-
PDF
- Split View
-
Views
-
Cite
Cite
Gideon Wasserberg, L. White, A. Bullard, J. King, R. Maxwell, Oviposition Site Selection in Aedes albopictus (Diptera: Culicidae): Are the Effects of Predation Risk and Food Level Independent?, Journal of Medical Entomology, Volume 50, Issue 5, 1 September 2013, Pages 1159–1164, https://doi.org/10.1603/ME12275
Close - Share Icon Share
Abstract
For organisms lacking parental care and where larval dispersal is limited, oviposition site selection decisions are critical fitness-enhancing choices. However, studies usually do not consider the interdependence of the two. In this study, we evaluated the effect of food level on the oviposition behavior of Aedes albopictus (Skuse) in the presence or the absence of a nonlethal predator (caged dragonfly nymph). We also attempted to quantify the perceived cost of predation to ovipositioning mosquitoes. Mosquitoes were presented with oviposition cups containing four levels of larval food (fermented leaf infusion) with or without a caged libellulid nymph. By titrating larval food, we estimated the amount of food needed to attract the female mosquito to oviposit in the riskier habitat. As expected, oviposition rate increased with food level and decreased in the presence of a predator. However, the effect of food level did not differ between predator treatments. By calculating the difference in the amount of food for points of equal oviposition rate in the predator-present and predator-absent regression lines, we estimated the cost of predation risk to be 1950 colony-forming-units per milliliter. Our study demonstrated the importance of considering the possible interdependence of predation risk and food abundance for oviposition-site-seeking insects. This study also quantified the perceived cost of predation and found it to be relatively low, a fact with positive implications for biological control.
The issue of resource-dependent antipredator behavior is a topic extensively studied within the context of foraging ecology (Lima and Dill 1990, Brown and Kotler 2004), showing, generally, that animals in a poor energetic state are more willing to accept predation risk than animals in a better energetic state. This topic, however, has not been well-studied for insect oviposition behavior. For organisms lacking parental care and where larval dispersal is limited, oviposition site selection decisions are critical choices (Bentley and Day 1989, Brodin et al. 2006, Vonesh and Blaustein 2010). With mosquitoes, it has been demonstrated that, generally, gravid females avoid sites with predators (Blaustein 1999, Brodin et al. 2006, Vonesh and Blaustein 2010) and are attracted to resource-rich sites (Blaustein and Kotler 1993, Bond et al. 2005). Most studies, however, examined the effect of predation risk and food level in isolation. The interdependence among them remains poorly understood.
Most mosquito studies on the interdependence of food and safety were conducted with respect to larval survival (Orr and Resh 1992, Lounibos et al. 1993, Fincke et al. 1997, Wallace and Merritt 1999, Bond et al. 2005, Beketov and Liess 2007) and presented circumstantial evidence suggesting independence between predation risk and food level. Two studies that addressed this issue experimentally are those of Fincke et al. (1997) and Beketov and Liess (2007). Fincke et al. (1997) studied the effect of predation by odonate nymphs on mosquito larvae survival rate and population size in artificial tree holes in Panama at low and high nutrient levels. They showed that odonate nymphs effectively controlled mosquito larvae population at low nutrient levels but were less effective at high nutrient levels. Beketov and Liess (2007) showed that predator-conditioned water affected larvae survival only when food was scarce. This result indicates that the perception of predation risk could be affected by food abundance and demonstrated state-dependent behavioral effect of predator presence on mosquito larvae survival. Unfortunately, the interdependence of food and safety in the context of mosquito oviposition behavior remains poorly understood, particularly with respect to oviposition site selection.
In this study, we investigated the oviposition behavior of mosquitoes when faced with conflicting demands of food for—versus safety of—their progeny. Specifically, the goal of this study was to evaluate if food abundance affects the perception of predation risk of oviposition-site-seeking mosquitoes. If the perception of predation risk does not depend on food level (Hypothesis 1), then oviposition rate should increase at the same rate with resource level for both predator-present and predator-absent treatments (Fig. 1, Hypothesis 1). If predation risk is valued low when food is scarce and valued high when food is abundant (Brown 1988), then diverging lines would be predicted (Fig. 1, Hypothesis 2). If predation risk is valued high at low food abundance and low in high food abundance (Beketov and Liess 2007), converging lines would be predicted (Fig. 1, Hypothesis 3). Furthermore, in this study, we attempted to quantify the perceived cost of predation. We achieved that by titrating different levels of larval food and estimating the food level at which oviposition level is equal between the predator-present and predator-absent treatments.
