-
PDF
- Split View
-
Views
-
Cite
Cite
Michael J Overman, Renata Ferrarotto, Kanwal Raghav, Binsah George, Wei Qiao, Karime K Machado, Leonard B Saltz, Thibault Mazard, J N Vauthey, Paulo M Hoff, Brian Hobbs, Evelyn M Loyer, Scott Kopetz, The Addition of Bevacizumab to Oxaliplatin-Based Chemotherapy: Impact Upon Hepatic Sinusoidal Injury and Thrombocytopenia, JNCI: Journal of the National Cancer Institute, Volume 110, Issue 8, August 2018, Pages 888–894, https://doi.org/10.1093/jnci/djx288
Close - Share Icon Share
Abstract
Oxaliplatin-based chemotherapy can cause hepatic sinusoidal injury (HSI), portal hypertension, and splenic sequestration of platelets. Evidence suggests that bevacizumab may protect against HSI.
Two cohorts of metastatic colorectal cancer (CRC) were analyzed: a nonrandomized exploratory cohort of 184 patients treated at a single institution from 2003 to 2010 and a confirmatory cohort of 200 patients from a multi-institutional randomized trial (NO16966). All patients were treated with frontline fluoropyrimidine and oxaliplatin with or without bevacizumab. Changes in splenic volumes and platelet counts were compared by treatment, two-sided log-rank test.
In the exploratory cohort, the bevacizumab-treated patients (n = 138) compared with the nonbevacizumab-treated patients (n = 46) demonstrated a longer median time to splenic enlargement (≥30%, P = .02) and reduced rate of thrombocytopenia (<150 000/mm3, P = .04). In the confirmatory cohort (106 bevacizumab arm and 94 placebo arm), the median time to a spleen enlargement of 30% or more was 7.6 vs 5.4 (P = .01), and six-month cumulative incidence of thrombocytopenia (platelets < 100 000/mm3) was 19% vs 51% (P < .001) for bevacizumab compared with placebo. The development of an increasing spleen size was associated with the risk of either grade 1 or grade 2 thrombocytopenia (P < .001). The cumulative rate of grade 1 or grade 2 thrombocytopenia was statistically less in the bevacizumab arm, with six-month grade 2 thrombocytopenia rates of 4% vs 23% (P < .001). Patients with a large spleen prior to chemotherapy initiation appeared to be at highest risk of this toxicity.
In metastatic CRC, the addition of bevacizumab to oxaliplatin-based chemotherapy reduces the frequency of splenic enlargement and the rate of thrombocytopenia.
Oxaliplatin-based chemotherapies represent a mainstay of systemic treatment for many gastrointestinal cancers, such as colorectal, gastroesophageal, and pancreatic cancer. The development of oxaliplatin-induced hepatic sinusoidal injury (HSI) is a known dose-dependent toxicity, which can be identified radiographically by increases in spleen size that result from an increase in portal venous pressure (1–5).
This toxicity has been best characterized in the adjuvant setting, where 45% of colorectal cancer (CRC) patients following six months of adjuvant fluoropyrimidine and oxaliplatin chemotherapy will develop splenic enlargement and resultant chronic reductions in platelet counts that normalize in approximately two years (1). The impact of this toxicity in the metastatic setting has been best characterized in CRC patients undergoing liver resection, where the development of oxaliplatin-induced HSI and resultant portal hypertension result in increased bleeding risk and postoperative morbidity (2,3,6,7).
The suggestion that bevacizumab may protect against the development of oxaliplatin-induced HSI has been noted in both the adjuvant and metastatic settings. In the NSABP C-08 study, a statistically significant reduction in thrombocytopenia was seen in the arm that received bevacizumab in addition to FOLFOX for the adjuvant treatment of CRC (1.4% vs 3.4%, P < .01) (8). A number of small nonrandomized reports in patients undergoing hepatic resection of CRC liver metastases have suggested that bevacizumab reduces the rate of histologically determined hepatic sinusoidal injury (1,9–12). In addition, in a rat model of sinusoidal injury, VEGF tyrosine kinase inhibitors were able to protect against the development of this toxicity (13,14).
Given these findings, we sought to determine if bevacizumab can reduce the rate of hepatic sinusoidal injury, as manifested as a reduced rate of splenic enlargement. In addition, we sought to determine the clinical relevance of this bevacizumab effect by examining the impact on platelet counts in metastatic CRC patients during frontline oxaliplatin-based chemotherapy.
Methods
Patients
This study was conducted under the approval of the University of Texas MD Anderson Institutional Review Board, and due to the retrospective nature of these analyses, a waiver of informed consent was obtained.
