-
PDF
- Split View
-
Views
-
Cite
Cite
Willemijne A M E Schrijver, Karijn P M Suijkerbuijk, Carla H van Gils, Elsken van der Wall, Cathy B Moelans, Paul J van Diest, Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis, JNCI: Journal of the National Cancer Institute, Volume 110, Issue 6, June 2018, Pages 568–580, https://doi.org/10.1093/jnci/djx273
Close - Share Icon Share
Abstract
In metastatic breast cancer, hormone and/or human epidermal growth factor receptor 2 (HER2)–targeted therapy decision-making is still largely based on tissue characteristics of the primary tumor. However, a change of estrogen receptor alpha (ERα), progesterone receptor (PR), and HER2 status in distant metastases has frequently been reported. The actual incidence of this phenomenon has been debated.
We performed a meta-analysis including 39 studies assessing receptor conversion from primary breast tumors to paired distant breast cancer metastases. We noted the direction of change (positive to negative or vice versa) and performed subgroup analyses for different thresholds for positivity, the type of test used to assess HER2 receptor status, and metastasis location–specific differences (two-sided tests).
Overall, the incidence of receptor conversion varied largely between studies. For ERα, PR, and HER2, we found that random effects pooled positive to negative conversion percentages of 22.5% (95% confidence interval [CI] = 16.4% to 30.0%), 49.4% (95% CI = 40.5% to 58.2%), and 21.3% (95% CI = 14.3% to 30.5%), respectively. Negative to positive conversion percentages were 21.5% (95% CI = 18.1% to 25.5%), 15.9% (95% CI = 11.3% to 22.0%), and 9.5% (95% CI = 7.4% to 12.1%). Furthermore, ERα discordance was statistically significantly higher in the central nervous system and bone compared with liver metastases (20.8%, 95% CI = 15.0% to 28.0%, and 29.3%, 95% CI = 13.0% to 53.5%, vs 14.3%, 95% CI = 11.3% to 18.1, P = .008 and P < .001, respectively), and PR discordance was higher in bone (42.7%, 95% CI = 35.1% to 50.6%, P < .001) and liver metastases (47.0%, 95% CI = 41.0% to 53.0%, P < .001) compared with central nervous system metastases (23.3%, 95% CI = 16.0% to 32.6%).
Receptor conversion for ERα, PR, and HER2 occurs frequently in the course of disease progression in breast cancer. Large prospective studies assessing the impact of receptor conversion on treatment efficacy and survival are needed. Meanwhile, reassessing receptor status in metastases is strongly encouraged.
Despite advances in breast cancer treatment during the last decades, most metastatic breast cancer patients still have poor life expectancy. Acquiring more profound insights into the phenotypic and molecular composition of metastatic tumors is of the utmost importance to pave the way for more effective therapeutic regimens.
Estrogen receptor alpha (ERα), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status have proven their clinical utility in guiding therapeutic decision-making in (metastatic) breast cancer (1). Prescription of endocrine or HER2-targeted therapies is mainly directed at the biomarker status of the primary tumor. However, increasing evidence shows extensive differences between immunohistochemically assessed tissue characteristics of primary breast tumors and their paired metastases (2–6). For ERα, PR, and HER2, widely varying discordance rates have been reported so far: 3%–54% for ERα, 5%–78% for PR, and 0%–34% for HER2 (7–9). This change of hormone receptor and/or HER2 status between primary tumor and paired metastasis within a patient is usually denoted “receptor conversion.”
Several guidelines have now consented that patients with accessible breast cancer metastases should be offered a biopsy or resection to confirm the diagnosis of metastases and to re-assess ERα, PR, and HER2 status (1,10,11). Obtaining metastatic material is, however, not without risk, introducing potential hemorrhage and infection. Furthermore, to date, there is insufficient evidence supporting improved survival outcomes when treatment regimens are based on the receptor status of the metastasis instead of the primary tumor (1). This can be explained by the fact that randomized controlled trials stratifying patients for treatment based on receptor status of either the primary tumor or the metastasis could be deemed unethical and are, to our knowledge, therefore not performed.
Although previous studies have summarized available data and literature, a solid systematic review addressing receptor conversion in distant metastases including meta-analysis to date is lacking. Other studies only included lymph node metastases (12) or assessed receptor conversion in pooled loco-regional and distant metastases (1), despite large differences between these two groups (13). In this study, we focus on distant metastases because they are the major cause of breast cancer–related mortality (14). Furthermore, tissue characteristics of distant sites are not always reassessed due to difficulty obtaining a biopsy, leading to potential suboptimal treatment. Also, distant metastases are commonly treated with systemic therapy, whereas resection and radiotherapy are preferred treatments for loco-regional metastases.
We set out to systematically evaluate the frequency of receptor conversion between primary breast tumors and distant breast cancer metastases (excluding regional lymph nodes) in the published peer-reviewed articles, paying special attention to thresholds for positivity (1% vs 10% for ERα and PR), the type of test used to assess HER2 status (immunohistochemistry, fluorescence in situ hybridization, or a combination of both) and metastasis location–specific differences.
Methods
Selection of Studies
The Embase, Cochrane, and PubMed databases were searched on July 11, 2016, for relevant studies, covering a time period from 1986 until 2016. The literature search used the following terms (with synonyms, MeSH terms, and closely related words): “breast cancer” and “metastasis,” combined with “estrogen receptor/ERα,” “progesterone receptor/PR,” “HER2/neu,” “immunohistochemistry/IHC” or “in situ hybridization/ISH,” and “receptor conversion/dis- or concordance.” We needed a broad search to include all articles with distant metastases. However, this also resulted in many articles only addressing local or lymph node metastases that were excluded during the screening process. Duplicates were eliminated using RefWorks. The search strategy is listed in the Supplementary Materials (available online). All articles were screened for relevance. Original full-text research articles directly describing immunohistochemically assessed ERα, PR, or HER2 status in primary breast tumors compared with paired distant metastases were included. Exclusion criteria were case reports, meta-analyses, and reviews, cytology specimens (or circulating tumor cells or tissue collected by fine needle aspiration), male patients, axillary lymph node or loco-regional metastases, methodology other than immunohistochemistry (IHC) or in situ hybridization (ISH), receptors other than ERα, PR, and HER2, and languages other than English.
Data Collection
A total of 5521 unique articles were identified and screened. When no full text was available online, printed copies of these articles were requested by sending an e-mail to the corresponding author. Titles and abstracts were screened for relevance; 3733 articles were excluded because the title and abstract did not meet the selection criteria (no full text/abstract only, no comparison primary vs matched metastasis, no original article, review/case report, lymph node/locoregional metastases, cytology/circulating tumor cells, other receptors, other tumor types) (Figure 1). Reference lists of the papers of interest were screened manually and using Scopus to ensure sensitivity of the search strategy and to identify additional relevant studies, leading to 11 additional articles. Checking the titles and abstracts of these articles did not lead to new search terms.
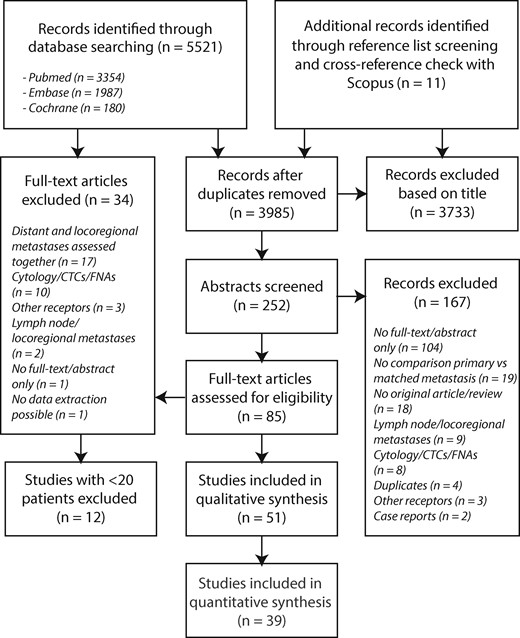
Flow diagram of study selection for this meta-analysis. CTC = circulating tumor cells; FNAs = fine needle aspirates.
