-
PDF
- Split View
-
Views
-
Cite
Cite
Alexios Matikas, Theodoros Foukakis, Jonas Bergh, RE: Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis, JNCI: Journal of the National Cancer Institute, Volume 110, Issue 11, November 2018, Pages 1280–1281, https://doi.org/10.1093/jnci/djy046
Close - Share Icon Share
We read with interest the systematic review and meta-analysis by Schrijver et al. on receptor conversion during breast cancer progression to metastatic disease, when compared with the primary tumor (1). While we agree with the conclusions of the study, we would like to express our concerns regarding the chosen methodology, namely the exclusion of studies that relied on cytology for the assessment of receptor changes.
While one might claim that issues regarding the availability of tumor material and reproducibility may arise due to the use of cytology specimens, we submit that this is not the case. The authors provide three references that support their reasoning behind the by-default exclusion of all cytology-based trials (references 55, 56, and 57 in the article). However, two of the studies that are cited (Rao et al., Ganott et al.) did not show any statistically significant difference in terms of sensitivity between core and fine needle aspiration biopsies (FNABs). Importantly, the cited publications concern locoregional breast cancer and not metastatic disease. Published data on the advanced disease setting do not support these assumptions. In a population-based study on the biology of breast cancer progression, our group demonstrated that there is high concordance between the detected-via-core biopsy and FNAB changes in the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki67 in the relapse site compared with the primary tumor (Figure 1) (2).
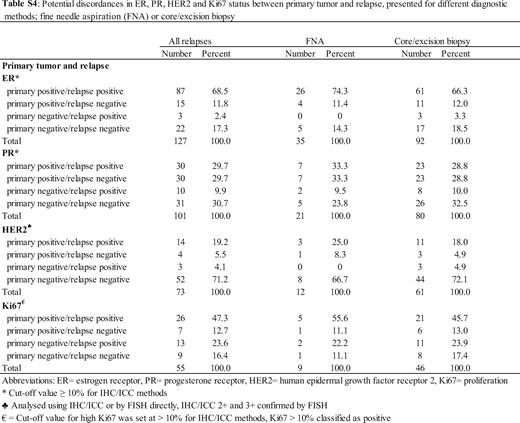
Discordance in receptor expression between primary tumor and relapse, as assessed by fine needle aspiration or core/excision biopsy. This was originally published as a supplementary table (2). Reprinted with permission from Elsevier. ER = estrogen receptor; FISH = fluorescence in situ hybridization; FNA = fine needle aspiration; HER2 = human epidermal growth factor receptor 2; ICC = immunocytochemistry; IHC = immunohistochemistry; Ki67 = proliferation; PR = progesterone receptor.
On the other hand, the blanket exclusion of all studies based on the cytological assessment of the receptors and not depending on an individual assessment of the study quality led to uncertainty regarding the heterogeneity of the results obtained by various methods of measurement of ER/PR expression. This issue has been previously examined by our group in one of the largest published studies on receptor changes in breast cancer relapse (3). Specifically, there were no systematic differences when estrogen receptor expression was assessed by immunohistochemistry/immunocytochemistry compared with biochemical assessment.
Furthermore, the authors correctly recognize the decalcification of bone biopsies as a source of bias and excluded it from their analysis such studies. However, this may not accurately represent routine clinical practice. In contrast, bone FNAB is a widely used alternative to bone core biopsies, without the disadvantage of decalcification; thus it can properly capture the evolution of disease biology during progression (4).
Finally, the authors state that there is a need for prospective trials to assess the clinical implications of receptor conversion. Two such studies, which are cited by the authors, have already been published (references 38 and 51 in the article). Taking into account the available evidence, further prospective studies with random assignment to no biopsy as a control arm could be considered unethical (5,6).
In conclusion, while we agree with the results and conclusions of this meta-analysis, we remain concerned regarding the study inclusion criteria and the message conveyed that FNAB of metastatic sites is not an appropriate diagnostic modality and that further investigation is warranted, because it is clear that routine metastatic biopsies should be integrated into clinical practice, as suggested by contemporary guidelines (7).
Note
Affiliation of authors: Department of Oncology-Pathology, Karolinska Institutet, Breast Cancer Group and Radiumhemmet, Cancer Theme, Karolinska University Hospital, Stockholm, Sweden.


