-
PDF
- Split View
-
Views
-
Cite
Cite
Joel L. Lutomiah, Hellen Koka, James Mutisya, Santos Yalwala, Milka Muthoni, Albina Makio, Samson Limbaso, Lillian Musila, Jeffrey W. Clark, Michael J. Turell, Elizabeth Kioko, David Schnabel, Rosemary C. Sang, Ability of Selected Kenyan Mosquito (Diptera: Culicidae) Species to Transmit West Nile Virus Under Laboratory Conditions, Journal of Medical Entomology, Volume 48, Issue 6, 1 November 2011, Pages 1197–1201, https://doi.org/10.1603/ME11062
Close - Share Icon Share
Abstract
West Nile virus (WNV) is currently active in Kenya as evidenced by the detection of antibodies in birds bled as part of an avian influenza surveillance program in 2009. Although WNV has been isolated from several mosquito species in Kenya, no studies have ever been conducted to determine which of these species are competent vectors of this virus. Therefore, we allowed Kenyan mosquitoes to feed on 2- or 3-d-old chickens that had been infected with a Lineage one strain of WNV 24–48 h earlier. These mosquitoes were tested ≈2 wk later to determine infection, dissemination, and transmission rates. All five species [Culex quinquefasciatus Say, Culex univittatus Theobald, Culex vansomereni Edwards, Mansonia africana (Theobald), and Mansonia uniformis (Theobald)] were susceptible to infection, but disseminated infections were detected only in the three Culex, and not the two Mansonia species. Culex mosquitoes with a disseminated infection readily transmitted virus by bite, but even when inoculated with WNV, the two Mansonia failed to transmit virus, indicating a salivary gland barrier. These studies indicate that the three Culex species may play a role in the transmission of WNV in Kenya.
West Nile virus (WNV) is an arbovirus of public health importance that belongs to family Flaviviridae and genus Flavivirus. It was first isolated in the West Nile province of Uganda in 1937 from the blood of a febrile patient (Smithburn et al. 1940). Since then, WNV has caused a large number of epidemics with numerous fatalities worldwide (Sang and Dunster 2001, Gubler 2007, Petersen and Hayes 2008). West Nile virus (WNV) is enzootic in Africa, Asia, Europe, Australia, North and South America (Hayes 1989, Gubler 2007), with the largest reported single outbreak occurring in South Africa in 1974 where Culex univittatus Theobald mosquitoes were involved (Jupp 2001). Since then, the ongoing outbreak in the Americas has resulted in >10,000 human cases. The virus is maintained in a natural transmission cycle between Culex mosquitoes and birds. Although large mammals are considered incidental, dead-end hosts (Hayes 1989), certain squirrels and lagomorphs may produce a viremia high enough to infect some mosquitoes (Platt et al. 2008). Most infections with WNV in humans are subclinical; however, ≈20% result in a mild febrile illness, West Nile fever, and a small percentage (<1%) of infections develop a meningoencephalitis, with about a 10% case fatality rate (Hayes et al. 2005). There is no vaccine or treatment currently available for humans.
Although WNV epidemics have not been reported in Kenya, the virus has been isolated from several species of mosquitoes collected from different geographical regions including Cx. univittatus from the Rift Valley province (Miller et al. 2000), Aedes sudanensis (Theobald) from the North Eastern province of Kenya (Crabtree et al. 2009), and from several Culex and Aedes species collected in the North Eastern province during the Rift Valley fever outbreak in 2007 (LaBeaud et al. 2011). WNV antibodies have also been detected in bird serum, including resident, nonmigrant species collected from the Rift Valley province as part of an avian influenza monitoring program conducted between January and March 2009 (S. L., unpublished data) and a human case was detected in Mombasa in 2005 (R. S., unpublished data). The isolation of virus, detection of infections in birds, and occurrence of a human case suggest that WNV is a potential public health concern in Kenya.
Although WNV has been isolated from numerous species of mosquitoes in several different genera, it has primarily been associated with members of the genus Culex (Hubálek and Halouzka 1999). However, not all mosquitoes found with viruses in the wild are capable of transmitting arboviruses because of midgut infection, midgut escape, and salivary gland barriers (Hardy 1988). Despite being aware of the presence of this virus in Africa for many years, there is little data available about which African mosquitoes are competent vectors, and no data exist about the potential of Kenyan mosquitoes to transmit WNV.
Therefore, we initiated studies to evaluate the potential of five Kenyan mosquito species, Culex quinquefasciatus Say, Cx. univittatus and Culex vansomereni Edwards, Mansonia africana (Theobald), and Mansonia uniformis (Theobald) to transmit a lineage one strain of WNV under laboratory conditions. Identifying which species are competent vectors and the factors that influence their ability to transmit WNV is the first important step in assessing the risk of WNV transmission and outbreaks in Kenya and developing appropriate targeted monitoring and control strategies.