Fifty-one selected publications were independently reviewed by two of the authors (WAMES and KPMS) to determine the eligibility of each article in the meta-analysis. Because the heterogeneity (assessed using the Q test and Higgins I2, as described below) was perceived to be higher in studies with small samples sizes, we excluded 12 articles describing fewer than 20 patients. Quality assessment of the 39 included studies was performed by critical appraisal, based on standardized criteria for diagnostic research using the QUADAS-2 tool for quality assessment of diagnostic accuracy studies (Table 1) (15). This tool consists of four key domains covering patient selection, index test, reference standard, and flow of patients through the study (timing of the index test and reference standard). For patient selection, we considered the prospective or retrospective nature of data collection, the consecutive inclusion of patients, and the presence of clear in- and exclusion criteria. Considering the receptor status of the metastasis as the index test, we took into account standardization and clear description of the analysis (assay, threshold of positivity, blinding). The receptor status of the primary tumor was considered the reference standard and assessed for the same criteria. Each domain was assessed in terms of the risk of bias, and the first three were also assessed in terms of concerns regarding applicability. Risk of bias and concern of applicability for each domain were rated as low, high, or unclear. Studies with two or more high or unclear ratings were excluded from this meta-analysis.
| Study . | Risk of bias . | Concerns regarding applicability . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patientselection . | Index test . | Referencestandard . | Flow and timing . | . | Patientselection . | Index test . | Referencestandard . | . | |
| Amir et al., 2012b† (38,51,54) | + | + | + | + | Low | + | + | + | Applicable for review |
| BRITS study Thompson et al., 2010 | |||||||||
| DESTINY study Amiret al., 2012a | |||||||||
| Bogina et al., 2011 (37) | + | + | + | + | Low | + | + | + | Applicable for review |
| Brogi et al., 2011 (69) | + | + | + | + | Low | + | + | + | Applicable for review |
| Chan et al., 2012 (70) | + | + | + | + | Low | + | + | + | Applicable for review |
| Cummings et al., 2014 (71) | + | + | + | + | Low | + | + | + | Applicable for review |
| Duchnowska et al., 2012 (32) | + | + | + | + | Low | + | + | + | Applicable for review |
| Edgerton et al., 2003 (47) | + | + | + | + | Low | + | + | + | Applicable for review |
| Fabi et al., 2011 (39) | + | + | + | + | Low | + | + | + | Applicable for review |
| Fuchs et al., 2006 (72) | + | + | + | + | Low | + | + | + | Applicable for review |
| Gaedcke et al., 2007 (73) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Gonzalez-Angulo et al., 2011 (74) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hilton et al., 2010 (61) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hoefnagel et al., 2013‡ (2) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hoefnagel et al., 2010 & 2012 (3,4) | + | + | + | + | Low | + | + | + | Applicable for review |
| Jensen et al., 2010 (34) | + | + | + | + | Low | + | + | + | Applicable for review |
| Karagoz Ozenet al., 2014 (49) | + | + | + | + | Low | + | + | + | Applicable for review |
| Kulka et al., 2016 (62) | + | + | + | + | Low | + | + | + | Applicable for review |
| Nakamura et al., 2013 (35) | + | + | + | + | Low | + | + | + | Applicable for review |
| Regitnig et al., 2004 (63) | + | + | + | + | Low | + | + | + | Applicable for review |
| Santinelli et al., 2008 (75) | + | + | + | + | Low | + | + | + | Applicable for review |
| Simmons et al., 2009 (53) | + | + | + | + | Low | + | + | + | Applicable for review |
| Thomson et al., 2016 (76) | + | + | + | + | Low | + | – | + | Applicable for review |
| Yonemori et al., 2008 (36) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Zidan et al., 2005 (77) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Aurilio et al., 2013 (9) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Bachmann et al., 2013 (78) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Botteri et al., 2012 (79) | ? | + | ? | + | Moderate | + | + | + | Applicable for review |
| Cabioglu et al., 2009 (80) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Chang et al., 2011 (46) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Curigliano et al., 2011 (52) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Curtit et al., 2013 (31) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Gancberg et al., 2002 (81) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Idirisinghe et al., 2010 (33) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Lorincz et al., 2006 (82) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Lower et al., 2009 (48) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Omoto et al., 2010 (83) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Shen et al., 2015 (50) | + | + | + | – | Moderate | + | + | + | Applicable for review |
| St. Romain et al., 2012 (84) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Vincent-Salomon et al., 2002 (85) | + | + | ? | + | Moderate | – | + | + | Applicable for review |
| Shao et al., 2011 (86) | + | + | + | + | Low | + | ? | ? | Not applicable for review |
| Wu et al., 2008 (87) | + | + | + | + | Low | – | – | – | Not applicable for review |
| Lower et al., 2005 (88) | + | + | – | + | Moderate | + | – | + | Not applicable for review |
| Amir et al., 2008 (89) | – | + | – | – | High | – | + | + | Not applicable for review |
| Gullo et al., 2013 (90) | ? | ? | ? | – | High | – | – | – | Not applicable for review |
| Kalinsky et al., 2015 (91) | + | ? | – | – | High | – | – | – | Not applicable for review |
| Kamby et al., 1989 (92) | + | ? | ? | – | High | – | – | – | Not applicable for review |
| Koo et al., 2010 (93) | ? | ? | ? | – | High | – | – | ? | Not applicable for review |
| Lear-Kaul et al., 2003 (94) | + | ? | ? | + | High | – | + | – | Not applicable for review |
| Nogami et al., 2014 (95) | + | ? | ? | + | High | – | ? | + | Not applicable for review |
| Schwarz et al., 2004 (96) | + | ? | ? | + | High | + | – | – | Not applicable for review |
| Welter et al., 2008 (97) | + | – | ? | – | High | + | – | – | Not applicable for review |
| Study . | Risk of bias . | Concerns regarding applicability . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patientselection . | Index test . | Referencestandard . | Flow and timing . | . | Patientselection . | Index test . | Referencestandard . | . | |
| Amir et al., 2012b† (38,51,54) | + | + | + | + | Low | + | + | + | Applicable for review |
| BRITS study Thompson et al., 2010 | |||||||||
| DESTINY study Amiret al., 2012a | |||||||||
| Bogina et al., 2011 (37) | + | + | + | + | Low | + | + | + | Applicable for review |
| Brogi et al., 2011 (69) | + | + | + | + | Low | + | + | + | Applicable for review |
| Chan et al., 2012 (70) | + | + | + | + | Low | + | + | + | Applicable for review |
| Cummings et al., 2014 (71) | + | + | + | + | Low | + | + | + | Applicable for review |
| Duchnowska et al., 2012 (32) | + | + | + | + | Low | + | + | + | Applicable for review |
| Edgerton et al., 2003 (47) | + | + | + | + | Low | + | + | + | Applicable for review |
| Fabi et al., 2011 (39) | + | + | + | + | Low | + | + | + | Applicable for review |
| Fuchs et al., 2006 (72) | + | + | + | + | Low | + | + | + | Applicable for review |
| Gaedcke et al., 2007 (73) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Gonzalez-Angulo et al., 2011 (74) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hilton et al., 2010 (61) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hoefnagel et al., 2013‡ (2) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hoefnagel et al., 2010 & 2012 (3,4) | + | + | + | + | Low | + | + | + | Applicable for review |
| Jensen et al., 2010 (34) | + | + | + | + | Low | + | + | + | Applicable for review |
| Karagoz Ozenet al., 2014 (49) | + | + | + | + | Low | + | + | + | Applicable for review |
| Kulka et al., 2016 (62) | + | + | + | + | Low | + | + | + | Applicable for review |
| Nakamura et al., 2013 (35) | + | + | + | + | Low | + | + | + | Applicable for review |
