-
PDF
- Split View
-
Views
-
Cite
Cite
M. Obara-Nagoya, T. Yamauchi, M. Watanabe, S. Hasegawa, M. Iwai-Itamochi, E. Horimoto, T. Takizawa, I. Takashima, H. Kariwa, Ecological and Genetic Analyses of the Complete Genomes of Culex Flavivirus Strains Isolated From Culex tritaeniorhynchus and Culex pipiens (Diptera: Culicidae) Group Mosquitoes, Journal of Medical Entomology, Volume 50, Issue 2, 1 March 2013, Pages 300–309, https://doi.org/10.1603/ME12159
Close - Share Icon Share
Abstract
Culex flavivirus (CxFV) is an insect-specific flavivirus that was first reported in 2007 in Japan. CxFV strains were isolated from Culex tritaeniorhynchus Giles and Culex pipiens L. group mosquitoes and genetically characterized in Toyama Prefecture, Japan, from 2004 to 2009, to reveal host specificity, mode of transmission, and seasonal and geographical distribution. The minimum infection rate (MIR) of CxFV within Cx. tritaeniorhynchus populations was 0.3 and much lower than that within Cx. pipiens group (17.9). The complete genome sequences of 11 CxFV isolates (four from Cx. tritaeniorhynchus and seven from Cx. pipiens group) consisted of 10,835–10,837 nucleotides. When these 11 isolates and five reference strains (NIID-21-2 and Tokyo strains from Japan, Iowa07 and HOU24518 strains from the United States, H0901 strain from China) were compared, there were 95.2–99.2% nucleotide and 98.1–99.8% amino acid identities. Phylogenetic analysis showed that the 11 isolates were divided into four clusters. One cluster consisted of five isolates from Cx. pipiens group and Cx. tritaeniorhynchus from one site and their nucleotide sequences almost completely matched. One cluster consisted of an isolate with a unique sequence from a Cx. pipiens group mosquito captured in an aircraft from Taiwan, suggesting that it was introduced from abroad. CxFV strains were divided into several groups according to countries when nucleotide sequences of CxFV available in GenBank and 11 Toyama isolates were compared. These results suggest that CxFV is maintained in nature among Culex mosquitoes in a mosquito habitat-specific but not a species-specific manner.
Many mosquito-borne and tick-borne pathogenic viruses, such as Japanese encephalitis virus (JEV), West Nile virus (WNV), and tick-borne encephalitis virus (TBEV), belong to the genus Flavivirus within the family Flaviviridae (Gubler et al. 2007). The genome is a single-stranded, positive-sense RNA molecule of about 10–11 kb, which comprises a single open reading frame and 5′- and 3′-untranslated regions (UTRs). The open reading frame encodes structural (capsid (C)-premembrane (prM)-envelope (E)) and nonstructural (NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5) proteins.
Flavivirus is distributed globally with distinct mosquito or tick vectors in each region (Gubler et al. 2007). For example, JEV is mainly transmitted by Culex tritaeniorhynchus Giles in Asia, yellow fever virus is transmitted by Aedes and Haemagogus mosquitoes in tropical Africa and South America, and WNV is transmitted by Culex mosquitoes in Africa, West Asia, the Middle East, Europe, and the United States.
There are also insect-specific viruses in the genus Flavivirus, such as cell fusing agent virus (CFAV; Stollar et al. 1975), Kamiti River virus (KRV; Crabtree et al. 2003, Sang et al. 2003), Quang Binh virus (Crabtree et al. 2009), Aedes flavivirus (Hoshino et al. 2009), and Nakiwogo virus (Cook et al. 2009).