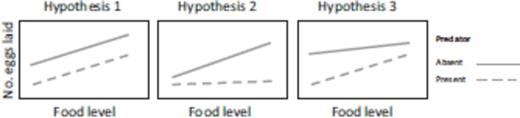
Competing hypotheses. Hypothesis 1: Independent effects of predation risk and food level should be expressed as two parallel regression lines. Hypothesis 2: Diverging regression lines indicate small effect of predation at low food level and large effect at high food levels. Hypothesis 3: Converging lines indicate large effect of predation at low food level and small effect at high food levels.
Materials and Methods
The experiment was conducted at Peabody-Park, University of North Carolina at Greensboro, comprising five 5-d-long replicate trials (hereafter, sessions) between 3 September and 12 October 2010 (3 September, 10 September,17 September,24 September, and 8 October). We used six parallel transects containing eight oviposition traps (ovitraps), each interspersed at 10-m intervals between traps and 20 m between transects. The two outer transects (transects 1 and 6) happened to occur close to the forest edge, whereas the inner ones (transects 3 and 4) close to a small creek. Each transect contained a single replicate of all eight treatment combinations (four food levels and two predator levels: presence or absence). Oviposition traps (ovitraps) were 650-ml black plastic cups (14.3 cm height, 6.5 and 9 cm diameter bottom and top, respectively) filled two-thirds with dechlorinated tap water and containing a rolled germination paper as an oviposition substrate (ovistrip) that was secured to the cup's lip with a black binder clip. Cups were punctured 5 cm below the lip to prevent overflow because of rain (Richards et al. 2006). Treatment location along each transect was randomized at each trapping session. In the laboratory, eggs on the ovistrip were counted using a dissecting scope. To confirm species identity, we flooded ovistrips of the 28 September 2010 collection and reared larvae to adulthood.
Predation Risk.
We used dragonfly nymphs that are known as effective predators of mosquito larvae (Blaustein 1999, Vonesh and Blaustein 2010) and were observed to overlap with container-breeding mosquitoes in used automobile tires (Akram and Lee 2004) and in tropical regions in tree holes (Fincke et al. 1997). Additionally, dragonfly nymphs are commonly found in naturally occurring rock pools that, when unoccupied by the nymphs, are often inhabited by immature mosquitoes (B. Byrd and G. O'Meara, personal communication), including, occasionally, Aedes albopictus (Skuse) (O'Meara et al. 1997). Hence, owing to this limited overlap between Ae. albopictus mosquitoes and libellulid nymphs, we expected low level of apprehension by the former, an attribute that should enable nymphs to constitute an effective biological control agent against mosquito larvae. Dragonfly nymphs were collected from ponds around Burlington, NC, and fed, ad libitum, in the laboratory with live Ae. albopictus larvae. As the identification of dragonfly nymph species required killing them, we could not sort the nymphs by species before experimental deployment and had to group them at the genus level. In addition, we could not obtain sufficient number of nymphs to keep size class constant and used a mixture of size classes. However, the majority were small and likely second instars. Both of these factors might increase variation in the relative magnitude of predator presence. However, as at each sampling session, nymphs were assigned randomly to the different food-level groups, this heterogeneity should not introduce any bias. Nymphs were placed in rectangular 8 by 6 by 2.5-cm aluminum cages (2-mm mesh). Predator cages were replaced weekly with fresh nymphs to ensure presence of a live predator. We verified the absence of a confounding effect of the cage in a preliminary experiment where we placed 21 pairs of ovitraps, with and without a cage, and compared number of eggs per ovitrap. No significant difference was found (paired-t = 0.288, P = 0.778). A sample of 15 dragonfly nymphs was sent to David L. Stephan at the Entomology Department of the North Carolina State University for species identification and was found to consist of Erythemis simplicicollis Say (54%), Plathemis Lydia Drury (38%), and Pachydiplax longipennis Burmeister (8%).