Single-Institution Nonrandomized Exploratory Cohort
The University of Texas MD Anderson Cancer Center tumor registry and pharmacy database were queried for metastatic colorectal cancer patients who underwent frontline chemotherapy with a fluoropyrimidine and oxaliplatin with or without bevacizumab for three or more months from January 1, 2003, to January 1, 2010. The following exclusion criteria were then applied to the 306 identified cases: prior adjuvant oxaliplatin therapy, lack of available imaging, prior liver resection, presence of known hepatitis or cirrhosis, and absence of spleen on imaging. The final population analyzed consisted of 184 patients (138 bevacizumab-treated and 46 nonbevacizumab-treated).
NO16966 Randomized Confirmatory Clinical Trial Cohort
The N016966 clinical trial was a multi-institutional phase III randomized placebo controlled trial in which metastatic unresectable CRC patients were treated with frontline fluoropyrimidine and oxaliplatin (CAPOX or FOLFOX-4) with or without bevacizumab from February 2004 to February 2005 (NCT00069095) (15). Imaging assessments were conducted every six weeks, and platelet counts were obtained every two to three weeks according to treatment schedule. Our prior experience with four to six months of oxaliplatin treatment demonstrated a normal distribution of the log-transformed fold-change in spleen values, with a mean of 0.26 and standard deviation of 0.26. Preliminary data from our exploratory cohort demonstrated a reduction in this splenic enlargement by approximately 50%, corresponding to an expected mean log-fold change of 0.13 in the bevacizumab-treated patients. A sample size of 170 patients would provide a 90% power to detect an absolute difference in the means of 0.13, with a two-sided alpha of .05. Given the potential for up to 20% of the samples to not be evaluable for various reasons (including prior spleen resection, technical difficulties, or poor quality of imaging), we utilized 200 patients, randomly selected from the 1401 enrolled patients. Platelet values were available for all patients, but 12 patients were excluded from splenic size analysis due to either the absence of a spleen on imaging or lack of a baseline examination.
Thrombocytopenia was recorded prospectively and categorized by CTCAE v4 toxicity, with grade 1 thrombocytopenia defined as less than the lower limit of normal to 75 000/mm3 or greater and grade 2 thrombocytopenia defined as less than 75 000/mm3 to 50 000/mm3 or greater.
Splenic Size Analysis
For both the exploratory and NO16966 cohorts, computed tomography (CT) images were loaded into an Advantage Workstation (General Electic Medical Systems, Milwaukee, WI), and splenic volume was measured using the Volume Viewer software (General Eclectic Medical Systems, Milwaukee, Wisconsin). For the exploratory cohort, contrast-enhanced CT studies had been performed with a multidetector row 4- or 16-slice CT scanner (Light-Speed, GE Healthcare, Piscataway, NJ) using a collimation of 5 mm and reconstruction at 2.5 mm. For the NO16966 cohort, contrast-enhanced CT studies with single venous-phase contrast enhancement had been performed according to the practice at each center using a collimation of 5 or 7.5 mm and reconstruction at 2.5 or 3.8 mm.
Statistical Analysis
For both cohorts, the predefined coprimary end points were the comparison of the time to the development of splenomegaly (≥30% spleen size) between bevacizumab and nonbevacizumab treatment cohorts and the time to a platelet count of less than 100 000/mm3. This threshold was chosen as this is frequently used as a threshold for the administration of the next cycle of chemotherapy. All changes in spleen volume and platelet counts were determined by comparison with the baseline pretreatment value. The distribution of each continuous variable was summarized by its median and range. The distribution of each categorical variable was summarized in terms of its frequencies and percentages. The Fisher exact test and Wilcoxon rank-sum test were used for hypothesis tests comparing treatment groups. Kaplan-Meier curves were used to estimate time-to-event distributions (eg, cumulative incidence). Log-rank tests were used to compare time-to-event variables between treatment groups. The Cox proportional hazards regression model was used to characterize associations between patient characteristics and the incidences of thrombocytopenia and splenomegaly. Goodness of fit for the Cox survival regression model was assessed using the Grambsch-Therneau test. To account for potential selection bias, analyses of the exploratory cohort were adjusted for propensity scores derived from patient clinical and demographic information (such as total dose of oxaliplatin, diabetes status, body mass index [BMI], sex, and age). The c-index (AUC) for the logistic regression model is 0.61. Piecewise polynomial regression was used to characterize mean trends over time, with 95% confidence bands representing interval estimators that characterize the extent of uncertainty at a statistical significance level of .05 over the entire follow-up time domain observed in our study population (16). R package AdaptFitOS was used to compute the confidence bands. All computations were carried out in SAS version 9.4, TIBCO Spotfire S+ version 8.2, or R version 3.1.3. All the statistical tests were two-sided. The cut-point for statistical significance was a P value of .05 or less.