In 2007, novel “Culex flavivirus” (CxFV) strains were reported from Culex pipiens L. and Cx. tritaeniorhynchus in Japan and Culex quinquefasciatus Say in Indonesia (Hoshino et al. 2007). Phylogenetic analysis showed that they were most genetically related with the other insect flaviviruses. CxFV reportedly replicates only in mosquito cells and has been isolated from both male and female mosquitoes, suggesting vertical transmission (Hoshino et al. 2007, Farfan–Ale et al. 2009). Because CxFV strains have been isolated from many species of mosquito including Cx. pipiens, Cx. tritaeniorhynchus, Cx. quinquefasciatus, Culex restuans Theobold, Culex tarsalis Coquillett, and Culex interrogator Dyar and Knab across a vast area including the United States, Latin America (Mexico, Guatemala, Trinidad, Brazil), Africa (Uganda), Indonesia, Japan, and China (Hoshino et al. 2007, Morales–Betoulle et al. 2008, Farfan–Ale et al. 2009, Kim et al. 2009, Blitvich et al. 2009, Cook et al. 2009, Saiyasombat et al. 2010; GenBank accession no. HQ678513 and HQ605702–HQ605704), they seem to be globally distributed within Culex mosquitoes. According to the country of isolation, CxFV strains were divided into two clades (Kim et al. 2009, Blitvich et al. 2009, Saiyasombat et al. 2010). Although CxFV reportedly infects many species of Culex mosquito, it is not clear whether CxFV exists in nature in a mosquito-species-specific or habitat-specific manner.
The complete genome sequences were determined in CxFV strains from Cx. pipiens (strains NIID-21-2 and Tokyo (Hoshino et al. 2007), Iowa07 (Blitvich et al. 2009), and H0901 (unpublished data, accession no. HQ678513)) and Cx. quinquefasciatus (strains CxFV-Mex07 [Farfan–Ale et al. 2009] and HOU24518 [Kim et al. 2009]). These CxFV strains were found to be similar to each other. In comparison with other flavivirus strains (CFAV, KRV, JEV, dengue virus [DENV], Yokose virus, TBEV, and Apoi virus), CxFV was most similar to cell fusing agent virus, in particular, in the prM and E genes (69.0–69.5% nucleotide identities) (Hoshino et al. 2007). According to the E and NS5 genes, the strains isolated from Cx. tritaeniorhynchus are similar to those from Cx. pipiens (Hoshino et al. 2007, 2009); however, the nucleotide sequences in other regions were not determined and might be different.
Cx. tritaeniorhynchus is the major vector of JEV, the causative agent of severe encephalitis in humans. Because both CxFV and JEV are harbored by Culex species of mosquitoes in Japan, they may either affect or share host mosquitoes. For example, they may interfere or help each other to increase. Therefore, clarification of the mechanism of maintenance of CxFV is important not only ecologically but also from a public health perspective.
Mosquito and JEV surveillance have been conducted continuously from 1969 in Toyama Prefecture, Japan, and habitat partitioning of Cx. tritaeniorhynchus, Cx. pipiens group mosquitoes (containing Cx. pipiens pallens and Cx. pipiens molestus), and Aedes albopictus Skuse has been precisely characterized (Obara et al. 2011, Watanabe et al. 2011). Whereas Cx. tritaeniorhynchus was mainly distributed on farms, Cx. pipiens group and Ae. albopictus were distributed at other sites such as the gardens of private houses. In this study, the complete genome sequences of CxFV strains isolated from Cx. tritaeniorhynchus were determined and compared with those isolated from Cx. pipiens group in Toyama Prefecture as well as representative isolates of strains previously reported. From these data, CxFV maintenancein Culex mosquitoes and their habitats are discussed.
Materials and Methods
Mosquitoes.
To isolate viruses, mosquitoes were collected once a week using CO2 traps from 2004 to 2009 at a total of 21 sites as previously reported (Obara et al. 2011) (Fig. 1). The traps were battery-operated light traps (Inokuchi–Tekko, Nagasaki, Japan), Centers for Disease Control and Prevention Miniature Light Traps (John W. Hock Company, Gainesville, FL), 12 V battery-operated light traps (FHK, Tokyo, Japan), or six V battery-operated traps (Rakuno Gakuen University, Hokkaido, Japan), which were set with dry ice or a CO2 cylinder and left overnight. Some mosquitoes were collected with a net in aircraft at Toyama Airport. Mosquitoes were classified according to collection site, date of collection, species, and sex. Mosquito species were morphologically identified according to Tanaka et al. (1979). In this study, Cx. pipiens group contains Cx. pipiens pallens and Cx. pipiens molestus because these two species could not be discriminated morphologically. Mosquitoes were then pooled into groups of <50 individuals and stored at −80°C.