Materials and Methods
Mosquitoes.
We collected adult Ma. africana, Ma. uniformis, Cx. quinquefasciatus, Cx. univittatus, and Cx. vansomereni, in dry ice-baited CDC miniature light traps (John W. Hock, Gainesville, FL) in the vicinity of Baringo and Naivasha in the Rift Valley province and the Sukari ranch in the Central province between 14 and 15 November 2008. The mosquitoes were transferred to 4-liter plastic cages and transported to the Kenyan Medical Research Institute (KEMRI) where they were maintained in the 4-liter plastic cages in the biological safety level-2 insectary at 27°C and a photoperiod of 12:12 (L:D) h.* They were provided either a 10% glucose solution on cotton wicks or sliced apples as a carbohydrate source until used in the study. In addition to these field-collected specimens, we also tested some Cx. quinquefasciatus from a colony (F51) established from specimens captured inside houses in Kahawa West, Central province, Kenya, in 2004, and maintained at the International Centre of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya.
Virus and Virus Assays.
MMP1047, a Lineage one strain of West Nile virus, was isolated from mosquitoes collected from Malindi, Kenya, in 1977 as part of a routine arbovirus surveillance program. The working stock virus was passaged two times in Vero (African green monkey kidney) cells before use in these studies. To determine infection status and viral titer, serial 10-fold dilutions of specimens were made in Minimum Essential Medium Eagle (EMEM), (Sigma-Aldrich, St. Louis, MO) supplemented with 15% heat-inactivated fetal bovine serum (FBS), (Sigma-Aldrich), Earle's salts, reduced NaHCO3, and 1% antibiotic/antimycotic solution with 10,000 U penicillin, 10 mg streptomycin, and 25 μg amphotericin B per ml (Sigma-Aldrich) without L-glutamine (EMEM diluent) and tested for the presence of virus on Vero cell monolayers by plaque assay.
Experimental Animal Models and Vector Competence Studies.
Young (1- to 2-d-old) chickens (Gallus gallus L.) were purchased from a commercial chicken breeding farm in Nairobi and transported to KEMRI. Chickens were inoculated subcutaneously with 104.3 or 106.3 plaque-forming units (PFU) of stock WNV in a total volume of 0.1 ml (105.3 or 107.3 PFU/ml). Chickens were maintained on starter food and water ad libitum. Individual chickens were bled (0.1 ml) from the jugular vein at 24-h intervals to determine a virus replication curve. Additional inoculated chickens were not handled after being infected to serve as handling controls to determine mortality associated with WNV infection in these chickens. At 24 and 48 h after infection, individual chickens were restrained and placed on cages containing the field-collected mosquitoes. To ensure that the various mosquito species received the same virus exposure, nonfed females of several species were combined into a single cage before feeding on the infected chicken. Immediately after mosquito feeding, 0.1 ml of blood was obtained from the jugular vein of each chicken by using a 27 gauge needle and added to 0.9 ml of EMEM diluent. After being bled, chickens were euthanized with CO2. The blood suspensions were frozen at −70°C until tested for virus by plaque assay to determine the viremias in the chickens at the time of mosquito feeding. Engorged mosquitoes were transferred to either 0.9- or 4-liter screen-topped cardboard or plastic cages, depending on the number of engorged mosquitoes, and held at 27°C at a photoperiod of 12:12 (L:D) h. After an incubation period of 7–21 d, they were allowed to refeed individually on 1- to 2-d-old naïve chickens to determine if they could transmit virus by bite. Immediately after the transmission attempt, the mosquitoes were killed by freezing, identified to species using keys by Edwards (1941) and Jupp (1986), and their engorgement status determined. The legs and bodies of each mosquito were triturated separately in 1 ml of EMEM diluent and frozen at −70°C until assayed for WNV by plaque assay on Vero cell monolayers. Infection was determined by detecting virus in the mosquito tissue homogenates. If virus was detected in its body, but not its legs, the mosquito was considered to have a nondisseminated virus infection limited to its midgut. In contrast, if virus was found in both the body and leg homogenates, the mosquito was considered to have a disseminated infection (Turell et al. 1984). We defined the infection rate as the percentage of mosquitoes tested that contained virus in their body and the dissemination rate as the percentage of infected (body positive) mosquitoes that contained virus in their legs, respectively. Chickens used in the transmission attempts were bled from the jugular vein 1 d after mosquito feeding and the blood screened for virus as described above. Based on previous studies (Turell et al. 2000), infected chickens should have a viremia of >105 PFU/ml at this time. Detecting virus in these blood samples indicated transmission.