Results
Exploratory Cohort
Of the 184 patients treated with fluoropyrimidine and oxaliplatin, 138 received bevacizumab. The majority of patients, 93%, received FOLFOX chemotherapy, with the remainder receiving CAPOX. There were no statistical differences between the two groups (Table 1). In particular, the cumulative oxaliplatin exposure was similar between the bevacizumab- and nonbevacizumab-treated cohorts. The median time to splenic enlargement of 30% or greater was longer in the bevacizumab cohort (7.6 vs 5.5 months, P = .02). The six-month cumulative incidence rates of splenic enlargement of 30% or greater were 63% in the nonbevacizumab cohort and 44% in the bevacizumab cohort (P = .08) (Supplementary Figure 1A, available online). There was no difference when using a higher threshold of 50% or greater (P = .82). The six-month cumulative incidence of thrombocytopenia (platelet count < 100 000/mm3) did not differ between the nonbevacizumab cohort and the bevacizumab cohort (17% vs 17%, P = .69) (Supplementary Figure 1B, available online). Due to the low event rate, an exploratory analysis using a platelet count threshold below the lower limit of normal (<150 000/mm3) was conducted, and the six-month cumulative incidence was 70% in the nonbevacizumab cohort and 56% in the bevacizumab cohort (P = .04) (Supplementary Figure 1C, available online). When comparing patients with and without splenic enlargement of 30% or greater, the rate of thrombocytopenia (platelet count < 100 000/mm3) at three months was 40% vs 16%, respectively (P < .001). In the Cox model regarding the time to splenic enlargement, where propensity score was used to adjust for covariate bias and difference, an estimated 1.61-fold reduction in the rate of splenomegaly was observed with the addition of bevacizumab (hazard ratio [HR] = 0.62, 95% confidence interval [CI] = 0.38 to 1.02, P = .06). Similarly, the use of bevacizumab was associated with an estimated 1.59-fold reduction in the rate of thrombocytopenia (platelet count < 150 000/mm3; HR = 0.63, 95% confidence interval [CI] = 0.40 to 1.00, P = .05) (Supplementary Table 1, available online).
| Characteristics . | Bevacizumab arm . | Nonbevacizumab arm . | P* . |
|---|---|---|---|
| (n = 138) . | (n = 46) . | ||
| Age, median (range), y | 56 (30–82) | 58 (25–83) | .73 |
| Sex, No. (%) | 1.00 | ||
| Female | 62 (44.9 | 21 (45.7) | |
| Male | 76 (55.1) | 25 (54.3) | |
| BMI, median (range), kg/m2 | 25.8 (18.5–49.5) | 26.6 (15.9–55.2) | .72 |
| Tumor site, No. (%) | .07 | ||
| Colon | 112 (81.1) | 31 (67.4) | |
| Rectum | 26 (18.9 | 15 (32.6) | |
| Diabetes mellitus, No. (%) | 17 (12.3) | 7 (15.2) | .62 |
| Chemotherapy, No. (%) | .05 | ||
| FOLFOX | 131 (94.9) | 39 (84.8 | |
| CAPOX | 7 (5.1) | 7 (15.2) | |
| Oxaliplatin cycles, median (range) | 9 (6–19) | 9 (6–14) | .79 |
| Cumulative oxaliplatin dose, median (range), mg | 1414 (570–3480) | 1423 (696–3300) | .73 |
| Cumulative 5-FU dose, median (range), mg* | 45901 (28 576–94 470) | 47789 (26 111–84 869) | .4 |
| Baseline spleen size, median (range), cm3 | 217 (59–684) | 231 (81–683) | .84 |
| Baseline platelet count, median (range), K/µL | 320 (161–1013) | 304 (151–887) | .91 |
| Characteristics . | Bevacizumab arm . | Nonbevacizumab arm . | P* . |
|---|---|---|---|
| (n = 138) . | (n = 46) . | ||
| Age, median (range), y | 56 (30–82) | 58 (25–83) | .73 |
| Sex, No. (%) | 1.00 | ||
| Female | 62 (44.9 | 21 (45.7) | |
| Male | 76 (55.1) | 25 (54.3) | |
| BMI, median (range), kg/m2 | 25.8 (18.5–49.5) | 26.6 (15.9–55.2) | .72 |
| Tumor site, No. (%) | .07 | ||
| Colon | 112 (81.1) | 31 (67.4) | |
| Rectum | 26 (18.9 | 15 (32.6) | |
| Diabetes mellitus, No. (%) | 17 (12.3) | 7 (15.2) | .62 |
| Chemotherapy, No. (%) | .05 | ||
| FOLFOX | 131 (94.9) | 39 (84.8 | |
| CAPOX | 7 (5.1) | 7 (15.2) | |
| Oxaliplatin cycles, median (range) | 9 (6–19) | 9 (6–14) | .79 |
| Cumulative oxaliplatin dose, median (range), mg | 1414 (570–3480) | 1423 (696–3300) | .73 |
| Cumulative 5-FU dose, median (range), mg* | 45901 (28 576–94 470) | 47789 (26 111–84 869) | .4 |
| Baseline spleen size, median (range), cm3 | 217 (59–684) | 231 (81–683) | .84 |
| Baseline platelet count, median (range), K/µL | 320 (161–1013) | 304 (151–887) | .91 |
For FOLFOX-treated patients. Two-sided P values are based on the Fisher exact test statistic for categorical variables and Wilcoxon rank-sum for continuous variables. BMI = body mass index; CAPOX = capecitabine and oxaliplatin; FOLFOX = infused fluorouracil, folinic acid, and oxaliplatin.