| Regitnig et al., 2004 (63) | + | + | + | + | Low | + | + | + | Applicable for review |
| Santinelli et al., 2008 (75) | + | + | + | + | Low | + | + | + | Applicable for review |
| Simmons et al., 2009 (53) | + | + | + | + | Low | + | + | + | Applicable for review |
| Thomson et al., 2016 (76) | + | + | + | + | Low | + | – | + | Applicable for review |
| Yonemori et al., 2008 (36) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Zidan et al., 2005 (77) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Aurilio et al., 2013 (9) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Bachmann et al., 2013 (78) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Botteri et al., 2012 (79) | ? | + | ? | + | Moderate | + | + | + | Applicable for review |
| Cabioglu et al., 2009 (80) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Chang et al., 2011 (46) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Curigliano et al., 2011 (52) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Curtit et al., 2013 (31) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Gancberg et al., 2002 (81) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Idirisinghe et al., 2010 (33) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Lorincz et al., 2006 (82) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Lower et al., 2009 (48) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Omoto et al., 2010 (83) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Shen et al., 2015 (50) | + | + | + | – | Moderate | + | + | + | Applicable for review |
| St. Romain et al., 2012 (84) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Vincent-Salomon et al., 2002 (85) | + | + | ? | + | Moderate | – | + | + | Applicable for review |
| Shao et al., 2011 (86) | + | + | + | + | Low | + | ? | ? | Not applicable for review |
| Wu et al., 2008 (87) | + | + | + | + | Low | – | – | – | Not applicable for review |
| Lower et al., 2005 (88) | + | + | – | + | Moderate | + | – | + | Not applicable for review |
| Amir et al., 2008 (89) | – | + | – | – | High | – | + | + | Not applicable for review |
| Gullo et al., 2013 (90) | ? | ? | ? | – | High | – | – | – | Not applicable for review |
| Kalinsky et al., 2015 (91) | + | ? | – | – | High | – | – | – | Not applicable for review |
| Kamby et al., 1989 (92) | + | ? | ? | – | High | – | – | – | Not applicable for review |
| Koo et al., 2010 (93) | ? | ? | ? | – | High | – | – | ? | Not applicable for review |
| Lear-Kaul et al., 2003 (94) | + | ? | ? | + | High | – | + | – | Not applicable for review |
| Nogami et al., 2014 (95) | + | ? | ? | + | High | – | ? | + | Not applicable for review |
| Schwarz et al., 2004 (96) | + | ? | ? | + | High | + | – | – | Not applicable for review |
| Welter et al., 2008 (97) | + | – | ? | – | High | + | – | – | Not applicable for review |
In case of disagreement, the study was discussed, resulting in consensus about inclusion among the two investigators. + = low risk, - = high risk; ? = unclear.
Amir et al. (54) describe two clinical studies, namely the BRITS study (38) and the DESTINY study (51). For total conversion, the data of both clinical studies were used. However, for the direction of conversion (positive to negative and vice versa), discordance percentages were only presented in the pooled study (54).
| Study . | Risk of bias . | Concerns regarding applicability . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patientselection . | Index test . | Referencestandard . | Flow and timing . | . | Patientselection . | Index test . | Referencestandard . | . | |
| Amir et al., 2012b† (38,51,54) | + | + | + | + | Low | + | + | + | Applicable for review |
| BRITS study Thompson et al., 2010 | |||||||||
| DESTINY study Amiret al., 2012a | |||||||||
| Bogina et al., 2011 (37) | + | + | + | + | Low | + | + | + | Applicable for review |
| Brogi et al., 2011 (69) | + | + | + | + | Low | + | + | + | Applicable for review |
| Chan et al., 2012 (70) | + | + | + | + | Low | + | + | + | Applicable for review |
| Cummings et al., 2014 (71) | + | + | + | + | Low | + | + | + | Applicable for review |
| Duchnowska et al., 2012 (32) | + | + | + | + | Low | + | + | + | Applicable for review |
| Edgerton et al., 2003 (47) | + | + | + | + | Low | + | + | + | Applicable for review |
| Fabi et al., 2011 (39) | + | + | + | + | Low | + | + | + | Applicable for review |
| Fuchs et al., 2006 (72) | + | + | + | + | Low | + | + | + | Applicable for review |
| Gaedcke et al., 2007 (73) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Gonzalez-Angulo et al., 2011 (74) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hilton et al., 2010 (61) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hoefnagel et al., 2013‡ (2) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hoefnagel et al., 2010 & 2012 (3,4) | + | + | + | + | Low | + | + | + | Applicable for review |
| Jensen et al., 2010 (34) | + | + | + | + | Low | + | + | + | Applicable for review |
| Karagoz Ozenet al., 2014 (49) | + | + | + | + | Low | + | + | + | Applicable for review |
| Kulka et al., 2016 (62) | + | + | + | + | Low | + | + | + | Applicable for review |
| Nakamura et al., 2013 (35) | + | + | + | + | Low | + | + | + | Applicable for review |
| Regitnig et al., 2004 (63) | + | + | + | + | Low | + | + | + | Applicable for review |
| Santinelli et al., 2008 (75) | + | + | + | + | Low | + | + | + | Applicable for review |
| Simmons et al., 2009 (53) | + | + | + | + | Low | + | + | + | Applicable for review |
| Thomson et al., 2016 (76) | + | + | + | + | Low | + | – | + | Applicable for review |
| Yonemori et al., 2008 (36) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Zidan et al., 2005 (77) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Aurilio et al., 2013 (9) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Bachmann et al., 2013 (78) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Botteri et al., 2012 (79) | ? | + | ? | + | Moderate | + | + | + | Applicable for review |
| Cabioglu et al., 2009 (80) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Chang et al., 2011 (46) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Curigliano et al., 2011 (52) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Curtit et al., 2013 (31) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Gancberg et al., 2002 (81) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Idirisinghe et al., 2010 (33) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Lorincz et al., 2006 (82) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Lower et al., 2009 (48) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Omoto et al., 2010 (83) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Shen et al., 2015 (50) | + | + | + | – | Moderate | + | + | + | Applicable for review |
| St. Romain et al., 2012 (84) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Vincent-Salomon et al., 2002 (85) | + | + | ? | + | Moderate | – | + | + | Applicable for review |
| Shao et al., 2011 (86) | + | + | + | + | Low | + | ? | ? | Not applicable for review |
| Wu et al., 2008 (87) | + | + | + | + | Low | – | – | – | Not applicable for review |
| Lower et al., 2005 (88) | + | + | – | + | Moderate | + | – | + | Not applicable for review |
| Amir et al., 2008 (89) | – | + | – | – | High | – | + | + | Not applicable for review |
| Gullo et al., 2013 (90) | ? | ? | ? | – | High | – | – | – | Not applicable for review |
| Kalinsky et al., 2015 (91) | + | ? | – | – | High | – | – | – | Not applicable for review |
| Kamby et al., 1989 (92) | + | ? | ? | – | High | – | – | – | Not applicable for review |
| Koo et al., 2010 (93) | ? | ? | ? | – | High | – | – | ? | Not applicable for review |
| Lear-Kaul et al., 2003 (94) | + | ? | ? | + | High | – | + | – | Not applicable for review |
| Nogami et al., 2014 (95) | + | ? | ? | + | High | – | ? | + | Not applicable for review |
| Schwarz et al., 2004 (96) | + | ? | ? | + | High | + | – | – | Not applicable for review |
| Welter et al., 2008 (97) | + | – | ? | – | High | + | – | – | Not applicable for review |
| Study . | Risk of bias . | Concerns regarding applicability . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patientselection . | Index test . | Referencestandard . | Flow and timing . | . | Patientselection . | Index test . | Referencestandard . | . | |