Larval Food.
Larval food was prepared by brewing 1 kg of deciduous tree leaf litter (mixture of ≈1:1 Hickory spp. and White Oak leaves) in a 20-liter container of dechlorinated tap water. This leaf infusion was prepared 1 wk before the beginning of the experiment and was reused for the duration of the study with dechlorinated tap-water added weekly to replenish the solution used. We obtained four food levels by diluting this source water at 1/10 and 1/100 ratios. Undiluted source-water was used for the highest food level and dechlorinated tap-water was used for the lowest level (control). As bacteria constitute an important food source for mosquito larvae (Merritt et al. 1992), we quantified food level in terms of bacterial count as colony-forming-units [CFU] in each of the four resource levels using a standard serial dilution and incubation scheme (Reynolds 2012). This also enabled us to account for the temporal fluctuation in resource level that occurred during the study.
Data Reduction and Statistical Analysis.
Data from stations where ovitraps were disturbed or nymphs died were discarded (n = 16) (Ntotal = 224). To account for potential leverage problems because of high bacterial count at the high food concentration, bacterial counts were Log10(x + 1) transformed. Our response variable was number of mosquito eggs per ovitrap. We used multiple regression to test the effect of bacterial concentration (log[CFU + 1]), predator presence, and their interaction as the predictive variables. We used transect number and session number as control variables. We fitted these variables as second-order polynomial variables to account for potential edge effect because of transect location and weather-related variability. Data were analyzed using R-software (R Development Core Team 2012).
Results and Discussion
In total, 16,163 mosquito eggs were collected, with an average of 72.16 eggs per ovitrap. After flooding and rearing of the 29 September 2011 ovistrip collection, we found it contained 100% Ae. albopictus, which is typical to this site (G.W., unpublished data). Overall, after controlling for spatial (transect location) and temporal (trapping session) effects, a significant negative effect of predator presence and positive effect of food level were observed (Table 1). Unfortunately, despite the highly significant value of the statistical model, its explanatory power is fairly limited (R2 = 20.3%). It is possible that some of the unexplained variation could be ascribed to the uncontrolled dragonfly species and size class effects. Future studies will address these potential sources of variation.
The effect of food level, expressed as Log10 (CFU + 1); presence of a caged predator; sampling transect location; and sampling session on the oviposition rate of female Aedes albopictus in oviposition cups
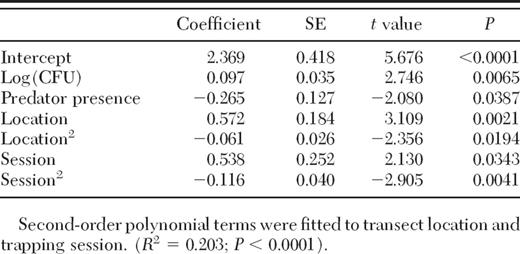
The effect of food level, expressed as Log10 (CFU + 1); presence of a caged predator; sampling transect location; and sampling session on the oviposition rate of female Aedes albopictus in oviposition cups

The Effect of Food Level, Predation Risk, and Their Interaction.
Egg numbers increased from an average of 62.30 (SE = 5.58) eggs per ovitrap at the lowest food concentration of 0 CFU to 92.45 (SE = 9.68) eggs at the highest food concentration of 223,000 CFU (Fig. 2). Predator presence had a negative effect on oviposition (Fig. 2), with average number of eggs decreasing from 79.69 eggs per ovitrap (SE = 5.82) in the predator-absent treatment to 64.75 (SE = 4.37) in the predator-present treatment. This suggests that gravid Ae. albopictus females are able to gauge food resource level as well as the presence of larvae predator. As shown in other studies, this is done most likely by the detection of specific chemical cues (kairomones) such as metabolites of microbial decomposition of organic matter as indicators of food abundance (Bentley and Day 1989, Trexler et al. 2003, Ponnusamy et al. 2008) or as kairomones emanating directly from the predator (Wisenden 2000, Silberbush and Blaustein 2008, Zuharah and Lester 2010). In our case, we assume that gravid females were not able to directly observe the dragonfly nymph because it was enclosed within a tight-mesh cage in the bottom of the cup and, therefore, assume they detected it through predator-specific kairomones.