| Characteristics . | Bevacizumab arm . | Nonbevacizumab arm . | P* . |
|---|---|---|---|
| (n = 138) . | (n = 46) . | ||
| Age, median (range), y | 56 (30–82) | 58 (25–83) | .73 |
| Sex, No. (%) | 1.00 | ||
| Female | 62 (44.9 | 21 (45.7) | |
| Male | 76 (55.1) | 25 (54.3) | |
| BMI, median (range), kg/m2 | 25.8 (18.5–49.5) | 26.6 (15.9–55.2) | .72 |
| Tumor site, No. (%) | .07 | ||
| Colon | 112 (81.1) | 31 (67.4) | |
| Rectum | 26 (18.9 | 15 (32.6) | |
| Diabetes mellitus, No. (%) | 17 (12.3) | 7 (15.2) | .62 |
| Chemotherapy, No. (%) | .05 | ||
| FOLFOX | 131 (94.9) | 39 (84.8 | |
| CAPOX | 7 (5.1) | 7 (15.2) | |
| Oxaliplatin cycles, median (range) | 9 (6–19) | 9 (6–14) | .79 |
| Cumulative oxaliplatin dose, median (range), mg | 1414 (570–3480) | 1423 (696–3300) | .73 |
| Cumulative 5-FU dose, median (range), mg* | 45901 (28 576–94 470) | 47789 (26 111–84 869) | .4 |
| Baseline spleen size, median (range), cm3 | 217 (59–684) | 231 (81–683) | .84 |
| Baseline platelet count, median (range), K/µL | 320 (161–1013) | 304 (151–887) | .91 |
| Characteristics . | Bevacizumab arm . | Nonbevacizumab arm . | P* . |
|---|---|---|---|
| (n = 138) . | (n = 46) . | ||
| Age, median (range), y | 56 (30–82) | 58 (25–83) | .73 |
| Sex, No. (%) | 1.00 | ||
| Female | 62 (44.9 | 21 (45.7) | |
| Male | 76 (55.1) | 25 (54.3) | |
| BMI, median (range), kg/m2 | 25.8 (18.5–49.5) | 26.6 (15.9–55.2) | .72 |
| Tumor site, No. (%) | .07 | ||
| Colon | 112 (81.1) | 31 (67.4) | |
| Rectum | 26 (18.9 | 15 (32.6) | |
| Diabetes mellitus, No. (%) | 17 (12.3) | 7 (15.2) | .62 |
| Chemotherapy, No. (%) | .05 | ||
| FOLFOX | 131 (94.9) | 39 (84.8 | |
| CAPOX | 7 (5.1) | 7 (15.2) | |
| Oxaliplatin cycles, median (range) | 9 (6–19) | 9 (6–14) | .79 |
| Cumulative oxaliplatin dose, median (range), mg | 1414 (570–3480) | 1423 (696–3300) | .73 |
| Cumulative 5-FU dose, median (range), mg* | 45901 (28 576–94 470) | 47789 (26 111–84 869) | .4 |
| Baseline spleen size, median (range), cm3 | 217 (59–684) | 231 (81–683) | .84 |
| Baseline platelet count, median (range), K/µL | 320 (161–1013) | 304 (151–887) | .91 |
For FOLFOX-treated patients. Two-sided P values are based on the Fisher exact test statistic for categorical variables and Wilcoxon rank-sum for continuous variables. BMI = body mass index; CAPOX = capecitabine and oxaliplatin; FOLFOX = infused fluorouracil, folinic acid, and oxaliplatin.
Confirmatory NO16966 Cohort
Of the 200 randomly selected patients, 106 received bevacizumab (54 FOLFOX/bevacizumab and 52 CAPOX/bevacizumab) and 94 did not receive bevacizumab (49 FOLFOX/placebo and 45 CAPOX/placebo). Baseline characteristics for the bevacizumab- and placebo-treated arms were similar (Table 2).