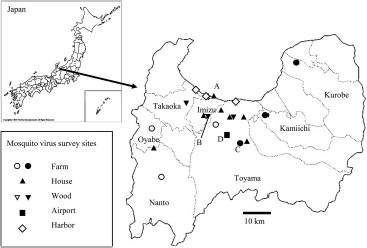
Map showing survey sites for virus isolation in Toyama Prefecture from 2004 to 2009. Marks denote the corresponding sites as indicated in the box where mosquitoes were collected for virus isolation. Filled marks indicate the sites where CxFV-positive mosquitoes were collected.
Virus Isolation.
CxFV isolation was performed at the same time as JEV isolation (Obara et al. 2011). Pools of mosquitoes were homogenized in 0.5–1.0 ml of maintenance medium (Eagle's Minimum Essential Medium containing 2% fetal bovine serum or 0.11% bovine serum albumin fraction V) and centrifuged at 5,867 × g for 5 min. The supernatants were then passed through 0.45 μm filters (Ultrafree MC, Millipore Corp., Bedford, MA). The filtrates were diluted 10-fold with medium and inoculated onto monolayers of C6/36 Ae. albopictus cells (No. IFO50010; obtained from Health Sciences Research Resources Bank, Osaka, Japan). These cultures were incubated at 28°C for 2 h in 5% CO2. After maintenance media were added, the cells were incubated for 6–8 d. Because toxicity in the homogenate hindered cytopathic effects (CPE) by virus, two or three cell passages were performed and culture media were collected when CPE appeared.
Detection of CxFV.
Viral RNA was extracted from culture supernatants that exhibited CPE with a QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA) in accordance with the manufacturer's instructions. Reverse transcription-polymerase chain reaction (RT-PCR) was carried out with either QIAGEN OneStep RT-PCR Kit (Qiagen) or TaKaRa One Step RNA PCR Kit (AMV) (TaKaRa Bio Inc., Otsu, Japan). The 258 bp of the NS5 gene were amplified with primers MA and cFD2 (Kuno 1998; Table 1). For PCR, the mixture was incubated at 53°C for 30 min, and then 40 cycles of 94°C for 1 min, 53°C for 1 min, and 72°C for 1 min.
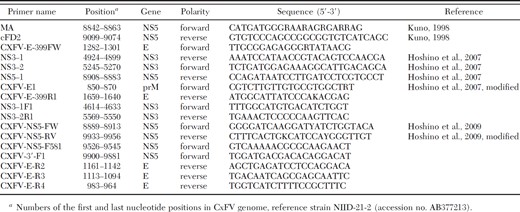

After purification of the amplicons, the nucleotide sequences were determined with the BigDye Terminator v1.1 or v3.1 Cycle Sequencing Kit and ABI 3100 or 3130 sequencer (Applied Biosystems, Branchburg, NJ). The nucleotide sequences were aligned using Sequencher software version 4.7 (Gene Codes Co., Ann Arbor, MI) and compared with those of CxFV in the GenBank database. The minimum infection rate (MIR) was defined in terms of CxFV-positive pool number per 1,000 mosquitoes tested.
Analysis of the Complete Genomes of CxFV Isolates.
