two-way ANOVA, Tukey’s multiple comparisons test).
![Antitumor activity of HSP90i in vivo. Mice were maintained in accordance with local guidelines, and therapeutic interventions were approved by the Animal Care and Use Committees of Vall d´Hebron Institute of Oncology. A tumor fragment from the germline BRCA1 PDX127 TNBC model was implanted subcutaneously onto the flank of six-week-old female nude mice. Mice were supplemented with 1 mmol/L estradiol (Sigma) in the drinking water. A) Immunofluorescence of gH2AX (DNA DSB marker), BRCA1, or RAD51 (HRR marker) costained with geminin (as marker of the S/G2 phase of the cell cycle). Nuclear foci of gH2AX, BRCA1, and RAD51 are shown in red; geminin pan-nuclear staining is shown in green. DAPI staining is shown in blue. Three thin sections of formalin-fixed, paraffin-embedded archival samples of the breast and lung tumors of the patient were stained parallel to sections of PDX127 and a BRCA1 wild-type tumor (Ct+), which was used as positive control for all markers. The scale bar represents 5 µm. B) PDX127 was subcutaneously implanted in NMRI mice (vehicle n = 3, HSP90i n = 5, cisplatin plus gemcitabine n = 5, cisplatin plus gemcitabine plus HSP90i n = 6), and when tumors reached an average size of 200 mm3, mice were treated with vehicle, NVP-AUY922 (mg/kg six days/wk by oral gavage), cisplatin (6 mg/kg, intravenously [i.v.]) plus gemcitabine (6 mg/kg, i.v.) at the indicated time points (#), or the combination of the three agents. Tumors were measured by caliper twice weekly. Average (SD) tumor volumes on day 23 were vehicle 8.38 (7.07) mm3, HSP90i 4.18 (1.93) mm3, cisplatin plus gemcitabine 3.31 (1.95) mm3, cisplatin plus gemcitabine plus HSP90i 0.065 (0.076) mm3. Error bars indicate SD. P values using two-way analysis of variance with Tukey’s post hoc test are summarized in the box. NSCLC = non–small cell lung cancer; PDX = patient-derived xenograft.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/110/8/10.1093_jnci_djy012/1/m_djy012f2.jpeg?Expires=1747908811&Signature=WIsSpBmQ7Lq46DOR1xa~2ntxR4zBiMVMLIz57mJo--zBUzcaJLeAp4lPt7sno5FQZMeX~PgErVPT-xNuBlT0e7mCapLG-3c3MKt1ZVlPy72bSybRzWQEJtqK3IuDNapPK3J7L~GiwMP~-GPUOjLyNOw1RaM51hjQ8x-76UdSbrBiDais7ZoxPEzxT0tnkkEWvnF0mENvCEcwNO88wCi89mk7HegOKSuBph80mXz2iRIoS-6mVv8vm9WUXCWdBFDOejAuMOh5QWmgpWiHlJbORenM4jqPB-awGtxNndG9vqDdepK~hbP2yVrtJD~SQcjI0gyIB4j8nhtlqLWU24~10Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Antitumor activity of HSP90i in vivo. Mice were maintained in accordance with local guidelines, and therapeutic interventions were approved by the Animal Care and Use Committees of Vall d´Hebron Institute of Oncology. A tumor fragment from the germline BRCA1 PDX127 TNBC model was implanted subcutaneously onto the flank of six-week-old female nude mice. Mice were supplemented with 1 mmol/L estradiol (Sigma) in the drinking water. A) Immunofluorescence of gH2AX (DNA DSB marker), BRCA1, or RAD51 (HRR marker) costained with geminin (as marker of the S/G2 phase of the cell cycle). Nuclear foci of gH2AX, BRCA1, and RAD51 are shown in red; geminin pan-nuclear staining is shown in green. DAPI staining is shown in blue. Three thin sections of formalin-fixed, paraffin-embedded archival samples of the breast and lung tumors of the patient were stained parallel to sections of PDX127 and a BRCA1 wild-type tumor (Ct+), which was used as positive control for all markers. The scale bar represents 5 µm. B) PDX127 was subcutaneously implanted in NMRI mice (vehicle n = 3, HSP90i n = 5, cisplatin plus gemcitabine n = 5, cisplatin plus gemcitabine plus HSP90i n = 6), and when tumors reached an average size of 200 mm3, mice were treated with vehicle, NVP-AUY922 (mg/kg six days/wk by oral gavage), cisplatin (6 mg/kg, intravenously [i.v.]) plus gemcitabine (6 mg/kg, i.v.) at the indicated time points (#), or the combination of the three agents. Tumors were measured by caliper twice weekly. Average (SD) tumor volumes on day 23 were vehicle 8.38 (7.07) mm3, HSP90i 4.18 (1.93) mm3, cisplatin plus gemcitabine 3.31 (1.95) mm3, cisplatin plus gemcitabine plus HSP90i 0.065 (0.076) mm3. Error bars indicate SD. P values using two-way analysis of variance with Tukey’s post hoc test are summarized in the box. NSCLC = non–small cell lung cancer; PDX = patient-derived xenograft.