| Characteristic . | Bevacizumab + FOLFOX or CAPOX (n = 106) . | Placebo + FOLFOX or CAPOX (n = 94) . | P* . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Sex | |||
| Male | 64 (60.4) | 52 (55.3) | .47 |
| Female | 42 (39.6) | 42 (44.7) | |
| Age, median (range), y | 61 (18–82) | 60 (33–83) | .81 |
| ECOG performance status | .61 | ||
| 0 | 66 (62.3) | 57 (60.6) | |
| 1 | 39 (36.8) | 37 (39.4) | |
| 2 | 1 (0.9) | 0 (0) | |
| Primary tumor site | .96 | ||
| Colon | 66 (62.3) | 60 (63.8) | |
| Rectal | 30 (28.3) | 25 (26.6) | |
| Colorectal | 10 (9.4) | 9 (9.6) | |
| Stage at first diagnosis | .75 | ||
| Local regional | 28 (26.4) | 23 (24.5) | |
| Metastatic | 78 (73.6) | 71 (75.5) | |
| No. of metastatic sites | .33 | ||
| 1 | 35 (33.0) | 34 (36.2) | |
| 2 | 49 (46.3) | 33 (35.1) | |
| 3 | 17 (16.0) | 23 (24.5) | |
| ≥4 | 5 (4.7) | 4 (4.3) | |
| Prior fluoropyrimidine adjuvant therapy | .13 | ||
| No | 88 (83.0) | 85 (90.4) | |
| Yes | 18 (17.0) | 9 (9.6) |
| Characteristic . | Bevacizumab + FOLFOX or CAPOX (n = 106) . | Placebo + FOLFOX or CAPOX (n = 94) . | P* . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Sex | |||
| Male | 64 (60.4) | 52 (55.3) | .47 |
| Female | 42 (39.6) | 42 (44.7) | |
| Age, median (range), y | 61 (18–82) | 60 (33–83) | .81 |
| ECOG performance status | .61 | ||
| 0 | 66 (62.3) | 57 (60.6) | |
| 1 | 39 (36.8) | 37 (39.4) | |
| 2 | 1 (0.9) | 0 (0) | |
| Primary tumor site | .96 | ||
| Colon | 66 (62.3) | 60 (63.8) | |
| Rectal | 30 (28.3) | 25 (26.6) | |
| Colorectal | 10 (9.4) | 9 (9.6) | |
| Stage at first diagnosis | .75 | ||
| Local regional | 28 (26.4) | 23 (24.5) | |
| Metastatic | 78 (73.6) | 71 (75.5) | |
| No. of metastatic sites | .33 | ||
| 1 | 35 (33.0) | 34 (36.2) | |
| 2 | 49 (46.3) | 33 (35.1) | |
| 3 | 17 (16.0) | 23 (24.5) | |
| ≥4 | 5 (4.7) | 4 (4.3) | |
| Prior fluoropyrimidine adjuvant therapy | .13 | ||
| No | 88 (83.0) | 85 (90.4) | |
| Yes | 18 (17.0) | 9 (9.6) |
Two-sided P values are based on the Fisher exact test statistic for categorical variables and Wilcoxon rank-sum for continuous variables. CAPOX = capecitabine and oxaliplatin; ECOG = Eastern Cooperative Group; FOLFOX = infused fluorouracil, folinic acid, and oxaliplatin.
| Characteristic . | Bevacizumab + FOLFOX or CAPOX (n = 106) . | Placebo + FOLFOX or CAPOX (n = 94) . | P* . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Sex | |||
| Male | 64 (60.4) | 52 (55.3) | .47 |
| Female | 42 (39.6) | 42 (44.7) | |
| Age, median (range), y | 61 (18–82) | 60 (33–83) | .81 |
| ECOG performance status | .61 | ||
| 0 | 66 (62.3) | 57 (60.6) | |
| 1 | 39 (36.8) | 37 (39.4) | |
| 2 | 1 (0.9) | 0 (0) | |
| Primary tumor site | .96 | ||
| Colon | 66 (62.3) | 60 (63.8) | |
| Rectal | 30 (28.3) | 25 (26.6) | |
| Colorectal | 10 (9.4) | 9 (9.6) | |
| Stage at first diagnosis | .75 | ||
| Local regional | 28 (26.4) | 23 (24.5) | |
| Metastatic | 78 (73.6) | 71 (75.5) | |
| No. of metastatic sites | .33 | ||
| 1 | 35 (33.0) | 34 (36.2) | |
| 2 | 49 (46.3) | 33 (35.1) | |
| 3 | 17 (16.0) | 23 (24.5) | |
| ≥4 | 5 (4.7) | 4 (4.3) | |
| Prior fluoropyrimidine adjuvant therapy | .13 | ||
| No | 88 (83.0) | 85 (90.4) | |
| Yes | 18 (17.0) | 9 (9.6) |
| Characteristic . | Bevacizumab + FOLFOX or CAPOX (n = 106) . | Placebo + FOLFOX or CAPOX (n = 94) . | P* . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Sex | |||
| Male | 64 (60.4) | 52 (55.3) | .47 |
| Female | 42 (39.6) | 42 (44.7) | |
| Age, median (range), y | 61 (18–82) | 60 (33–83) | .81 |
| ECOG performance status | .61 | ||
| 0 | 66 (62.3) | 57 (60.6) | |
| 1 | 39 (36.8) | 37 (39.4) | |
| 2 | 1 (0.9) | 0 (0) | |
| Primary tumor site | .96 | ||
| Colon | 66 (62.3) | 60 (63.8) | |
| Rectal | 30 (28.3) | 25 (26.6) | |
| Colorectal | 10 (9.4) | 9 (9.6) | |
| Stage at first diagnosis | .75 | ||
| Local regional | 28 (26.4) | 23 (24.5) | |
| Metastatic | 78 (73.6) | 71 (75.5) | |
| No. of metastatic sites | .33 | ||
| 1 | 35 (33.0) | 34 (36.2) | |
| 2 | 49 (46.3) | 33 (35.1) | |
| 3 | 17 (16.0) | 23 (24.5) | |
| ≥4 | 5 (4.7) | 4 (4.3) | |
| Prior fluoropyrimidine adjuvant therapy | .13 | ||
| No | 88 (83.0) | 85 (90.4) | |
| Yes | 18 (17.0) | 9 (9.6) |
Two-sided P values are based on the Fisher exact test statistic for categorical variables and Wilcoxon rank-sum for continuous variables. CAPOX = capecitabine and oxaliplatin; ECOG = Eastern Cooperative Group; FOLFOX = infused fluorouracil, folinic acid, and oxaliplatin.
