-
PDF
- Split View
-
Views
-
Cite
Cite
Rachel M. Mohr, Bradley A. Mullens, Alec C. Gerry, Diel Patterns of Female Host Seeking, Male Swarming, and Sugar Feeding in Fannia conspicua (Diptera: Muscidae) in Southern California, Journal of Medical Entomology, Volume 48, Issue 2, 1 March 2011, Pages 188–195, https://doi.org/10.1603/ME10135
Close - Share Icon Share
Abstract
Adult Fannia conspicua Malloch were captured by sweep net at a southern California coastal mountain community to determine diel flight activity and crop/gut sugar (gut sugar) concentration. Male swarming activity was monitored by visual estimation of swarm numbers. Gut sugar content of captured flies was determined by cold anthrone assay. Peak host seeking by female flies generally occurred in early morning (0700–0800) and early evening (1900–2000). Variation in female host-seeking activity was significantly explained by the time elapsed since sunrise or time remaining until sunset, with temperature, humidity, and wind speed having small, but significant effects on activity. Male swarming activity occurred more generally throughout the day, with peaks in mid-morning and mid-afternoon and reduced swarming during periods of highest female host-seeking activity. Male swarming behaviors were only minimally explained by environmental variables. Flies of both sexes commonly fed on fructose sugars with 99.94% of host-seeking female flies (n = 1,647), and 98.93% of swarming male flies (n = 1,398) with gut sugar levels exceeding those of starved flies. Host-seeking female flies had significantly higher overall sugar content than swarming male flies. Male flies had peak gut sugar levels at 0800–0900 and 2000. Female flies had broad peaks in gut sugar level from 0700 to 1200 and 1600 to 1900. Stepwise regression showed that variation in gut sugar level was poorly explained by environmental variables for both sexes.
During the summer months, “canyon flies” of the Fannia benjamini Malloch complex are encountered frequently in the dry canyon areas of inland and coastal southern California (Chillcott 1960). One member of this complex, Fannia conspicua Malloch, emerges in large numbers from May through July (Mullens and Gerry 2006). Adult female flies swarm around humans and animals intermittently, attempting to land and feed on sweat, tears, or other skin secretions (Garcia and Radovsky 1962). Adult female flies also respond positively to traps baited with mammalian host odors, including carbon dioxide and ammonia (Gerry and Mullens 2006, Mohr et al. 2011). Although they do not seem capable of piercing vertebrate skin on their own, the persistent efforts of these flies to land on a host render them extremely irritating and make outdoor activities unpleasant or unbearable during periods of peak fly activity. Although F. conspicua has not yet been implicated in the transmission of any pathogens, another member of the species complex is capable of transmitting a nematode eyeworm in the genus Thelazia (Turner 1976). F. conspicua is an emerging pest, primarily because of larval development within the thatch accumulation of the introduced landscape plant known as red apple (Aptenia cordifolia [L.f.] Schwant) (Mullens and Gerry 2006). Red apple is widely planted for erosion and fire control in southern California and is especially common on hillside slopes of many coastal mountain communities.
Gerry and Mullens (2006) observed that diel host seeking by female F. conspicua appeared to be bimodal, with peak activity in early morning and early evening. However, this observation occurred on a single day during the peak activity season. Cyclic patterns of behavior are common among host-seeking insects (Foster 1995) and can be affected by physiologic state (Browne 1993, Gary and Foster 2006) or by environmental factors (Grimstad and DeFoliart 1975). In many fly species, sugar feeding is also known to exhibit a daily cycle that may coincide with other flight behaviors such as host seeking or swarming (Yee and Foster 1992).
Sugar feeding is widespread among hematophagous insects, with sugar providing the necessary flight energy for activities such as host seeking, finding oviposition sites, and swarming (Hocking 1953). Among adult mosquitoes, sugar feeding is fundamental, serving as the basic food for both sexes (Foster 1995). Sugar feeding by flies also increases survival of both sexes, and may be a way to increase male reproductive fitness by increasing their ability to inseminate mates (Stone et al. 2009). Sugar feeding has also been documented among other host-seeking flies, including Ceratopogonidae (Mullens 1985), Tabanidae (Magnarelli et al. 1979), Simuliidae (Burgin and Hunter 1997b), and Muscidae (Jones et al. 1985).
Knowledge of a pest species' biological cycles and the physiological or environmental influences of these cycles can aid in developing control measures by identifying periods when these pests can be avoided or when they might be more susceptible to available control tactics. This study examined the timing and environmental factors affecting three important behaviors of F. conspicua: female host seeking, male swarming, and sugar feeding by both sexes.
Materials and Methods
Study Site.
The study took place on the crest of a prominent hill in the city of La Habra Heights, Los Angeles County (33°57′N, 117°57′W, 280 m elevation). This location was well known to have high adult F. conspicua activity and minimal human activity that might disrupt sampling (Mullens and Gerry 2006). Notably present near the sampling site was a large amount of the red apple plant that serves as an immature development site for F. conspicua in southern California.
Collection and Observation.
Host-seeking female flies were captured by sweep net at a single location at the crest of the hill. Male flies were collected from a recurring swarm site under the eaves of a garden shed in close proximity to the hilltop. Two additional sites of recurring swarms of male F. conspicua in the near vicinity were observed for swarm size. The observed male swarm sites were located on the steeply sloped eastern face of the hill, along fences planted with red apple and separated from each other by ≈15 m. One swarm was in a sunny location, and the other in a shady location near the foot of a coast live oak (Quercus agrifolia Née). Swarming male flies at both sites exhibited similar behaviors with roughly oblong swarms 0.25–1.5 m above the red apple groundcover. Sampling and observation sites were within 25 m of one another.