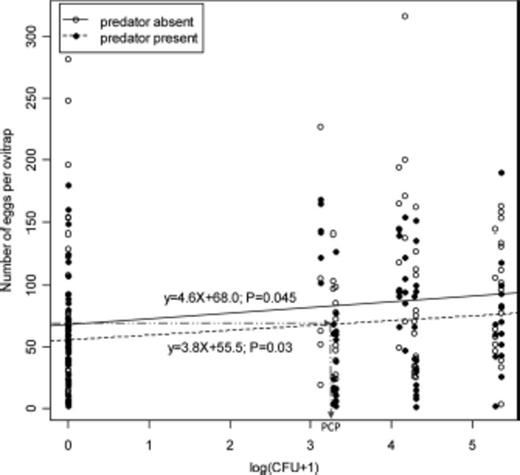
The effect of food level, expressed as Log10(bacterial count + 1), in the presence (P = 0.03) or absence of a caged dragonfly nymph on the number of eggs laid by Aedes albopictus females per oviposition trap. Calculation of the perceived cost of predation risk is depicted by extending a horizontal line from the y-axis intercept of the predator-present regression line to where it crosses the predator-absent regression line. Bacterial level at this point could be interpreted as the amount of food at which the risky-but-rich habitat is perceived equitable to the safe-but-poor habitat. This is the perceived cost of predation because of the presence of a single dragonfly nymph. The fact that the two regression lines are practically parallel indicates that the perception of predation risk does not depend on food resource level.
Another interesting observation is the fact that many eggs were laid at the risky and poor cups even though better alternatives were available (e.g., predator-free food-stacked cups). This could potentially suggest that site-seeking mosquitoes use some kind of density-dependent oviposition site selection rule in a manner that balances resource level and intraspecific competition (Fretwell 1972, Ellis 2008). Indeed, researchers observed that when given a choice, some mosquito genera tend to prefer oviposition site devoid of conspecific eggs over sites containing conspecific eggs (Kitron et al. 1989). In a different field study, where we presented mosquitoes with pairs of oviposition traps with or without conspecific eggs, we observed that >50 eggs-per-trap Ae. albopictus females start exhibiting avoidance from oviposition traps containing conspecific eggs and that the degree of this avoidance increases with conspecific egg density (G.W., unpublished data).
Our multiple regression model failed to find a significant statistical interaction between food level and predator presence (t = −0.006; P = 0.99) and was, therefore, dropped from the final statistical model (Table 1). This result supports the independence hypothesis (Fig. 1, Hypothesis 1), suggesting that the perception of predation risk by oviposition-site-seeking Ae. albopictus females does not depend on food level. Specifically, even though the slope of the predator-absent regression line is 0.8 larger than that of the predator-present line (Fig. 1), biologically, this difference is probably not meaningful because it indicates a difference of <1 egg per every log-bacterial-unit difference or ≈4.4 eggs for the entire resource range used here. Hence, for all practical purposes, it is reasonable to infer that resource level does not affect the perception of predation risk of site-seeking gravid Ae. albopictus mosquitoes.
Estimation of the Perceived Cost of Predation.
Given that the two regression lines can be assumed to be parallel, setting the y-value of the predator-presence line equation equal to the y-intercept of the predator-absence line equation (Fig. 2) 68.0 = 3.8X+55.5 enabled the calculation of the perceived cost of predation. Solving for X results in X = 3.29 in Log10(CFU + 1) units or 1950 CFU per milliliter of solution.
Spatial and Temporal Patterns.