To examine for the presence of a salivary gland barrier in mosquitoes with a known disseminated infection, some of the mosquitoes that did not feed on the viremic chickens were inoculated intrathoracically (Rosen and Gubler 1974) with 0.3 μl of virus suspension containing 106.3 PFU of WNV/ml) (102.6 PFU/mosquito) and allowed to feed on 1- to 2-d-old chickens 14 d later. Mosquitoes and blood samples from these chickens were processed as described for the orally exposed mosquitoes. Infection and dissemination rates were compared by Fisher exact tests at the 95% confidence level.
Results
Chicken Viremias.
All 1 or 2-d-old chickens inoculated subcutaneously with 105.3–7.3 PFU/ml (104.3–6.3 PFU/chicken) became viremic, with viremias peaking 2–3 d after infection (Table 1). Inoculation with a higher dose was associated with a slightly more rapid rise in titer and 100% (three of three) fatality within 5 d of those chickens inoculated with 106.3 PFU, whereas only three of five inoculated with the lower doses died or were euthanized when ill. Viremias in the eight chickens used to expose mosquitoes to WNV ranged from 105.8 to 107.2 PFU/ml of blood.
Viremia profiles in chickens inoculated with a Kenyan strain of West Nile virus
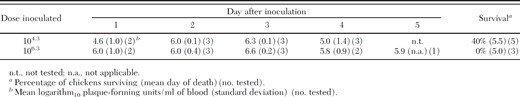
Viremia profiles in chickens inoculated with a Kenyan strain of West Nile virus

Susceptibility to Infection.
All five mosquito species were susceptible to infection with WNV after feeding on a viremic chicken with infection rates ranging from 42 to 82%. Because infection and dissemination rates were not statistically different between the field-collected or laboratory-reared Cx. quinquefasciatus (Fisher exact test, P ≥ 0.28), data from these two groups were combined for analysis. For each species, infection rates were not significantly different (Fishers exact test, P ≥ 0.17) for specimens tested 7, 13–14, or 20–21 d after feeding on the eight viremic chickens (data not shown). Infection rates for four of the species (Cx. univittatus, Cx. vansomereni, Ma. africana, and Ma. uniformis) were similar and ≈50% (Table 2). However, infection rates in Cx. quinquefasciatus were significantly higher than those for each of these species (Fisher exact test, P ≤ 0.03).
Infection and dissemination rates for mosquitoes after feeding on chickens with viremias of 105.8−7.2 PFU/ml
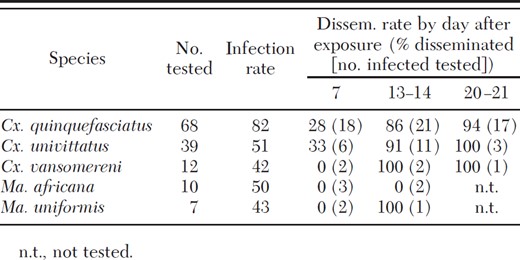
Infection and dissemination rates for mosquitoes after feeding on chickens with viremias of 105.8−7.2 PFU/ml

Dissemination of WNV to the Hemocoel.
Disseminated infections were detected in four of the five species tested, and while we did not detect virus in the legs of Ma. uniformis, we were only able to test two infected specimens ≥14 d after the infectious blood meal. For those species that produced disseminated infections, dissemination rates at days 13–21 were greater than those at day 7, with <30% of the infected specimens having virus detectable in their legs at 7 d after the viremic blood meal as compared with >90% at ≥13 d (Table 2).
Transmission of WNV.
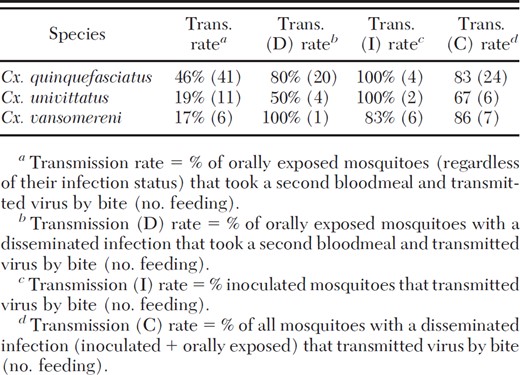
Each of the three Culex species successfully transmitted WNV, with ≈80% of the Culex mosquitoes with a disseminated infection transmitting WNV, regardless of species (Table 3). For each of these species, the percentage of orally exposed mosquitoes with a disseminated infection that transmitted virus was similar to that of those mosquitoes inoculated with virus.