Viral RNA was extracted from culture supernatants at passage four with a QIAamp Viral RNA Mini Kit to obtain a sufficient virus titer for analysis. RT-PCR was performed with the primers shown in Table 1. The mixture with primers CXFV-NS5-FW and CXFV-NS5-RV was incubated at 50°C for 45 min, at 94°C for 2 min, and then 45 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 2 min, and finally at 72°C for 10 min. PCR with primers CXFV-E-399FW and NS3–1, NS3–2, and NS5–1 was performed as follows. Viral RNA (12 μl) was treated with 3 μl of DNase mixture (containing 1 U of DNase I) at 37°C for 30 min and then at 75°C for 5 min, followed by the addition of 15 μl of a mixture containing Super Script II RNase H- transcriptase XL (Invitrogen Corp., Carlsbad, CA) or Super Script III RNase H- transcriptase XL (Invitrogen Corp.). RT was performed at 42°C for 60 min, and the enzyme was inactivated at 99°C for 5 min. A 50 μl PCR reaction mixture contained 2 μl of cDNA, 2.5 U of TaKaRa LA Taq (TaKaRa Bio Inc.), 10x LA PCR buffer II 5 μl, 2.5 mM MgCl2, and 0.4 mM of each dNTP. Amplification was performed under the following conditions: 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 6 min. RT-PCR with primers CXFV-E1 and CXFV-E-399R1, NS3–1F1 and NS3–2R1, CXFV-NS5-FW and CXFV-NS5-RV was performed as described in “Detection of CxFV.” The sequences were determined by primer walking.
The extreme 3′ ends of CxFV isolates were determined using poly(A) Polymerase Tailing Kit (EPICENTRE Biotechnologies, Madison, WI) and 3′-Full RACE Core Set (TaKaRa Bio Inc.) with primer CXFV-NS5-F581 (Table 1). Nested PCR was performed by TaKaRa Ex Taq (TaKaRa Bio Inc.) with primers CXFV-3′-F1 (Table 1) and three sites Adaptor Primer in 3′-Full RACE Core Set. The extreme 5′ ends of CxFV isolates were determined by 5′ RACE System for Rapid Amplification of cDNA Ends, Version 2.0 (Invitrogen Corp.), with primers CXFV-E-R2, CXFV-E-R3, and CXFV-E-R4 (Table 1). CxFV isolated in this study have been submitted to GenBank under accession numbers AB701766–AB701776 and AB772323–AB772405.
Phylogenetic Analysis.
The nucleotide and amino acid sequences of the reference strains were obtained from GenBank. Alignment and phylogenetic analysis were performed using MEGA 3.1 software (Kumar et al. 2004). A phylogenetic tree was constructed by the neighbor-joining method and genetic distances were calculated according to Kimura's two-parameter method (Kimura 1980). The reliability of the tree was estimated by performing 1,000 bootstrap replications. The homologies among CxFV strains were analyzed by GENETYX software (version 10, Genetyx Corp. 2009). The similarities versus position with the reference strains were plotted using SimPlot program version 3.5.1 (Lole et al. 1999) (distributed by the author Ray at http://www.welch.jhu.edu/).
Results
Infection Rate of CxFV.
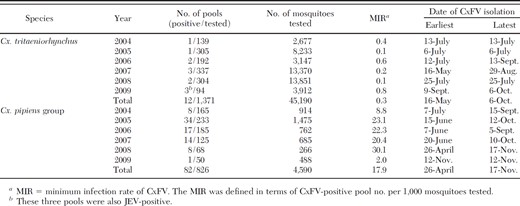
In total, 94 CxFV strains were isolated: 12 pools from 45,190 Cx. tritaeniorhynchus (45,184 females and 6 males) and 82 pools from 4,590 Cx. pipiens group (4,564 females and 26 males) (Table 2). The MIRs of Cx. tritaeniorhynchus and Cx. pipiens group were calculated as 0.3 and 17.9, respectively, with the latter being significantly higher than the former (P < 0.001). They were exclusively isolated from female mosquitoes. Three CxFV strains were isolated from three pools in which JEV were isolated (Obara et al. 2011).
The numbers of Culex tritaeniorhynchus or Culex pipiens group from which CxFV were isolated
The numbers of Culex tritaeniorhynchus or Culex pipiens group from which CxFV were isolated

CxFV-positive mosquitoes were collected in the sites indicated in Fig. 1, which included three farms, seven gardens of private houses, three woods, and Toyama Airport. These sites were widely distributed in Toyama Prefecture.