The waterfall plot of maximal percentage change in spleen size is shown in Figure 1 for bevacizumab- and placebo-treated patients. At six months, the cumulative incidence rates of a 30% or greater increase in spleen size for bevacizumab compared with placebo arms were 41% vs 58% (P = .01), and for a 50% or greater increase, the incidence rates were 21% vs 48% (P < .001, respectively) (Figure 2). The median time to the development of a 30% or greater increase in spleen size was 5.4 months (95% CI = 4.8 to 6.5 months) for placebo-treated patients and 7.6 months (95% CI = 6.3 to 8.8 months) for bevacizumab-treated patients (P = .01). The median time to the development of splenic enlargement using a higher threshold of 50% or greater was 6.3 months (95% CI = 5.6 to 7.3 months) for placebo-treated patients and 11.3 months (95% CI = 9 months to not reached) for bevacizumab-treated patients, respectively (P < .001).
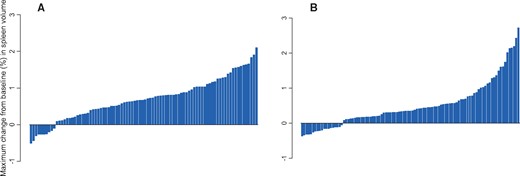
Maximal percentage change in spleen size in the confirmatory cohort stratified by (A) placebo (n = 89) or (B) bevacizumab (n = 99).
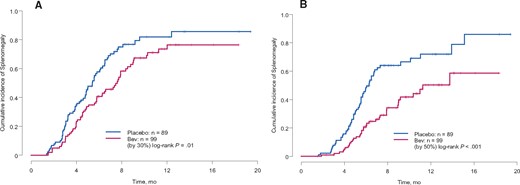
Cumulative rate of developing splenomegaly defined as (A) 30% or greater increase in spleen size or (B) 50% or greater increase in spleen in the confirmatory cohort (two-sided log-rank test).
The median time to the development of a platelet count lower than 100 000/mm3 was six months (95% CI = 5.0 to 9.8 months) for placebo-treated patients and not reached for bevacizumab-treated patients (P < .001). The six-month cumulative incidence of thrombocytopenia (platelet count < 100 000/mm3) was 51% in the placebo arm and 19% in the bevacizumab arm (P < .001). The cumulative incidence of developing grade 1 or grade 2 thrombocytopenia was statistically less in the bevacizumab arm compared with the placebo arm (Figure 3). Grade 3 thrombocytopenia was rare, occurring in only four patients, and did not differ between the bevacizumab and placebo arms.
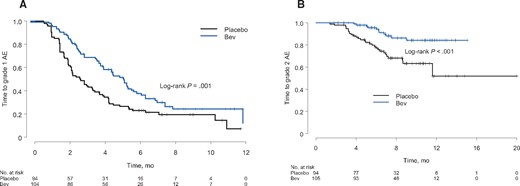
Time to the development of (A) grade 1 (<lower limit of normal to ≥ 75 000/mm3) or (B) grade 2 (<75 000/mm3 to ≥ 50 000/mm3) thrombocytopenia in the confirmatory cohort stratified by placebo or bevacizumab (Bev) treatment.
In the entire confirmatory cohort, the development of splenic enlargement was associated with the development of thrombocytopenia (Figure 4, A and B). When further stratified by treatment arm, the group that demonstrates the greatest reduction in mean platelet counts is the subset of the placebo-treated patients who developed splenic enlargement of 30% or greater (Figure 4C).