| Amir et al., 2012b† (38,51,54) | + | + | + | + | Low | + | + | + | Applicable for review |
| BRITS study Thompson et al., 2010 | |||||||||
| DESTINY study Amiret al., 2012a | |||||||||
| Bogina et al., 2011 (37) | + | + | + | + | Low | + | + | + | Applicable for review |
| Brogi et al., 2011 (69) | + | + | + | + | Low | + | + | + | Applicable for review |
| Chan et al., 2012 (70) | + | + | + | + | Low | + | + | + | Applicable for review |
| Cummings et al., 2014 (71) | + | + | + | + | Low | + | + | + | Applicable for review |
| Duchnowska et al., 2012 (32) | + | + | + | + | Low | + | + | + | Applicable for review |
| Edgerton et al., 2003 (47) | + | + | + | + | Low | + | + | + | Applicable for review |
| Fabi et al., 2011 (39) | + | + | + | + | Low | + | + | + | Applicable for review |
| Fuchs et al., 2006 (72) | + | + | + | + | Low | + | + | + | Applicable for review |
| Gaedcke et al., 2007 (73) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Gonzalez-Angulo et al., 2011 (74) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hilton et al., 2010 (61) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hoefnagel et al., 2013‡ (2) | + | + | + | + | Low | + | + | + | Applicable for review |
| Hoefnagel et al., 2010 & 2012 (3,4) | + | + | + | + | Low | + | + | + | Applicable for review |
| Jensen et al., 2010 (34) | + | + | + | + | Low | + | + | + | Applicable for review |
| Karagoz Ozenet al., 2014 (49) | + | + | + | + | Low | + | + | + | Applicable for review |
| Kulka et al., 2016 (62) | + | + | + | + | Low | + | + | + | Applicable for review |
| Nakamura et al., 2013 (35) | + | + | + | + | Low | + | + | + | Applicable for review |
| Regitnig et al., 2004 (63) | + | + | + | + | Low | + | + | + | Applicable for review |
| Santinelli et al., 2008 (75) | + | + | + | + | Low | + | + | + | Applicable for review |
| Simmons et al., 2009 (53) | + | + | + | + | Low | + | + | + | Applicable for review |
| Thomson et al., 2016 (76) | + | + | + | + | Low | + | – | + | Applicable for review |
| Yonemori et al., 2008 (36) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Zidan et al., 2005 (77) | + | + | + | + | Low | + | ? | + | Applicable for review |
| Aurilio et al., 2013 (9) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Bachmann et al., 2013 (78) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Botteri et al., 2012 (79) | ? | + | ? | + | Moderate | + | + | + | Applicable for review |
| Cabioglu et al., 2009 (80) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Chang et al., 2011 (46) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Curigliano et al., 2011 (52) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Curtit et al., 2013 (31) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Gancberg et al., 2002 (81) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Idirisinghe et al., 2010 (33) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Lorincz et al., 2006 (82) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Lower et al., 2009 (48) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Omoto et al., 2010 (83) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Shen et al., 2015 (50) | + | + | + | – | Moderate | + | + | + | Applicable for review |
| St. Romain et al., 2012 (84) | + | + | ? | + | Moderate | + | + | + | Applicable for review |
| Vincent-Salomon et al., 2002 (85) | + | + | ? | + | Moderate | – | + | + | Applicable for review |
| Shao et al., 2011 (86) | + | + | + | + | Low | + | ? | ? | Not applicable for review |
| Wu et al., 2008 (87) | + | + | + | + | Low | – | – | – | Not applicable for review |
| Lower et al., 2005 (88) | + | + | – | + | Moderate | + | – | + | Not applicable for review |
| Amir et al., 2008 (89) | – | + | – | – | High | – | + | + | Not applicable for review |
| Gullo et al., 2013 (90) | ? | ? | ? | – | High | – | – | – | Not applicable for review |
| Kalinsky et al., 2015 (91) | + | ? | – | – | High | – | – | – | Not applicable for review |
| Kamby et al., 1989 (92) | + | ? | ? | – | High | – | – | – | Not applicable for review |
| Koo et al., 2010 (93) | ? | ? | ? | – | High | – | – | ? | Not applicable for review |
| Lear-Kaul et al., 2003 (94) | + | ? | ? | + | High | – | + | – | Not applicable for review |
| Nogami et al., 2014 (95) | + | ? | ? | + | High | – | ? | + | Not applicable for review |
| Schwarz et al., 2004 (96) | + | ? | ? | + | High | + | – | – | Not applicable for review |
| Welter et al., 2008 (97) | + | – | ? | – | High | + | – | – | Not applicable for review |
In case of disagreement, the study was discussed, resulting in consensus about inclusion among the two investigators. + = low risk, - = high risk; ? = unclear.
Amir et al. (54) describe two clinical studies, namely the BRITS study (38) and the DESTINY study (51). For total conversion, the data of both clinical studies were used. However, for the direction of conversion (positive to negative and vice versa), discordance percentages were only presented in the pooled study (54).
In case of disagreement, the study was discussed until consensus was reached among the two investigators. In case of persistent disagreement, a third opinion was obtained (CBM). The following details were extracted: total number of patients evaluated, clinic-pathologic characteristics of the primary tumor (if reported), site of and time to relapse, ERα, PR, and HER2 discordance rates with direction of conversion (positive to negative or vice versa), and information about treatment and survival. The technique used to define endocrine receptor or HER2 status (IHC and/or ISH) and the specific antibodies or probes were also registered.
According to the European Society for Medical Oncology and American Society of Clinical Oncology clinical practice guidelines, using a standardized assessment methodology (1% or 10% cutoff, Allred or H-score) for defining ERα and PR positivity (16) is a prerequisite. Unfortunately, H-score/Allred score was not reported in the majority of studies, and we therefore focused on 1% and 10% cutoffs. HER2 ISH should be used on all samples or in case of an ambiguous (2+) IHC score (17). Therefore, we focused on studies that met these criteria, but due to low numbers we also included studies that did not perform ISH. To correct for this bias, we included subanalyses to check for conversion differences between used techniques.
For complete and transparent reporting of the results of this review, we used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement checklist (18).
Statistical Analysis
The percentages of ERα, PR, and HER2 changes and 95% confidence intervals (CIs) were calculated for each study. Subanalyses were performed for thresholds for positivity (1% vs 10% for ERα and PR), the type of test used to assess receptor status (IHC, fluorescence in situ hybridization [FISH], or a combination of both), and location of metastasis. For HER2 immunohistochemistry, 0 and 1+ were considered negative, 2+ equivocal, and 3+ positive.
For meta-analysis, the conservative random effects model was used to calculate the pooled percentage, and statistical significance was determined using the Z-test (19). Heterogeneity across studies was assessed using both the Q test and Higgins I2 (20,21). I2 values of 0%, 25%, 50%, and 75% are indicated as “no,” “low,” “moderate,” and “high” heterogeneity, respectively. Comparison of subgroups was performed using the chi-square test.
All statistical tests for meta-analyses were performed using Comprehensive Meta-Analysis 2.0 software (Biostat, Englewood, NJ) and IBM SPSS Statistics 23. P values of less than .05 (two-sided) were considered statistically significant.
Results
Study Characteristics
The main characteristics of the remaining 39 studies are reported in Supplementary Tables 1–4 (available online). Data on ERα, PR, and HER2 status in the primary tumor and corresponding distant metastasis were available in 27, 24, and 35 studies, respectively. The discordance rate was assessed in 1948 patients for ERα, in 1730 patients for PR, and in 2440 patients for HER2. The mean age at diagnosis of the primary tumors was 51 years (26 studies, range = 22–93 years), 86.2% of tumors were of the ductal type (14 studies, 1038/1204 tumors), and the mean time between primary tumor and matched distant metastasis was 51 months (28 studies, range = 0–432 months).