CxFV strains were isolated from Cx. tritaeniorhynchus and Cx. pipiens group from May to October and from April to November, respectively (Table 2). The strains were most frequently isolated in July (39 strains, 41.5%). The MIR and the number of Cx. pipiens group peaked at almost the same time in July to August (Fig. 2), when they were compared at one site (A in Fig. 1) in 2005. The number of CxFV-positive pools in Cx. tritaeniorhynchus increased in July (four isolates, 33.3%) at the same time as that in Cx. pipiens group, although only a small number of CxFV were isolated from Cx. tritaeniorhynchus and the number of Cx. tritaeniorhynchus in July was less than those in August and September (Obara et al. 2011).
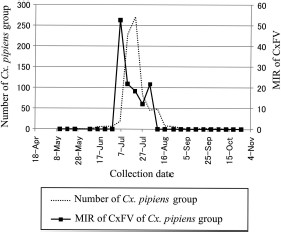
The MIR of CxFV and number of Cx. pipiens group in site A (Fig. 1) in 2005. The MIR means CxFV-positive pool number per 1,000 mosquitoes tested. The numbers of mosquitoes were calculated from the weekly collected numbers.
The Complete Genomes of CxFV Isolates.
The complete genomes of 11 Toyama isolates were sequenced and the characteristics of Toyama strains and the reference strains were summarized (Table 3). Three isolates from Cx. tritaeniorhynchus and four from Cx. pipiens group consisted of 10,837 nucleotides, containing 91 nucleotides in the 5′UTR and 654 nucleotides in the 3′UTR, which were the same as the reference strains, except H0901 (Table 3). One isolate from Cx. tritaeniorhynchus and three from Cx. pipiens group had one nucleotide deletion at position 10,316 (3′UTR), and “Toyama1431” had an additional nucleotide deletion at position 82 (5′UTR).
The characteristics of CxFV isolated in this study and the reference strains
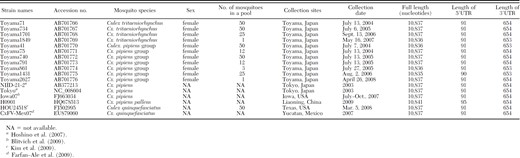
The characteristics of CxFV isolated in this study and the reference strains

The nucleotide and amino acid identities of the entire genome sequences were compared among Toyama and reference strains (Table 4). The results showed the differences of nucleotide and amino acid identities among Toyama strains and reference strains. Eleven Toyama strains and five reference strains (NIID-21-2, Tokyo, Iowa07, H0901, and HOU24518) showed higher identities in both nucleotides and amino acids (95.2–99.2% and 98.1–99.8%, respectively) than the Mexican reference strain (CxFV-Mex07) (90.7–90.9% and 96.8–97.1%, respectively).

To identify whether there are specific regions in the CxFV genome that might be responsible for the above differences observed among CxFV from different species or countries, their complete genomes were compared by a similarity plot program. No specific region with markedly different homology was observed among these strains, except part of the NS5 region (about no. 9,050–9,100) of the reference “Tokyo” strain (data not shown). Because this region in the reference Tokyo strain had low similarity with other reference strains except CxFV-Mex07, it might be unique.
To determine the way in which CxFV is maintained in nature, phylogenetic analysis was performed using 11 Toyama isolates and reference strains. The 11 Toyama isolates were divided into four clusters in the phylogenetic tree (Fig. 3). One cluster consisted of four isolates (Toyama41, 861, 1431, and 1849) from Cx. pipiens group or Cx. tritaeniorhynchus captured at site A or B. Their nucleotide sequences matched at 99.6–99.8% (Table 4). Two of them, Toyama 1849 and 861 isolated from Cx. tritaeniorhynchus and Cx. pipiens group, respectively, were captured at site B. Another cluster consisted of five isolates (Toyama71, 75, 734, 791, and 1701) from Cx. pipiens group or Cx. tritaeniorhynchus exclusively captured at site C. Their nucleotide sequences almost completely matched (Table 4). The other two clusters consisted of two unique isolates of either Toyama740 or Toyama2627 from Cx. pipiens group captured at A or D (airport) site, respectively (Fig. 3).
