-
PDF
- Split View
-
Views
-
Cite
Cite
Lindsey R Faw, Kasie Raymann, Nayma Romo Bechara, Gideon Wasserberg, Larval Conditioning and Aging of Sand Fly Rearing Medium Affect Oviposition Site Selection in Phlebotomus papatasi (Diptera: Psychodidae) Sand Flies, Journal of Medical Entomology, Volume 58, Issue 4, July 2021, Pages 1931–1935, https://doi.org/10.1093/jme/tjab063
Close - Share Icon Share
Abstract
Sand fly larvae develop in sheltered humid habitats containing decaying organic matter on which they feed. Previously, we showed that gravid females of Phlebotomus papatasi Scopoli (Diptera: Psychodidae) are attracted to and stimulated to lay eggs on larval rearing medium containing larvae. That study, however, did not control for the possible effect of medium aging. Our goal in this study was to evaluate the effect of larval substrate conditioning on attraction and oviposition responses of Ph. papatasi sand flies while controlling for the effect of substrate aging. Initially, we confirmed that the pretreatment fresh larval food sources (to be used as larval conditioned and unconditioned media) did not differ with respect to their effect on attraction and oviposition responses. The larval conditioned medium was produced by rearing larvae to the second/third-instar stage over 3 wk using the same larval food source. To produce larval unconditioned medium, the same amount of fresh larval food was added to a control rearing cup that did not contain larvae but was aged under identical time and conditions. Two-choice bioassays were conducted to evaluate gravid female’s attraction and oviposition response to larval conditioned and unconditioned media. We found that gravid females were significantly attracted (P < 0.05) to larval conditioned medium when compared with unconditioned medium under the same amount of time and conditions. However, no such difference was found with respect to oviposition response. Both attraction and oviposition responses were significantly increased for larval conditioned and unconditioned media in comparison to the initial fresh larval food source.
Phlebotomine sand flies (Diptera: Psychodidae) transmit protozoan parasites (Leishmania spp.), as well as bacterial (Bartonella bacilliformis) and viral pathogens (Killick-Kendrick 1999, Ready 2013). Sand fly larvae develop in sheltered humid habitats such as burrows, tree holes, and caves containing decaying organic matter on which they feed (Feliciangeli 2004). Therefore, it is often hypothesized that gravid females would be attracted to and stimulated to lay eggs based on stimuli emanating from different sources of organic matter. This hypothesis was supported by many observations with both New-World and Old-World sand flies (Schlein et al. 1989, Elnaiem and Ward 1992, Dougherty et al. 1995, Radjame et al. 1997, Wasserberg and Rowton 2011, Marayati et al. 2015).
In a recent study with Phlebotomus papatasi Scopoli, a vector of Leishmania major, we evaluated the attraction and oviposition responses of gravid females to larval rearing substrate of various types and stages including rabbit feces, fresh larval food (made of mixture of fermented rabbit feces and rabbit chow), and larval food used for rearing larvae up to different instar stages (Marayati et al. 2015). The key finding was that gravid, Ph. papatasi sand flies, were attracted to and stimulated to lay a maximum number of eggs when presented with rearing medium containing second/third-instar larvae (Marayati et al. 2015). We also recently showed that several bacterial isolates cultured from this medium were highly attractive to gravid sand flies, suggesting that this attraction is mediated by bacterially produced kairomones (Kakumanu et al. 2020).
Attraction and oviposition stimulation by larval conditioned rearing medium suggests that the presence of actively foraging larvae in the medium improves the attractiveness of this medium to gravid females. However, it is also possible that it is not the presence of larvae in the medium that enhances oviposition response but rather it could be driven by the aging and decomposition of the substrate itself. This putative ‘substrate aging hypothesis’ was not evaluated in Marayati’s et al. (2015) study. The goal in this study was to evaluate the effect of larval medium conditioning while controlling for the effect of substrate aging. This was accomplished by evaluating the attraction and oviposition responses of gravid Ph. papatasi to two sets of larval food sources: one used for rearing larvae over 3 wk to the second/third instar and one containing larval food, but with no larvae, aged for the same amount of time under the same conditions.
Materials and Methods
Insects and Colony Maintenance
Phlebotomus papatasi sand flies originating from Abkuk, Turkey, were maintained following conventional rearing methods described elsewhere (Lawyer et al. 2017, Shymanovich et al. 2019, Kowacich et al. 2020). The flies were blood-fed at SoBran Biosciences in Greensboro, NC on live anesthetized ICR mice (SoBran Inc. IACUC protocol number: UNC-002-2016). Sand flies were maintained in insect rearing chambers (Caron, Marietta, OH) at 26°C, 85% RH under a 14:10 (L:D) h reverse photoperiod, with 1 h of crepuscular light.