Heterogeneity
Overall, variation between studies was high. For ERα, the heterogeneity for total conversion and conversion from positive to negative was high (I² = 72.8% and 78.3%, respectively), but for conversion from negative to positive, no heterogeneity was perceived (I² = 0%). This barely changed when studies were more specifically subdivided per threshold for positivity (Table 2). A similar trend was seen for PR conversion, but conversion from negative to positive did show moderate heterogeneity (61.3%). Less heterogeneity was perceived for the 10% threshold of positivity (I² = 41.1%, 62.8%, and 24.5% for total, positive to negative, and negative to positive conversion, respectively). For HER2, the smallest variation between studies was seen when IHC was used to assess receptor status (I²: 74.4%, 30.8%, and 28.2% for total, positive to negative, and negative to positive conversion, respectively). We performed a subanalysis to analyze if sample size could drive heterogeneity. We stratified studies in near equal groups according to number of described cases (n < 40, n = 40–70, n > 70). No statistically significant difference in total conversion percentages was seen between groups of different sample sizes for ERα, PR, and HER2 (data not shown).
| Conversion per IHC receptor studies . | All . | 1% . | 10% . | IHC . | FISH . | IHC + FISH . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | H . | % . | H . | % . | H . | % . | H . | % . | H . | % . | H . | |
| ERα | ||||||||||||
| total | 19.3 | 72.8 | 17.7 | 74.4 | 19.4 | 61.2 | — | — | — | — | — | — |
| +/− | 22.5 | 78.3 | 16.9 | 77.2 | 23.9 | 67.9 | — | — | — | — | — | — |
| −/+ | 21.5 | 0.0 | 22.6 | 0.0 | 17.3 | 34.3 | — | — | — | — | — | — |
| PR | ||||||||||||
| total | 30.9 | 69.8 | 33.2 | 75.3 | 31.0 | 41.1 | — | — | — | — | — | — |
| +/− | 49.4 | 77.0 | 47.9 | 88.2 | 47.0 | 62.8 | — | — | — | — | — | — |
| −/+ | 15.9 | 61.3 | 20.6 | 71.7 | 16.6 | 24.5 | — | — | — | — | — | — |
| HER2 | ||||||||||||
| total | 10.3 | 80.4 | — | — | — | — | 12.7 | 74.4 | 11.5 | 69.3 | 9.8 | 82.4 |
| +/− | 21.3 | 74.4 | — | — | — | — | 20.8 | 30.8 | 16.3 | 49.2 | 22.0 | 77.9 |
| −/+ | 9.5 | 42.1 | — | — | — | — | 12.1 | 28.2 | 12.5 | 52.2 | 8.9 | 48.2 |
| Conversion per IHC receptor studies . | All . | 1% . | 10% . | IHC . | FISH . | IHC + FISH . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | H . | % . | H . | % . | H . | % . | H . | % . | H . | % . | H . | |
| ERα | ||||||||||||
| total | 19.3 | 72.8 | 17.7 | 74.4 | 19.4 | 61.2 | — | — | — | — | — | — |
| +/− | 22.5 | 78.3 | 16.9 | 77.2 | 23.9 | 67.9 | — | — | — | — | — | — |
| −/+ | 21.5 | 0.0 | 22.6 | 0.0 | 17.3 | 34.3 | — | — | — | — | — | — |
| PR | ||||||||||||
| total | 30.9 | 69.8 | 33.2 | 75.3 | 31.0 | 41.1 | — | — | — | — | — | — |
| +/− | 49.4 | 77.0 | 47.9 | 88.2 | 47.0 | 62.8 | — | — | — | — | — | — |
| −/+ | 15.9 | 61.3 | 20.6 | 71.7 | 16.6 | 24.5 | — | — | — | — | — | — |
| HER2 | ||||||||||||
| total | 10.3 | 80.4 | — | — | — | — | 12.7 | 74.4 | 11.5 | 69.3 | 9.8 | 82.4 |
| +/− | 21.3 | 74.4 | — | — | — | — | 20.8 | 30.8 | 16.3 | 49.2 | 22.0 | 77.9 |
| −/+ | 9.5 | 42.1 | — | — | — | — | 12.1 | 28.2 | 12.5 | 52.2 | 8.9 | 48.2 |
% = percentage of conversion; FISH = fluorescence in situ hybridization; H = heterogeneity according to the I2%; IHC = immunohistochemistry.
| Conversion per IHC receptor studies . | All . | 1% . | 10% . | IHC . | FISH . | IHC + FISH . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | H . | % . | H . | % . | H . | % . | H . | % . | H . | % . | H . | |
| ERα | ||||||||||||
| total | 19.3 | 72.8 | 17.7 | 74.4 | 19.4 | 61.2 | — | — | — | — | — | — |
| +/− | 22.5 | 78.3 | 16.9 | 77.2 | 23.9 | 67.9 | — | — | — | — | — | — |
| −/+ | 21.5 | 0.0 | 22.6 | 0.0 | 17.3 | 34.3 | — | — | — | — | — | — |
| PR | ||||||||||||
| total | 30.9 | 69.8 | 33.2 | 75.3 | 31.0 | 41.1 | — | — | — | — | — | — |
| +/− | 49.4 | 77.0 | 47.9 | 88.2 | 47.0 | 62.8 | — | — | — | — | — | — |
| −/+ | 15.9 | 61.3 | 20.6 | 71.7 | 16.6 | 24.5 | — | — | — | — | — | — |
| HER2 | ||||||||||||
| total | 10.3 | 80.4 | — | — | — | — | 12.7 | 74.4 | 11.5 | 69.3 | 9.8 | 82.4 |
| +/− | 21.3 | 74.4 | — | — | — | — | 20.8 | 30.8 | 16.3 | 49.2 | 22.0 | 77.9 |
| −/+ | 9.5 | 42.1 | — | — | — | — | 12.1 | 28.2 | 12.5 | 52.2 | 8.9 | 48.2 |
| Conversion per IHC receptor studies . | All . | 1% . | 10% . | IHC . | FISH . | IHC + FISH . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | H . | % . | H . | % . | H . | % . | H . | % . | H . | % . | H . | |
| ERα | ||||||||||||
| total | 19.3 | 72.8 | 17.7 | 74.4 | 19.4 | 61.2 | — | — | — | — | — | — |
| +/− | 22.5 | 78.3 | 16.9 | 77.2 | 23.9 | 67.9 | — | — | — | — | — | — |
| −/+ | 21.5 | 0.0 | 22.6 | 0.0 | 17.3 | 34.3 | — | — | — | — | — | — |
| PR | ||||||||||||
| total | 30.9 | 69.8 | 33.2 | 75.3 | 31.0 | 41.1 | — | — | — | — | — | — |
| +/− | 49.4 | 77.0 | 47.9 | 88.2 | 47.0 | 62.8 | — | — | — | — | — | — |
| −/+ | 15.9 | 61.3 | 20.6 | 71.7 | 16.6 | 24.5 | — | — | — | — | — | — |
| HER2 | ||||||||||||
| total | 10.3 | 80.4 | — | — | — | — | 12.7 | 74.4 | 11.5 | 69.3 | 9.8 | 82.4 |
| +/− | 21.3 | 74.4 | — | — | — | — | 20.8 | 30.8 | 16.3 | 49.2 | 22.0 | 77.9 |
| −/+ | 9.5 | 42.1 | — | — | — | — | 12.1 | 28.2 | 12.5 | 52.2 | 8.9 | 48.2 |
% = percentage of conversion; FISH = fluorescence in situ hybridization; H = heterogeneity according to the I2%; IHC = immunohistochemistry.