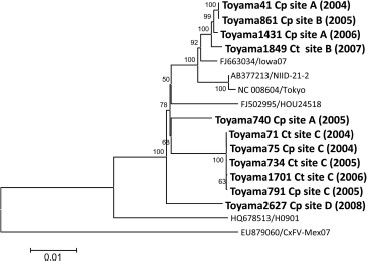
Phylogenetic tree of the complete genome of CxFV. The tree was constructed by the neighbor-joining method. CxFV isolates in Toyama Prefecture are denoted in bold and shown as strain name, isolation source (Ct, Cx. tritaeniorhynchus; Cp, Cx. pipiens group), site name (Fig. 1), and year of isolation. The reference strains are shown by “accession no./strain name.” The scale shows the genetic distance in nucleotide substitutions per site. Numbers at branches indicate bootstrap values (%) >50%. Bootstrap replications were performed 1,000 times.
Genetic Identities Among CxFV Strains Isolated in Toyama.
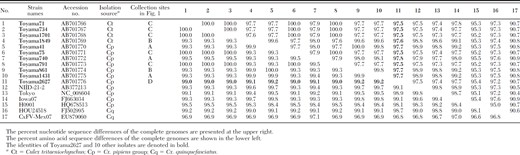
To characterize Toyama740 and Toyama2627 more precisely, 210 bp of NS5 genes were compared with all of the other Toyama isolates, because 210 bp of NS5 sequences of all Toyama CxFV isolates were obtained and this region was not characteristic in the complete genome sequence of Toyama isolates (data not shown). The result that 210 bp of the NS5 sequence of Toyama740 matched 100% with those of six out of 94 isolates indicates that Toyama740 is not unique. Toyama2627 formed a unique cluster according to both complete genome (Fig. 3) and 210 bp of the NS5 gene (data not shown), and its identities to 10 other Toyama isolates were relatively low (97.5–97.8% nucleotides). Because the strain Toyama2627 was isolated from Cx. pipiens group captured in an aircraft from Taiwan, it is highly possible that the mosquito originated from Taiwan. However, 210 bp of the NS5 gene of 20 out of 22 strains isolated at site C from 2004 to 2009 matched 100% with each other. The other two strains isolated in 2008 and 2009 had 99.5% nucleotide identity with 20 isolates. The above observations suggest that CxFV is maintained in a habitat-dependent manner in nature in Toyama Prefecture irrespective of the mosquito species.
Genetic Identities Among CxFV Strains Isolated From Different Regions of the World.
To date 61 nucleotide sequences of CxFV strains whose host mosquitoes have been identified have been deposited in GenBank, nucleotide identities among these sequences including 11 Toyama isolates were compared with reveal the relationship between the CxFV sequences and mosquito species (Table 5). When 30 sequences of CxFV strains isolated from Cx. quinquefasciatus were compared, five isolates (Cq-5 isolated in Indonesia and the United States) were revealed to be closely related to the isolates from Cx. pipiens group, Cx. restuans, and Cx. tritaeniorhynchus, while the other 25 isolates (Cq-25 from Mexico, Indonesia, Guatemala, Trinidad, and Uganda) were closely related to those from Cx. interrogator. The identities between Cq-5 and Cq-25 were 88.7–90.9%. Therefore, CxFV strains from Cx. quinquefasciatus seem to be divided into two types. However, sequences from Cx. pipiens group, Cx. restuans, and Cx. tritaeniorhynchus possessed >94.1% identities with each other. Thus, CxFV seems to possess closely related nucleotide sequences across species boundaries.