![Confirmatory cohort. (A) Scatter plot demonstrating the magnitude of the observed maximum percentage change in spleen volume in relation to the magnitude of the observed maximum percentage change in platelet counts (line represents the piecewise linear best unbiased estimate of the mean trend; two outlying values are not shown at points [x, y]: [1.16, 1.19] and [–0.15, 3.02]). (B) Mean platelet counts over time stratified by the development of a 30% or greater increase in spleen volume (gray shading represents 95% confidence intervals, analysis by piecewise polynomial regression). (C) Mean platelet counts over time stratified by the development of a 30% or greater increase in spleen volume and treatment arm (gray shading represents 95% confidence intervals, analysis by piecewise polynomial regression). (D) Cumulative incidence of developing a 30% or greater increase in spleen volume stratified by treatment arm and median baseline spleen volume. Bev = bevacizumab (two-sided log-rank test).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/110/8/10.1093_jnci_djx288/1/m_djx288f4.jpeg?Expires=1748346565&Signature=MziuVV-Gu6guOf2fFS-ze5eok98QSwfBgOgX95ikFE-cV4pbRZf2wv0xolzGfoPVY5ubTN8WyYzQ5vNE4qW~ywo7FEOn3INRDscpMljjISDlYKLIqQgn1HpXI4xiifKXOugmzT0lRAYvYNNe8sxsmkAmn1MEHJwXwF4ZaUqzgzGJjAasV7BRQFWHOwhnWX2EiU3COzF3IXjspiA5v6rRzspK205jqO1D6Pax~-2KN8-0Px~oLAPP3NKmTtX9bt1peK0eePIA5Hd3sVHrJBaPU5M7DYLJ45SPPRiggaVuA3U4dQyGiNq5zvwj7CDMiS33PxsFll9Wfs6RUpauyDQemQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Confirmatory cohort. (A) Scatter plot demonstrating the magnitude of the observed maximum percentage change in spleen volume in relation to the magnitude of the observed maximum percentage change in platelet counts (line represents the piecewise linear best unbiased estimate of the mean trend; two outlying values are not shown at points [x, y]: [1.16, 1.19] and [–0.15, 3.02]). (B) Mean platelet counts over time stratified by the development of a 30% or greater increase in spleen volume (gray shading represents 95% confidence intervals, analysis by piecewise polynomial regression). (C) Mean platelet counts over time stratified by the development of a 30% or greater increase in spleen volume and treatment arm (gray shading represents 95% confidence intervals, analysis by piecewise polynomial regression). (D) Cumulative incidence of developing a 30% or greater increase in spleen volume stratified by treatment arm and median baseline spleen volume. Bev = bevacizumab (two-sided log-rank test).
In order to determine determinants of splenomegaly development, we conducted an exploratory analysis evaluating the impact of spleen size prior to chemotherapy on the risk of subsequently developing splenomegaly and thrombocytopenia. When separated by the median baseline spleen size (203 cm3), patients with larger baseline spleens who were treated with bevacizumab had a reduced rate of splenomegaly development (HR = 0.66, 95% CI = 0.46 to 0.94) (Figure 4D). A similar trend was seen in our exploratory cohort using the median baseline spleen size (217 cm3; HR = 0.64, 95% CI = 0.39 to 1.05).
Discussion
In frontline therapy for metastatic CRC, this study shows that the rate of thrombocytopenia was associated with increases in splenic size as a result of oxaliplatin-induced HSI. Of greater interest is that bevacizumab therapy reduces the rate of grade 1 and 2 thrombocytopenia from fluoropyrimidine and oxaliplatin therapy by reducing splenic enlargement related to oxaliplatin-induced HSI.
The role of an adequate platelet count prior to each chemotherapy treatment is intended to ensure that bleeding complications due to severe thrombocytopenia do not occur at or around a patient’s platelet nadir. In the NO16966 clinical trial, a platelet count of less than 75 000/mm3 resulted in a treatment delay and a platelet count of less than 50 000/mm3 resulted in a chemotherapy dose reduction. As treatment delays were not ascribed to specific toxicities, the exact rate of delay due to grade 2 thrombocytopenia could not be determined. However, with a 17% difference in cumulative six-month grade 2 thrombocytopenia (23% vs 4%) between placebo and bevacizumab, it is likely that a reduction in treatment delays occurred in bevacizumab-treated patients. Whether this difference in treatment intensity between bevacizumab and placebo arms results in a difference in efficacy is not known. Prior clinical trials investigating recombinant thrombopoietin receptor agonists for the prevention of chemotherapy-induced thrombocytopenia demonstrated statistically significantly faster resolution of thrombocytopenia, but none has demonstrated an impact on efficacy related to increased dose intensity (17,18). While higher chemotherapy dose intensity in the treatment of breast cancer has been correlated with improved outcomes in some studies, the data for improved outcomes with higher dose intensity in colorectal cancer are limited (19–21). Though a reduction in thrombocytopenia in the bevacizumab arm was seen in the NO16966 trial, the overall toxicity was higher with the addition of bevacizumab, as indicted by more patients discontinuing on the bevacizumab arm (30% vs 21%) and more discontinuing due to grade 3/4 events (21% vs 15%).