Pooled Percentage of ERα Discordance Between Primary Breast Tumor and Distant Metastasis
The total discordance percentage for ERα varied between studies from 7.3% to 51.2%, with a pooled random effects percentage of 19.3% (95% CI = 15.8% to 23.4%) (Figure 2A). The percentage of conversion from positive to negative was 22.5% (95% CI = 16.4% to 30.0%), and from negative to positive it was 21.5% (95% CI = 18.1% to 25.5%) (Figure 2, B and C). We divided studies into two groups using the 1% or 10% thresholds for positivity, showing total pooled ERα conversion percentages of 17.7% (95% CI = 13.5% to 22.7%) and 19.4% (95% CI = 14.6% to 25.2%), respectively (Supplementary Figure 1, available online). No statistically significant difference between both cutoffs was perceived for total discordance percentages (P = .82). The frequency of conversion from positive to negative was 16.9% (95% CI = 11.5% to 24.2%) for the 1% threshold and 23.9% (95% CI = 15.7% to 34.7%) for the 10% threshold. Conversion from negative to positive occurred in 22.6% and 17.3% of tumors, respectively (95% CI = 17.9% to 28.0% for the 1% threshold, 95% CI = 11.7% to 24.8% for the 10% threshold).
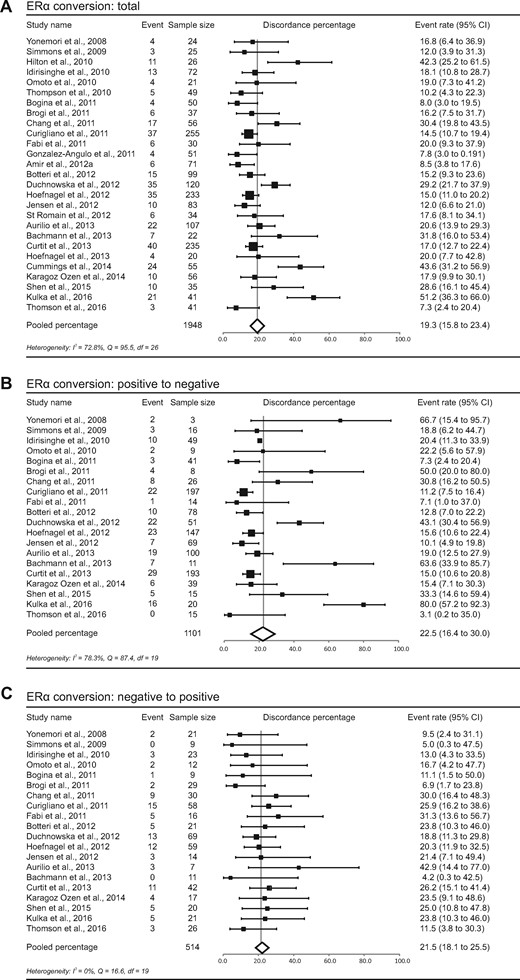
Study-specific and pooled estimate for estrogen receptor alpha (ERα) discordance percentages for studies reporting ERα immunohistochemistry in primary breast tumors and paired distant metastases. Study-specific data are ordered by date of publication. Discordance percentages are shown for total conversion (A), conversion from positive to negative (B), and conversion from negative to positive (C). Error bars indicate confidence intervals. Heterogeneity was assessed using I2 and Cochran’s Q. CI = confidence interval; df = degrees of freedom.
Pooled Percentage of PR Discordance Between Primary Breast Tumor and Distant Metastasis
The meta-analytic pooled percentage for PR was 30.9% (95% CI = 26.6% to 35.6%). The probability for positive receptors to change to negative was 49.4% (95% CI = 40.5% to 58.2%), and the probability for negative receptors to change to positive was 15.9% (95% CI = 11.3% to 22.0%) (Figure 3). Conversion from positive to negative occurred statistically significantly more often than from negative to positive (P < .001, P < .001, and P < .001 for the total group, 1% threshold, and 10% threshold for positivity, respectively). For the 1% threshold for positivity, the total percentage of discordance was 33.2% (95% CI = 27.2% to 39.7%), the conversion from positive to negative was 47.9% (95% CI = 34.4% to 61.7%), and the conversion from negative to positive was 20.6% (95% CI = 12.7% to 31.7%). For the 10% cutoff, these values were 31.0% (95% CI = 26.2% to 36.2%), 47.0% (95% CI = 36.5% to 57.7%), and 16.6% (95% CI = 11.8% to 22.7%), respectively (Supplementary Figure 2, available online).
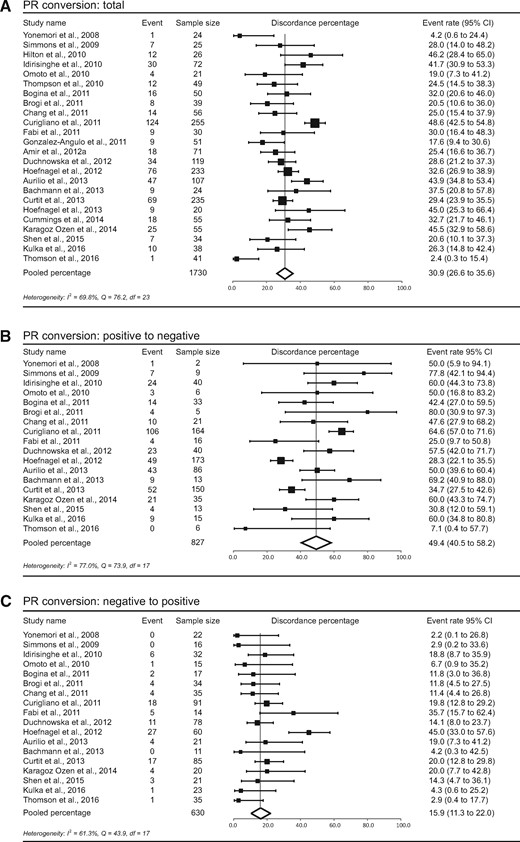
Study-specific and pooled estimate for progesterone receptor (PR) discordance percentages for studies reporting PR immunohistochemistry in primary breast tumors and paired distant metastases. Study-specific data are ordered by date of publication. Discordance percentages are shown for total conversion (A), conversion from positive to negative (B), and conversion from negative to positive (C). Error bars indicate confidence intervals. Heterogeneity was assessed using I2 and Cochran’s Q. CI = confidence interval; df = degrees of freedom.
Pooled Percentage of HER2 Discordance Between Primary Breast Tumor and Distant Metastasis
The pooled percentage of HER2 conversion was 10.3% (95% CI = 7.8% to 13.6%). Positive to negative conversion occurred in 21.3% (95% CI = 14.3% to 30.5%) and negative to positive in 9.5% (95% CI = 7.4% to 12.1%) in this meta-analysis (Figure 4). We subdivided studies into three groups—studies using FISH only, studies using IHC only, and studies using a combination of IHC and FISH (in case of 2+/equivocal IHC)—to assess receptor status. The total discordance percentages for FISH, IHC, and FISH+IHC were 11.5% (95% CI = 6.0% to 20.9%), 12.7% (95% CI = 7.1% to 21.6%), and 9.8% (95% CI = 6.9% to 13.6%), respectively. Conversion rates from positive to negative were 16.3% (95% CI = 5.2% to 40.7%), 20.8% (95% CI = 9.3% to 40.1%), and 22.0% (95% CI = 14.1% to 32.8%), and from negative to positive they were 12.5% (95% CI = 6.3% to 23.4%), 12.1% (95% CI = 6.7% to 20.9%), and 8.9% (95% CI = 6.6% to 11.9%) for the three groups (Supplementary Figure 3, available online). No statistically significant difference was seen between total discordance percentages of these groups (P = .25).