Ranges of nucleotide sequence identities of CxFV from mosquitoes within species and between species
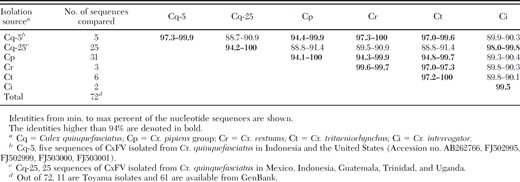
Ranges of nucleotide sequence identities of CxFV from mosquitoes within species and between species

Next, to reveal the relationship between the sequences and the country of isolation, 69 nucleotide sequences of CxFV available in GenBank and 11 Toyama isolates were compared (Table 6). The sequences of CxFV strains isolated in Japan and the United States were closely related (96.8–100% identical), and those isolated in Mexico, Guatemala, Trinidad, Brazil, and Uganda possessed 97.7–100% nucleotide identities. One of the nucleotide sequences of CxFV strains isolated in Indonesia (Indonesia-1 in Table 6) was closely related to those isolated in Japan and the United States (97.1–99.8% identical), whereas those of three and one CxFV strains isolated in Indonesia (Indonesia-3 in Table 6) and China, respectively, possessed relatively low identities with those in other countries. The above results indicated that CxFV strains were divided into several groups: Japan/United States including Indonesia-1, Latin America/Africa (including Mexico, Guatemala, Trinidad, Brazil, and Uganda), China, and Indonesia-3 types. Indonesia-1 might have been imported into Indonesia from abroad.
Ranges of nucleotide sequence identities of CxFV from mosquitoes within a country and between countries

Ranges of nucleotide sequence identities of CxFV from mosquitoes within a country and between countries

Discussion
CxFV has been sporadically isolated from mosquito species collected from several countries; however, it is not clear how CxFV is maintained in nature. Herein, CxFV was isolated from Cx. tritaeniorhynchus and Cx. pipiens group mosquitoes collected at several sites in Toyama Prefecture, Japan, over several years and the complete genome sequences of these isolates were compared with those in GenBank. The results of this study combined with those of others suggested that CxFVs are genetically stable over years in a habitat-dependent manner and that transmission of CxFVs occurs among different species of mosquitoes (inter-mosquito–species transmission). In particular, nucleotide sequences of CxFV at one site in Toyama Prefecture were strongly conserved from 2004 to 2009. CxFV may be stable because the virus may not be harmful to the host mosquito. Other reasons are that NS5 may not be influenced by the immunity of mosquitoes or that the nucleotide sequences analyzed in this study were short.
The above assertions of habitat-dependent maintenance and inter-mosquito–species transmission of CxFV were supported by the fact that the entire genome sequences of CxFV strains almost completely matched among Cx. tritaeniorhynchus and Cx. pipiens group mosquitoes at collection site C when the nucleotide sequences of CxFV strains from different sites in Toyama Prefecture were compared (Fig. 3). The comparison of nucleotide sequences of CxFV strains from different species of mosquito in different areas in the world (Table 5) showed the similar identities among the viruses from Cx. tritaeniorhynchus, Cx. pipiens group, Cx. quinquefaciatus, and Cx. restuans mosquitoes, which also supports the above assertions. However, to ask whether contamination of mosquitoes during collection was occurred, PCR was performed to detect Cx. pipiens group DNA from 12 Cx. tritaeniorhynchus pools with CxFV positive (Table 2) using the method as described (Kasai et al. 2008). The DNA of Cx. pipiens molestus were detected in eight out of 12 pools. If CxFV was contaminated from Cx. pipiens group, CxFV should have been isolated from not only pools of Cx. tritaeniorhynchus but also those of Cx. pipiens group collected in the same trap. Only two pools (strains Toyama71, Toyama734, Toyama1701, Toyama1849 in Table 3 did not contain these two pools) out of eight pools met this condition. Therefore, CxFV strains isolated from at least 10 pools of Cx. tritaeniorhynchus do not seem to be derived from Cx. pipiens group.
CxFV strains were found to be divided into Japan/United States, Latin America/Africa, China, and Indonesia types by comparison of the nucleotide sequences in GenBank and those of Toyama isolates. Previous studies also support our assertion that the nucleotide sequences of CxFV strains were divided by area independent of mosquito species (Blitvich et al. 2009, Kim et al. 2009).