Experimental Medium Preparation
Consistent with estimates of larval densities used in Marayati et al. (2015), approximately 9000 Ph. papatasi eggs were introduced into larval rearing containers made of 500-ml Nalgene jars with a plaster of Paris bottom (Marayati et al. 2015). Once hatched, the larvae were fed three times a week with fresh larval food for 3 wk until reaching second- to third-instar larval stage (Fig. 1A). This rearing medium is what we refer to as ‘larval conditioned medium’ (LCM). The unconditioned medium (UCM), which served as a control medium, was prepared by adding larval food at the same amount and the same frequency as that used for the LCM into the larval rearing jars that did not contain larvae (Fig. 1A). LCM and UCM jars were maintained under the same conditions as colony flies.
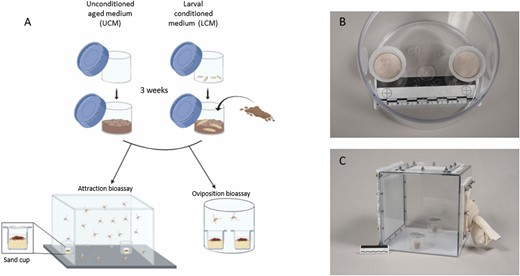
(A) Experimental design consisted of fresh larval rearing food that was used to rear sand fly larvae over 3 wk or aged in a similar manner but in the absence of larvae to form larval conditioned medium (LCM) and unconditioned aged medium (UCM), respectively. Gravid female sand flies were then presented with these two media types (placed in separate sand cups) in an oviposition arena (B) or in a free flight cage (C) to measure their oviposition or attraction responses, respectively.
Oviposition Bioassay
The basic bioassay design consists of a pair of sand cups, which were 10-ml disposable beakers (Thermo Fisher Scientific, Waltham, MA; Model: 08-732-121) filled with 8-ml autoclaved sand and 2-ml deionized water to keep the sand moist (Fig. 1B). We then added 0.01 g of our test materials on top of the sand surface, placed a moist filter paper on top, and inserted these sand cups into opposite sides of 500-ml Nalgene jars, which served as oviposition bioassay arenas (Fig. 1B). Jars were covered by a fine mesh fabric and secured with a pair of rubber bands. Using a mouth aspirator, 20 gravid sand flies (7 d postbloodmeal) were inserted into the arena and left to lay eggs over 3 d after which the flies were removed, and number of eggs laid on filter papers of each sand cup was counted (Fig. 1A). The experimental procedure comprised three stages: pretreatment oviposition testing, medium conditioning/aging, and experimental oviposition testing. For the pretreatment oviposition testing stage, samples from the rearing media designated to be LCM or UCM were inserted into oviposition bioassay arenas to confirm there was no initial difference in media condition. The LCM and UCM media were then formulated and reared/aged over 3 wk as described above. For the experimental oviposition testing stage, samples from both LCM and UCM were placed onto the sand cups in the experimental arenas and covered by a moist filter paper, flies were introduced, and the experiment ran over 3 d as described above. Two experimental sessions were conducted: one in November 2019 and another in February 2020. For the November 2019 session, three LCM and three UCM medium jar sources were made. These were used to prepare 10 experimental arena replicates for the baseline oviposition test and 20 arena replicates for the experimental oviposition test. For the February 2020 session, four LCM and four UCM medium jar sources were made and used to prepare 10 experimental arena replicates for the baseline oviposition test and 12 replicates for the experimental oviposition test.
Attraction Bioassays
The design of this experiment was similar to that used for the oviposition bioassay, except that the experimental arena was a transparent polycarbonate free flight cage (30 × 30 × 30 cm; Precision Plastics, Beltsville, Maryland; Fig. 1C). When testing for attraction, we placed LCM and UCM in separate sand cups inside separate 125-ml round, transparent jars (Nalgene, Rochester, New York) and placed a sticky aluminum screen disk (6 cm diameter), made of an aluminum window-screen sprayed with adhesive (Tanglefoot, Model 91992-MI-001, Marysville, OH), on top of the jar (hereafter, sticky traps; Kowacich et al. 2020; Fig. 1C). Free flight cages contained 20 gravid flies (6 d postbloodmeal) that were left to acclimate to cage conditions overnight. Then LCM and UCM containing sticky traps were introduced and placed in opposite sides of the cage and flies were given six hours to choose between the two media types. The number of flies attached to each adhesive metal screen was counted. The experiment was conducted in a dark walk-in environmental chamber, illuminated using reverse photoperiod (14:10 L:D) at 26°C and 65% RH. Timing and procedures of these bioassays were similar to those described above for the oviposition bioassay. For the November 2019 session, the three pairs of LCM and UCM medium jar sources were used to prepare 10 experimental free flight arenas for the pretreatment attraction test and 12 for the experimental attraction test. For the February 2020 session, the four LCM and four UCM medium jar sources were used to prepare 10 experimental free flight cage arenas for the pretreatment attraction test and 20 for the experimental attraction test.
Data Analysis
Given that our response variable in both bioassays is count data (number of eggs laid per cup or number of females trapped per sticky trap), we analyzed these data using negative binomial regression models (McCulagh and Nelder 1989). Specifically, we used two-way regression models to test for the effects of ‘medium aging’ (pretreatment vs experimental effect), ‘larval medium conditioning’ (LCM vs UCM), and their statistical interaction. The mean number of eggs laid, or number of flies trapped between pairs of sand cups or sticky traps within their respective arenas, were compared by paired t-tests.