Both control variables (transect location and trapping session time) exhibited a significant negative second-order polynomial relationship with respect to number of eggs laid (Table 1). As expected, spatially, the number of eggs laid increased going from the forest edge toward the central creek and then decreased toward the other forest edge (Fig. 3A). Temporally, egg numbers were lowest in sampling sessions 1 and 2 when the weather was relatively hot and dry and in sampling session 5 when the weather was relatively cool. Warm and humid conditions occurred during sampling sessions 3 and 4, when the highest egg numbers were recorded (Fig. 3B).
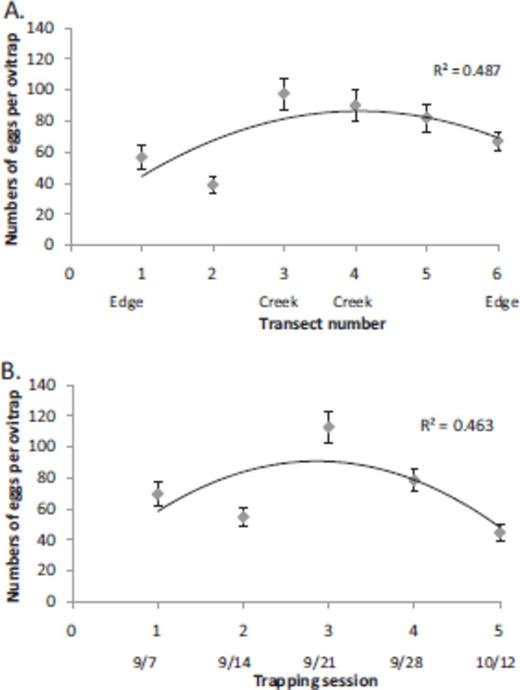
The effect of transect location (A) and time of the sampling session (B) on number of eggs laid per oviposition trap (mean ± SE). In both cases, hump-shape relations were observed, with higher egg numbers in transects most distant from the forest edge and adjacent to a creek (A) and during mid-September when ambient conditions were most favorable (B).
Our results strongly support the independence hypothesis (Hypothesis 1, Fig. 1), suggesting that the effects of food abundance and predation risk are independent of one another. Furthermore, our study was designed in a manner that enabled us to titrate different levels of food and estimate the amount of food needed such that the perceived benefit from using the risky-but-rich or the safer-but-poor habitat was equal. Compared with other studies showing 52% (Stav et al. 1999) or even 90% (Kiflawi et al. 2003) reduction in oviposition level in response to a dragonfly nymph or backswimmer, respectively, we observed a relatively small reduction of only 18.7%. This could be explained by the relative unfamiliarity of the invasive Ae. albopictus with the native libelluid predators because of their relatively recent co-occurrence (Gratz 2004) and limited habitat use overlap (O'Meara et al. 1997). Our observation that Ae. albopictus did respond to cues from a relatively unfamiliar predator is interesting, contrasting previous observations (Kesavaraju and Juliano 2004, Ohba et al. 2012). This observation suggests that the cue the nymphs emit is a general one. It could also be related to the fact that nymphs were fed before the experiment on Ae. albopictus larvae (Beketov and Liess 2007), although Kesavaraju and Juliano (2004), using water of Toxorhynchites rutilus (Coquillett) that fed on Ae. albopictus larvae, reported no antipredator response by Ae. albopictus larvae.
Finally, in terms of using libelluid nymphs for biological control of Ae. albopictus, the relative low apprehension of the control target (the mosquito) from the control agent (the libelluid nymph) might actually be encouraging because female mosquitoes will lay their eggs in the presence of the predator, hence making the dragonfly nymphs more effective in terms of mosquito control. Conceptually, we suggest that the relative simple study design presented here could be extended to other hematophagous insect systems to study the interdependence between the factors studied here as well as other biotic interactions (e.g., competition, parasitism) or abiotic effects (e.g., sublethal pollutants) and provide uniform insights of the organism's perception of the relative cost of these biotic and abiotic factors.
Acknowledgments
We thank Brian Byrd (Western Carolina University) for useful discussions and for reviewing an earlier draft of this article. We also thank the Biology Department, UNC-Greensboro, for providing support for this study, and David L. Stephan from the Entomology Department of the North Carolina State University for dragonfly nymph identification.
References Cited