Interestingly, thrombocytopenia resulting from splenic sequestration carries a lower bleeding risk than thrombocytopenia due to hypoproduction. While a peripheral reduction in platelet count exists in splenic sequestration, the total platelet pool remains near normal (22). These platelets are able to be rapidly mobilized from the spleen to replenish losses (23), though this suggests that a lower platelet threshold for chemotherapy administration in the face of splenic sequestration could be considered, determining that the respective contributions of hypoproduction and sequestration to a low platelet count are nontrivial. The data provided here do not provide guidelines on such platelet thresholds in the context of patients with oxaliplatin-induced HSI splenomegaly.
The protective impact of bevacizumab upon oxaliplatin-induced HSI has been previously noted in a number of small series evaluating liver resection specimens following preoperative chemotherapy (1,9–12). For example, in one retrospective study of 105 patients, preoperative fluoropyrimidine and oxaliplatin with bevacizumab resulted in a statistically significant reduction in histologically determined sinusoidal injury of 27% vs 54% (P < .01) when compared with fluoropyrimidine and oxaliplatin without bevacizumab (11). In another retrospective study evaluating oxaliplatin-induced HSI in liver resection specimens, a statistically significant reduction in mean preoperative platelet count was noted in patients with increasing grades of sinusoidal injury (10). The findings within this report are likely the explanation for the lower rate of thrombocytopenia seen in the C-08 study evaluating FOLFOX with or without bevacizumab for the adjuvant treatment of CRC. Though a number of other phase III randomized clinical trials have investigated the use of bevacizumab with oxaliplatin-based chemotherapy, the rates of thrombocytopenia were not reported (24,25).
Oxaliplatin is known to induce reactive oxygen species, which can trigger increased levels of VEGF-A, with increased fenestrations in the sinusoidal endothelium, leading to endothelial microdissections and the regional obstructions characteristic of sinusoidal injury (26–28). Studies investigating liver samples from patients who have developed oxaliplatin-induced sinusoidal injury and a preclinical rat model system of sinusoidal injury using monocrotaline, a pyrrolizidine alkaloid, have both demonstrated upregulation of VEGF-A as a key component of this toxicity (29–32). Using this rat model, two VEGF tyrosine kinase inhibitors, sorafenib and regorafenib, have demonstrated a protective effect with regards to monocrotaline-induced sinusoidal obstructive syndrome (13,14). These laboratory findings lend further support to the protective impact of VEGF-A blockade upon the development of oxaliplatin-induced HSI.
There are limitations to the cohorts studied in this report as the decision for bevacizumab use in the retrospective cohort was made by the treating physician and may have been influenced by additional factors. Though the rate of thrombocytopenia (platelet count < 100 000/mm3), a coprimary end point, did not differ in the exploratory cohort, a difference was seen using a platelet threshold of less than 150 000/mm3. The analysis of the NO16966 clinical trial represents an unplanned and thus exploratory analysis. However, the randomized and placebo-controlled design of this clinical trial represents the optimal data set for the determination of bevacizumab upon an end point. Given that other randomized clinical trial data sets of bevacizumab and oxaliplatin-based chemotherapy exist, examination of these data sets could provide further validation of these findings. As therapy delays and dose adjustments are not solely based upon a platelet count, but other laboratory and clinical factors, we were not able to isolate impact of thrombocytopenia upon chemotherapy dose intensity. In the future, efforts to identify patients at greatest risk for oxaliplatin-induced HSI may help to determine which patients may derive the greatest benefit from bevacizumab with regard to its ability to reduce the rate of thrombocytopenia.
In conclusion, the concurrent use of bevacizumab reduces oxaliplatin-induced his, with resultant reductions in thrombocytopenia, although the clinical impact of a reduction in thrombocytopenia upon efficacy outcomes is uncertain. Further efforts to identify those patients at greatest risk for this toxicity could help to identify a population that might benefit the most in terms of this toxicity from the addition of bevacizumab to oxaliplatin-based chemotherapy.
Funding
This work was supported by an independent research grant from Roche Pharmaceuticals.
Notes
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors thank and honor Dr. Chusilp Charnsangavej, who died November 23, 2013, for his support in initiating this project and his commitment to academic research. Drs. Overman, Raghav, Hoff, and Kopetz have recieved research funding from Roche. Dr. Hobbs has a consulting arrangement with Ignyta, Inc.
Portions of this work were previously presented at the American Society for Clinical Oncology 2012, Journal of Clinical Oncology 30, 2012 (suppl; abstr 3544), and Gastrointestinal Cancers Symposium 2012, Journal of Clinical Oncology 30, 2012 (suppl 4; abstr 564).