Results
Oviposition Bioassay
At the pretreatment test stage, no difference was found in the number of eggs laid in the to-be LCM and UCM media (paired t = −1.24, P = 0.23; Fig. 2A). The effect of ‘medium conditioning’ on the mean number of eggs was not significant (Z = −1.246, P = 0.212), with a slight nonsignificant trend (paired t = −0.54, P = 0.59) of higher numbers actually in the UCM (Fig. 2A). In contrast, the effect of ‘substrate aging’ was statistically significant (Z = 2.77, P = 0.0056) with overall mean number of eggs increasing between the pretreatment test stage (99.15 ± 13.21) and the experimental test stage (154.85 ± 14.25). This ‘substrate aging effect’ was consistent for both LCM and UCM but was only significant for the former (Fig. 2A). The statistical interaction between ‘substrate aging’ and ‘larval medium conditioning’ was not significant (Z = 0.676, P = 0.499).

The effect of larval medium conditioning and medium aging on Phlebotomus papatasi oviposition (A) and attraction (B) responses. Mean number of eggs laid (A) or sand flies trapped on a sticky mesh (B) were, initially, compared between the to-be larval conditioned medium and the to-be unconditioned medium in the pretreatment stage and following 3 wk (experimental test stage). In the experimental session, larval conditioned medium (LCM) contained mainly second- and third-instar larvae. Unconditioned medium (UCM) was aged to the same length of time and under same environmental conditions but did not contain larvae. Different uppercase letters indicate a significant ‘medium aging effect’ for UCM, and different lowercase letters indicate a significant ‘medium aging effect’ for LCM. Curly brackets pertain to significance values based on paired t-test comparisons between the two media types (NS = nonsignificant). Error bars = standard error.
Attraction Bioassay
At the pretreatment stage, no significant difference was found in the number of flies trapped in the to-be LCM and to-be UCM larval media (paired t = 1.30, df = 19, P = 0.21; Fig. 2B) and the overall percentage of flies trapped was very low (about 5%). The effect of ‘larval medium conditioning’ was statistically significant (Z = 2.254, P = 0.024), with higher number of flies trapped in the LCM cup compared with the UCM cup (paired t = 2.11, df = 31, P = 0.04; Fig. 2B). A highly significant effect of ‘substrate aging’ was also found (Z = 6.719, P < 0.0001), with overall mean number of flies trapped increasing from 0.75 ± 0.13 in the pretreatment test stage to 3.1 ± 0.27 in the experimental test stage. This effect was significant (P < 0.05) for both the LCM and UCM media (Fig. 2B). The statistical interaction between ‘time’ and ‘medium conditioning’ was not significant (Z = 0.137, P = 0.891).
Discussion
In a previous study, we showed that gravid, Ph. papatasi sand flies were attracted to and stimulated to lay a maximum number of eggs when presented with rearing medium containing second/third-instar larvae (Marayati et al. 2015). However, our reference medium in that study was fresh larval medium, and we did not control for the possible effect of medium aging and decomposition. Results of the current study are consistent with respect to the effect of larval medium conditioning on the attraction response, but not with respect to its effect on the oviposition response. Specifically, in the present study, we confirmed that medium conditioning by foraging and defecating larvae enhances attractiveness to gravid sand flies above and beyond the effect of the medium’s aging. However, our results contrast with Marayati et al. (2015) where the larval medium conditioning did not enhance sand fly’s oviposition response beyond that of the medium aging effect. Results indicate that larval food aging by itself strongly enhances both attraction and oviposition responses when compared with fresh, unused, larval food. The mechanism by which larval medium conditioning and medium aging and decomposition enhances the attraction and oviposition responses of sand flies is not yet known. It is also possible that these effects are not independent and that larval foraging might, actually, enhance medium decomposition rate. Based on a recent study, we hypothesize that these effects are microbially mediated (Kakumanu et al. 2020). We are currently conducting a longitudinal experiment in which we produce LCM and UCM series of jars and periodically sample from them for a metagenomic analysis of their microbiomes. This will shed light on the microbial succession of LCM and UCM from the time of their origin, through all immature and adult stages.
It is still unclear why sand flies are attracted to larval conditioned or aged rearing media and what is the adaptive advantage of being attracted to a certain subset of bacterial isolates. However, consistent with the ‘Preference—Performance Hypothesis’ (Jaenike 1978) and as shown by Peterkova-Koci et al. (2012), it is likely that some of these bacteria are essential for larval development (Peterkova-Koci et al. 2012). This type of a hypothesis has received support in the study of mosquitoes (Coon et al. 2016, 2017), but in sand flies, this issue is understudied and requires further investigation.
Acknowledgments
We acknowledge the late Matthew Miller for his preliminary metagenomic analysis. We also thank Loganathan Ponnusamy for his service on LRF graduate committee. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institute of Health (1R01AI123327). We also thank the University of North Carolina-Greensboro’s Department of Biology and Graduate School for their financial support to L.R.F. and N.R.B.