In contrast, the nucleotide sequences of CxFV isolates collected at sites A and C were different and the isolate from a mosquito in an aircraft at site D from a foreign country had relatively low nucleotide and amino acid identities with other Toyama isolates. This implies that nucleotide sequences of CxFV may vary with an area and CxFV may move across country borders by air.
Vertical transmission of CxFV is suggested because it replicates only in mosquito cells, has been isolated from both male and female mosquitoes, and detected in eggs and larvae of mosquitoes (Hoshino et al. 2007, Farfan–Ale et al. 2009, Bolling et al. 2011, Saiyasombat et al. 2011). However, to explain inter-mosquito–species transmission, CxFV may infect mosquitoes not only vertically but also horizontally via an oral route, such as drinking infected liquid, or eating microbes contaminated with virus. Because CxFV reportedly did not multiply in adult mosquitoes orally exposed to the virus (Kent et al. 2010), oral route transmission may only occur during the larval stage. Cx. tritaeniorhynchus and Cx. pipiens group share the same larval habitat (Tanaka et al. 1979). The larvae of Cx. tritaeniorhynchus are found most frequently in rice fields and various types of impounded water. Those of Cx. pipiens group occur in a very wide variety of artificial containers or other types of stagnant water. The larvae of Cx. tritaeniorhynchus and Cx. pipiens group were actually found in the same water (data not shown), so horizontal infection between Cx. tritaeniorhynchus and Cx. pipiens group might occur.
In this study, CxFV infection rate of Cx. tritaeniorhynchus was found to be much lower than that of Cx. pipiens group. There was no site where CxFV was isolated from only Cx. tritaeniorhynchus. Low infection rate of Cx. tritaeniorhynchus might be because of few infection opportunities, low efficiency of infection, or the presence of only a few colonies of infected mosquitoes. The infection rate of CxFV in Cx. pipiens group and Cx. tritaeniorhynchus peaked in July when the number of Cx. pipiens group peaked, while the number of Cx. tritaeniorhynchus peaked in August to September in Toyama Prefecture (Obara et al. 2011). Cx. tritaeniorhynchus might have opportunities to be infected with CxFV from Cx. pipiens group in July.
JEV and CxFV were simultaneously isolated from three pools in the same collection of mosquitoes. Flaviviruses reportedly show some influence on each other (Cologna et al. 2005, Pepin et al. 2008, Kent et al. 2010, Newman et al. 2011). Our preliminary results show that CxFV suppressed the growth of JEV maximally to about one-hundredth in a virus-dose-dependent manner (data not shown) when the mosquito cultured cells (C6/36) were simultaneously infected with CxFV and JEV. Further investigation is required to confirm the above result, using cell lines of genus Culex mosquitoes, but also mosquitoes in vivo.
In this study, the complete genome sequences of CxFV strains isolated from two species of mosquito in the same area were analyzed. Furthermore, ecological features of CxFV in terms of the host species of mosquitoes and the distributed area were characterized. Evolution of CxFV seemed to depend not on mosquito species but on mosquito habitat.
Thirty-three nucleotide sequences (accession no. AB639348–AB639349, HQ634589–HQ634598, JF938690, JQ023758, JQ065883, JQ308186–JQ308190, JQ409186–JQ409191, JQ518484) of CxFV have been deposited during the revision of this manuscript.
Acknowledgments
We thank Miyuki Maekawa for her technical assistance. We are also grateful to Yasufumi Ueda, Kentaro Matsuno, Toyama-Airport Detached Office of Niigata Quarantine Station, Takaoka Health Center and volunteers for collection of mosquitoes. We also thank the Department of Medical Entomology, National Institute of Infectious Diseases, for advice on virus isolation and PCR, and Department of Virology 1, National Institute of Infectious Diseases, for advice on virus isolation. This work was supported by a Health Labor Sciences Research Grant for Research on Emerging and Reemerging Infectious Disease (H17-shinkou-ippan-018, H20-shinkou-ippan-015) from the Japanese Ministry of Health, Labor and Welfare.
References Cited