Discussion
This is the first report on the ability of Kenyan mosquitoes to transmit WNV. All five mosquito species tested (Cx. quinquefasciatus, Cx. univittatus, Cx. vansomereni, Ma. africana, and Ma. uniformis) were susceptible to infection after feeding on a viremic chicken, and all three Culex species tested were moderately efficient vectors of WNV, with infection, dissemination, and transmission rates similar to those reported for Cx. quinquefasciatus (Jupp and McIntosh 1970) for specimens from South Africa and for Cx. pipiens and Cx. quinquefasciatus from the United States (Sardelis et al. 2001; Turell et al. 2000, 2001). However, the Cx. univittatus in our study were less susceptible than those from specimens collected in South Africa (Cornel and Jupp 1989, Cornel et al. 1993). Similarly, for the three Culex species tested, nearly all mosquitoes with a disseminated infection transmitted virus by bite, indicating a lack of a major salivary gland barrier (Hardy 1988). However, as with North American Culex (Turell et al. 2000, 2001; Sardelis et al. 2001), there appeared to be a time sensitive midgut escape barrier (Kramer et al. 1981) that was the principle determinant of the ability of these mosquitoes to transmit WNV by bite. Infection rates in Cx. quinquefasciatus were significantly higher than those observed in Cx. univittatus and Cx. vansomereni, indicating a greater midgut infection barrier in the latter two species. However, this was partially compensated by the slightly higher dissemination rates in the Cx. univittatus and Cx. vansomereni tested ≥13 d after virus exposure than in the Cx. quinquefasciatus tested at the same time period. Thus, Cx. quinquefasciatus, Cx. univittatus, and Cx. vansomereni should each be considered as potential vectors of WNV in Kenya.
Cx. univittatus is considered to be the principle WNV vector in much of Africa and the Middle East (Hubálek and Halouzka 1999). In Kenya, WNV was isolated from a male Cx. univittatus (Miller et al. 2000), indicating the ability of this species to transmit WNV vertically. There have also been recent detections of WNV antibodies from serum samples obtained from birds collected from the Marigat and Kisumu districts, including both resident (e.g., African mourning dove, Streptopelia decipiens Hartlaub and Finsch; Long-toed Plover, Vanellus crassirostris Hartlaub; and Greater Painted Snipe, Rostratula benghalensis L.) and migrant birds (S. L., unpublished data), suggesting continued low level circulation of WNV in various parts of Kenya.
This is the first reported attempt to evaluate the vector competence of Mansonia spp. with WNV in the laboratory. Both Ma. africana, and Ma. uniformis became infected when fed on a viremic chicken, and the single infected Ma. uniformis tested at 14 d after virus exposure did have a disseminated infection. However, because that mosquito and none of the inoculated ones fed on a naive chicken, it was not possible to tell if either of these species was actually able to transmit WNV by bite. However, based on the isolation of WNV from Ma. uniformis captured in Ethiopia (Hubálek and Halouzka 1999) and from multiple pools captured in Mauritania (Diallo et al. 2005) and the single demonstration of a disseminated infection in this study, these species should be considered as possible bridge vectors. Because of the lack of actual transmission data, additional studies are needed to determine whether these or other species are involved in the transmission cycle of WNV in Kenya.
In conclusion, this is the first report of the ability of Kenyan mosquitoes to transmit WNV. The collection of WNV seropositive birds and occasional isolation of WNV from mosquitoes indicates the continued presence of this virus in Kenya. Some limited serological surveys conducted in North Eastern Kenya have indicated significant exposure of human populations to WNV in that region (R. S., unpublished data). Therefore, additional information about potential vectors is needed to develop an appropriate and targeted response plan in the case of an outbreak or spread of infection with WNV. However, the ability of a mosquito to actually transmit a virus is only one factor in determining how important it will be in transmitting that virus in nature. Additional factors that need to be considered, especially when determining an appropriate disease suppression plan, include population density, host feeding preferences, availability of susceptible amplifying hosts, and association with human populations.
Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The views of the authors do not necessarily reflect the position of the Department of Defense or the Department of the Army. The procedures employed in this study were in compliance with KEMRI Health, Safety and Environment Advisory Committee approved standard operating procedures for work with West Nile virus at Biosafety Level two in Nairobi, Kenya.
Acknowledgments
We thank Dunstone Beti and Reuben Lugalia (KEMRI) for assisting in the collection of the mosquitoes from the field and for identifying the mosquitoes; Johnston Ingonga and Francis Mulwa (KEMRI) for their assistance in rearing the mosquitoes; the ICIPE for providing the Cx. quinquefasciatus mosquitoes from their colony for use in this study, and Caroline Ochieng (Walter Reed Project-GEIS program) for sequencing the strain of WNV used in this study. We also thank Kathy Kenyon (USAMRIID) for editorial suggestions. This study was made possible through the financial support provided for by USAMRU-K, Global Emerging Infections Surveillance and Response (GEIS), whom we also thank.
References Cited