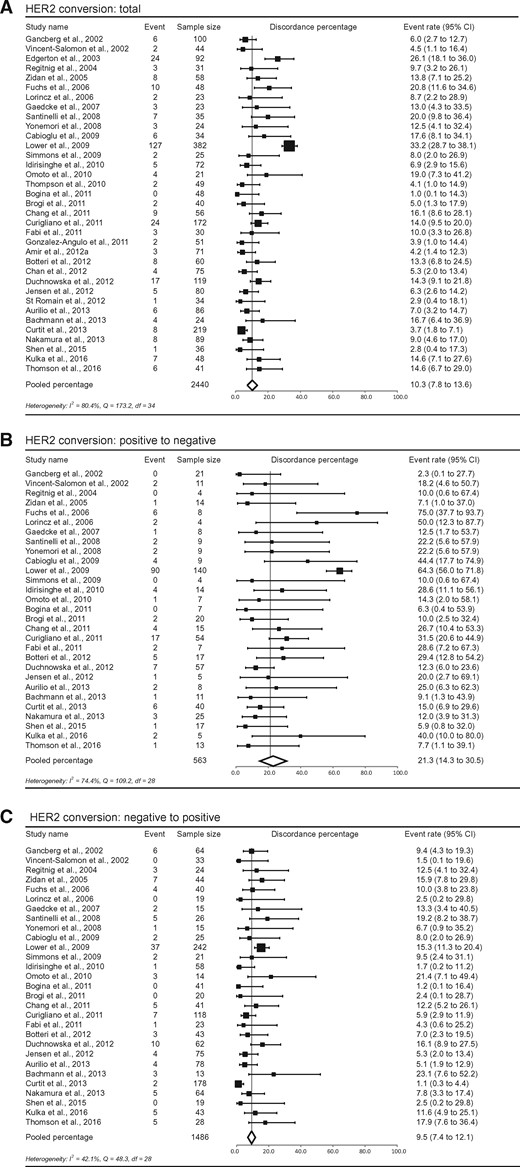
Study-specific and pooled estimate for human epidermal growth factor receptor 2 (HER2) discordance percentages for studies reporting HER2 immunohistochemistry in primary breast tumors and paired distant metastases. Study-specific data are ordered by date of publication. Discordance percentages are shown for total conversion (A), conversion from positive to negative (B), and conversion from negative to positive (C). Error bars indicate confidence intervals. Heterogeneity was assessed using I2 and Cochran’s Q. CI = confidence interval; df = degrees of freedom.
Pooled Percentage of Location-Specific Discordance Between Primary Breast Tumor and Distant Metastasis
Additionally, discordance analyses were performed within subgroups representing the most frequent distant metastatic sites. Central nervous system (CNS), bone, liver, skin, and lung metastases were described in 10, four, six, two, and two studies, respectively. Total discordance percentages for ERα, PR, and HER2 at each metastatic site are shown in Figure 5. A statistically significant difference in ERα discordance was seen between metastatic subsites (P < .001), with CNS (20.8%, 95% CI = 15.0% to 28.0%, P = .008) and bone metastases (29.3%, 95% CI = 13.0% to 53.5%, P < .001) scoring higher discordance values than liver metastases (14.3%, 95% CI = 11.3% to 18.1%). Also for PR, a statistically significant difference was observed between subsites (P < .001). PR discordance was statistically significantly higher in bone (42.7%, 95% CI = 35.1% to 50.6%, P < .001) and liver metastases (47.0%, 95% CI = 41.0% to 53.0%, P < .001), compared with CNS metastases (23.3%, 95% CI = 16.0% to 32.6%) (Figure 5B). For HER2, no statistically significant differences were observed for pooled discordance percentages between metastatic sites (P = .33). Because of the small numbers and the short description of groups in some articles, no subdivision for positive to negative and negative to positive conversion could be made.
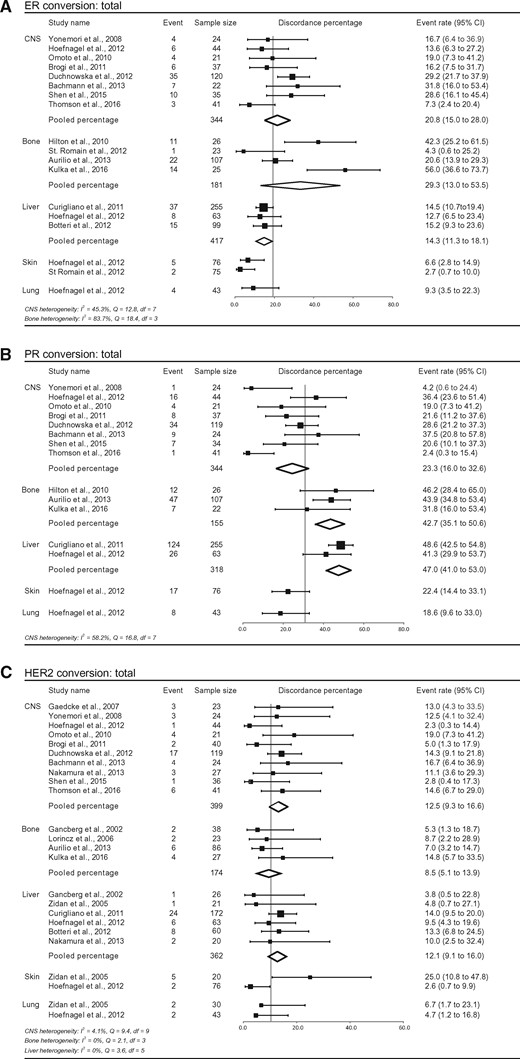
Study-specific and pooled estimates for metastasis location–specific discordance percentages for studies reporting estrogen receptor alpha (ERα), progesterone receptor (PR), and/or human epidermal growth factor receptor 2 (HER2) immunohistochemistry in primary breast tumors and paired distant metastases. Study-specific data are ordered by date of publication. Locations of metastases are grouped as follows: central nervous system, bone, liver, lung, and skin. The y-axis shows the total pooled discordance percentage of all locations. Discordance percentages are shown for total conversion of ERα (A), total conversion of PR (B), and total conversion of HER2 (C). Error bars indicate confidence intervals. Heterogeneity was assessed using I2 and Cochran’s Q. CI = confidence interval; CNS = central nervous system; df = degrees of freedom.
Discussion
At present, discordance of ERα, PR, and HER2 status between primary breast tumors and paired metastases is well recognized, with a majority of change from a positive to a negative receptor status (1). Clinically, both conversions are relevant. In case the receptor status is lost, chances are the patient will receive ineffective treatment at the cost of related toxicity. At the same time, lack of knowledge about metastases having gained a positive receptor status could potentially lead to wrongfully withholding effective treatments. Both could have their effects on outcome.
However, some notable questions remain unanswered: how frequent is this phenomenon, which factors influence its occurrence, and does a treatment switch based on the characteristics of the metastasis improve survival? With this meta-analysis, we aimed to specifically answer the first question and review literature on the last two. Moreover, little is known about the dynamics of receptor conversion in breast cancer: is conversion reversible, and to what extent does it differ between metastatic sites?
For ERα, PR, and HER2, we found random effects pooled discordance percentages of 19.3%, 30.9%, and 10.3%, respectively. Especially for PR, a switch from positive to negative receptor status occurred statistically significantly more often than from negative to positive. Similarly, HER2 changed twice as often from positive to negative than vice versa. Furthermore, metastasis location–specific differences were found, with more ERα discordance in CNS and bone metastases and higher diversity in bone or liver metastases. Together, these findings confirm the idea that breast cancer is a very heterogeneous disease, and they could stress the importance of assessing receptor status of metastases.