Here we report a lung cancer patient harboring a BRCA1 mutation who responded to six cycles of chemotherapy plus HSP90i, followed by HSP90i maintenance for more than three years, a longer than expected response when compared with standard treatment for NSCLC. Beyond downregulation of BRCA1 and impairing HRR, inhibition of HSP90 may compromise other DNA repair pathways relevant for cisplatin/gemcitabine toxicity, including the ICL repair/Fanconi Anemia pathway, non-homologous end joining (NHEJ), alternative NHEJ, or replication fork repair (14–18).
Indeed, cell line studies have demonstrated that HSP90i sensitizes BRCA1-mutant cells to DNA-damaging agents (ionizing radiation, platinum salts, or PARP inhibitors) (14–19). WES in our patient showed that both the NSCLC and the IDC tumors harbored the intact gBRCA1 mutation with associated loss of the wild-type allele. Recent reports have shown expression of BRCA1 hypomorphic isoforms conferring treatment resistance to platinum salts and PARP inhibitors in BRCA1-mutated tumors (20,21). Some BRCA1 isoforms rely on HSP90 for their stability. However, no functional BRCA1 isoform or RAD51 foci was detected in the patient by immunofluorescence, suggesting that the activity of HSP90i was independent of BRCA1 isoform stabilization (Figure 2; Supplementary Methods, available online). We further attempted to decipher the BRCA1-independent mechanism of action of this combination in PDX127. We postulated that HSP90i downmodulated homologous recombination (HR) downstream of BRCA1 or ICL repair, two major pathways involved in DNA repair after platinum plus gemcitabine treatment. To this end, we measured RAD51 and FANC2 foci formation after treatment, respectively (Supplementary Methods and Supplementary Figure 2, available online). Surprisingly, neither HR nor ICL repair was downmodulated by HSP90i in addition to chemotherapy in vivo, thus indicating that other pathways may be involved. The specific mechanism of this combination might therefore imply that there are other DNA repair pathways that BRCA1-deficient cells rely on.
This report raises several points. First, the study of such extraordinary responses in cancer patients could lead to be identification of mechanisms of sensitivity to novel therapies. We reported an exceptional response in the context of BRCA-related tumors. Second, our data support the antitumor activity of HSP90i in BRCA1-deficient tumors, as previously suggested in experimental models (14,19). Third, the patient was diagnosed with an IDC while being treated with maintenance therapy based on HSP90i. This observation indicates that the evolution of breast carcinoma with the continuous inhibition of HSP90 might have been less reliant on NHEJ for survival and therefore insensitive due to the lengthy inhibition of HSP90 monotherapy. The recent demonstration that HSP90 can modify environment susceptibility in genetic diseases reinforces our observation (22).
A limitation to our study is the absence of a PDX model with the tumor tissue of the patient due to the complete response. We therefore intended to extend our clinical observation into a PDX that closely recapitulates the BRCA1 status and clinical history of the reported patient.
Our results suggest that tumors with BRCA1 alterations may benefit from adding HSP90i to chemotherapy, suggesting the use of HSP90i-based therapy in patients with BRCA-related tumors or a BRCA-ness phenotype. Our model could have implications for the future development of HSP90i-based therapies against BRCA-related cancers.
Funding
This work was supported by the Spanish Cancer Association (AECC) Scientific Foundation (GCB14-2170) and partially by the Spanish Ministries of Health and Fondo de Investigación Sanitaria-Fondo Europeo de Desarrollo Regional (PI14/01248). Violeta Serra is funded by the Instituto de Salud Carlos III PI17-01080, CP14/00228, and the Catalan Agency AGAUR (2014 SGR 1331). Cristina Cruz is supported by a postdoctoral fellowship from the AECC.
Notes
Affiliations of authors: Cancer Genomic Group (FM, AV), Molecular Oncology Group (PN), Experimental Therapeutic Group (CC, ALG, VS), Thoracic Tumors Group, Vall d’hebron Institute of Oncology (SC, EF, AMdC, NP, AN, AMM, JR, JB), Vall d’hebron Institute of Oncology (JMM) Barcelona, Spain; Pathology Department (IS), Oncology Department, Vall d’hebron University Hsopital (SC, EF, CC, AMdC, NP, AN, AMM, JR, JZM, JB), Barcelona, Spain; Universidad Autonoma de Barcelona, Barcelona, Spain (EF, NP, AN, AMM, JB).
The funders had no role the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
We thank Dr. Cristina Saura for her support in the elaboration of manuscript and aacknowledge the CELLEX Foundation for providing research facilities and equipment.
All authors have declared no conflict of interest.
References
Author notes
See the Notes section for the full list of authors’ affiliations.
- apoptosis
- mutation
- carcinoma
- chemotherapy regimen
- cisplatin
- mutation analysis
- gemcitabine
- brca1 protein
- brca1 gene
- heat-shock proteins
- hsp90 heat-shock proteins
- lung neoplasms
- molecular chaperones
- molecular conformation
- transplantation, heterologous
- neoplasms
- breast cancer
- metastasis to the lung
- lung cancer stage iv
- brca1 mutation
- tumor volume
- cisplatin/gemcitabine
- whole exome sequencing