Next to heterogeneity, we intentionally did not mention publication bias. We are aware that publication bias can have a large effect on the validity of a meta-analysis, but a correct method to assess the amount of bias in diagnostic test accuracy analyses is lacking. Most procedures to investigate publication bias (especially focusing on small study effects) are built for intervention studies, and in other types of meta-analyses, different publication bias tests can show diverging results (22).
In this meta-analysis, we focused on immunohistochemically assessed receptor status of ERα, PR, and HER2 with a predefined threshold for positivity, as recommended by clinical practice guidelines to enable reliable treatment decision-making (16,17). Risk of relapse, prognosis, and response to treatment are attributed to the type of breast cancer determined by these markers (23–25). Especially ERα has been considered an important positive prognostic marker as well as a predictive marker of response to endocrine therapies (26). Although approximately 75% of breast cancers show ERα positivity, their outcome and response to therapy vary extremely (27).
Receptor conversion is thought to be the result of clonal selection and/or selective pressure of therapy (28–30). Some studies in this meta-analysis indeed reported an effect of chemotherapy exposure on ERα or PR receptor conversion and of previous trastuzumab therapy on HER2 conversion (31–37). Other articles, however, could not demonstrate such a correlation (38,39). In primary breast cancer, sequential biopsies have shown that ERα levels are reduced slightly with intervening endocrine therapy, while PR levels decrease more dramatically, with up to half of tumors completely losing PR expression when resistance develops (40). Therefore, PR loss in the metastasis may be an important additional hallmark of endocrine therapy response failure (41–43).
In recent years, clinical guidelines have increasingly started advising to re-assess metastatic tissue characteristics whenever possible (10,44,45). However, solid clinical evidence supporting these guidelines is currently lacking. One study reported responses to trastuzumab in two out of five patients with positive HER2 status after conversion (46). Other articles do show survival differences between concordant and discordant tumors, but the relation between therapy administration and discordance is poorly reported. For example, Chang et al. and Edgerton et al. showed a statistically significantly better overall survival in patients without HER2 conversion compared with patients with conversion (46,47), and Lower et al. reported that patients with negative to positive conversion performed better compared with conversion from positive to negative (48). Regarding ERα and/or PR, conversion from a positive primary tumor to a negative metastasis was associated with statistically significantly worse survival compared with patients remaining receptor positive. In contrast, no statistically significant survival difference was seen between patients showing conversion from negative to positive compared with patients remaining negative (4). Consensus about the influence of receptor conversion on survival is, however, not yet reached (32,49,50).
Modification of therapeutic plan based on biopsy of the metastasis has been reported in 14% to 62% of converted patients for ERα and PR and in 67% for HER2 (9,38,51–53), but the long-term effect of this therapy switch has not been reported. Change in therapy was more often seen when there was apparent gain of receptor status (54). Regarding the data available, randomized controlled trials in this setting no longer seem to be ethical. Moreover, large prospective studies with sufficient follow-up on survival and therapy response are very much needed to gain more insight into the real clinical significance for breast cancer patients.
A major limitation of describing immunohistochemical receptor conversion is the heterogeneity of the studies included. This could be attributed to the fact that many studies used retrospectively assessed data, potentially leading to differences in staining protocols, interobserver bias, and analytical errors (7). For example, analysis of HER2 IHC and FISH on the same primary breast tumors in different labs already showed discordance percentages of 18% and 12%, respectively (30). Other factors that could cause heterogeneous findings are differences in primary tumor characteristics (eg, tumor type, grading, and nodal status) (Supplementary Tables 1–4, available online) and time interval between primary tumor and metastasis. We did extract these variables from the included studies whenever possible, but they were often reported independently from the conversion statistics. Subanalyses based on these data were therefore impossible. Regarding the influence of the time interval between primary and metastasis, Aurilio et al. reported that the time interval did not statistically significantly affect the discordance rate for ERα, PR, or HER2. The median time interval in their cohort was 4.2 years (range = 0–18.9 years) (9). Furthermore, Bachmann et al. found that PR and HER2 discordance correlated to shorter interval to metastasis (median = 3 years, range = 0–6 years). A longer time interval could impose larger environmental and therapeutic influences, directing tumor cells toward conversion. Larger prospective studies should be performed to assess the influence of time interval on receptor conversion.
Several other limitations are heterogeneous study cohorts (age and race), small sample size, use of different techniques interchangeably (ligand-binding assay vs immunohistochemistry), influence of various systemic therapies, and use of different cutoffs for positivity (1% vs 10% for ERα and PR) (3). In order to minimize bias, we tried to select a group of studies that is as homogenous as possible. First, we excluded material obtained by fine needle aspiration, as insufficient sampling may potentially cause false-negative results (55–57). Furthermore, we chose to focus on solid metastases only, given that a clinically significant difference has been perceived between axillary lymph node metastases and distant metastases (8,13). We tried to primarily include studies that reassessed receptor status of the primary tumor together with the metastasis to exclude potential technical differences, but this was rarely reported.
Still, variation between studies remained high, which decreased by focusing on specific subgroups with matching thresholds for positivity (Table 2) or location of metastasis (Figure 5). Because discordance percentages did not statistically significantly differ between techniques, receptor conversion can be seen as a true biological phenomenon and is not solely the result of limited accuracy of receptor assays, as sometimes thought (45,58). Furthermore, the finding of metastatic subsite–specific differences in discordance frequency underlines the true biology. We demonstrated that ERα discordance was statistically significantly higher in CNS and bone compared with liver metastases and PR discordance was higher in bone and liver metastases compared with CNS metastases. Careful interpretation is advised, however, as these conclusions are based on studies with small samples sizes and potential selection bias (metastases are often only operated on when surgically well approachable and limited cancer burden is present).
Some studies claim that bone receptor status cannot be reliably assessed as antigenicity may be altered by decalcifying agents that enable sectioning of bone (59,60). However, bone was not the only site with high discordance rates. Moreover, we only included studies on bone metastases that were not decalcified (53,61) or decalcified with EDTA (9,62,63), as EDTA was shown not to alter ERα, PR, and HER2 immunohistochemistry (64). Discordance percentages per metastatic subsite were assessed before by Yeung et al. (65). For CNS/brain, bone, liver, and lung metastases, they found total discordance percentages for ERα of 17%, 47.5%, 15%, and 28%, and for PR of 22%, 36%, 45%, and 30.5%, respectively. For the same locations, we report discordance percentages of 21.8%, 29.3%, 14.3%, and 9.3% for ERα and 23.3%, 42.7%, 47.0%, and 18.6% for PR. Except for lung metastases, the pattern of discordance is roughly similar, with few overlapping studies between both analyses. These similarities suggest that discordance in distant metastases shows a location-specific pattern, potentially adjusting to micro-environmental needs of the target organ.
This systematic review confirms the frequent occurrence of ERα, PR, and HER2 receptor conversion. High heterogeneity was, however, seen between patients, receptors, techniques used, and subsites of metastasis, leading to dispersed discordance percentages. Although not yet prospectively examined, multiple studies report survival differences between patients with concordant and discordant receptor status, where the effect of treatment may be a confounder. Based on the present meta-analysis, we advise biopsy and re-assessment of receptor status in distant metastases whenever possible at each progression or change in therapy, in order to get more insight into the patterns and dynamics of hormone receptor conversion. Prospective studies with sufficient (post-treatment) follow-up are needed to assess the clinical implications of receptor conversion for breast cancer treatment. One such trial is the SAFIR01, in which genomic alterations were identified on metastatic biopsies (66). Furthermore, less invasive techniques as HER2 imaging and liquid biopsies can yield priceless information about the amount and mechanism of conversion with the potential to follow disease course during treatment (67,68).
Funding
This work was supported by Dutch Cancer Society grant UU 2011-5195; Philips Consumer Lifestyle.
Notes
The study sponsor had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.