Flies were observed and collected over 11 d between 8 June 2006 and 25 July 2006, generally under the warm, dry, sunny conditions typical of the area for this time of year. Special effort was made to include study days featuring unusual weather patterns including morning fog, precipitation, and extreme heat. Host-seeking female F. conspicua were captured by sweep net each hour from 0700 to 2000, 60–80 min after sunrise (0540–0558) through sunset (2002–1958). Based on trial sampling and Gerry and Mullens (2006), this encompassed essentially the entire adult activity period. Sampling consisted of 5-min collections taken at the beginning of each hour using a short-handled sweep net (45 cm diameter) to capture female flies attracted to the vicinity of the researcher. Immediately after female sampling, the two male swarms were observed to estimate male swarming activity. While standing ≈2.5 m from each swarm, the swarm size within a 1.5-m3 area was estimated on a categorical scale of 0–4 (0 flies, 1–5 flies, 5–10 flies, 10–20 flies, >20 flies). Up to 30 male flies were then collected from a third nearby swarm with a short-handled sweep net. Flies captured during each collection period were killed immediately by freezing with dry ice. Flies then were placed in a labeled vial and stored in a cooler filled with dry ice until they were returned to the laboratory to be stored at −30°C before identification (Turner 1976).
Ambient temperature, humidity, and wind speed were recorded after each sampling period. For each collection, the time elapsed since sunrise (morning collections) or time remaining until sunset (evening collections), based on official sunrise and sunset times from the United States Naval Observatory, was calculated and denoted crepuscular (crep) time. Crep time was used instead of crep units (Grimstad and DeFoliart 1975) because crep time is an exact measurement of time (min) from sunrise/sunset rather than a proportionate one, and thus was more appropriate for regression analysis.
After identification, 20 F. conspicua of each sex were randomly selected from each hourly field collection for analysis of crop/gut sugar (gut sugar) content. If a collection contained <20 flies of either sex, all the flies from that collection were used. Before analysis, flies were decapitated so that the heads might be used in a separate study. The heads were assumed to contain negligible amounts of sugar. No attempt was made to locate the actual source of the sugar within the body of the fly (e.g., in the crop or midgut), as detecting the presence of consumed sugar was sufficient to quantify sugar-feeding behavior.
Sugar Analysis.
The cold anthrone reagent technique, described by van Handel (1985) to quantify fructose within an insect gut, was adapted for analysis of individual F. conspicua. The reagent was prepared by mixing 1.94 g anthrone (Sigma-Aldrich, St. Louis, MO) with 1 liter 70% sulfuric acid and chilled to ≈2.5°C. Individual decapitated flies were placed into microcentrifuge tubes and macerated with a pestle grinder in 0.2 ml of 2% sodium sulfate solution. After grinding, 0.8 ml of reagent-grade methanol was added to each tube. Tubes were then vortexed for 5 s before being centrifuged at 8,160 × g for 8 min. A 0.2-ml aliquot was taken from each tube and placed into a 1.5-ml disposable plastic microcuvette, to which 1 ml of the cold anthrone reagent was then added. For the blank, 1 ml of anthrone reagent was added to 0.2 ml of methanol. After 45 min of room-temperature incubation, microcuvettes were vigorously agitated and absorbance was measured at 625 nm on a UV-VIS spectrophotometer (Spectronic 20; Milton Roy, Rochester, NY).
To establish baseline gut sugar content for starved F. conspicua, caged flies from a laboratory colony were allowed to emerge from pupae in the absence of food or water. At 24 h postemergence (22–24°C), 20 starved flies were killed, decapitated, and subjected to the anthrone assay. The 95th percentile absorbance of these flies was taken as the cutoff value for “sugar fed” in the field-collected flies. To determine the rate of diurnal sugar metabolism by F. conspicua, 4- to 5-d-old laboratory-reared adult flies were starved for 30 h, before being fed a 20% sucrose solution. Flies with visibly distended abdomens were assumed to have fed to repletion. Fed flies were divided into cohorts (n = 5) that were killed by freezing at 1, 2, 3, 4, 5, or 6 h after feeding. These flies were subjected to the anthrone sugar assay, as described above, and the measured absorbance values were subjected to linear regression to approximate the rate of sugar digestion.
To estimate fructose content from absorbance, standard solutions of 25, 50, 100, 150, and 250 μg/ml aqueous fructose solutions were prepared and subjected to the cold anthrone assay, as above, using deionized water as the blank. The absorbance values for each concentration (n = 5) were used to calculate a linear regression equation relating absorbance to fructose concentration.
Statistical Analysis.
To compensate for the seasonal change in population size, the number of female flies collected during each sampling period was transformed into a proportion of overall daily catch. Missing values (n = 4) were estimated using the mean of the catch at the same collection time from the sampling day immediately preceding and immediately after the collection date with the missing value. Estimation of missing data was necessary for the proportional transformation; however, estimated values were excluded from further analysis. To examine differences in female host seeking by time of day, proportional data were arcsine square root transformed to stabilize variance before analysis using a one-way analysis of variance, with means separated by Tukey's honestly significant difference test (SPSS 15.0; SPSS, Chicago, IL). Univariate regression analysis was used to calculate a preliminary model estimating female host-seeking activity (fly capture), using relative time of day (morning [700–1000], midday [1100–1600], afternoon [1700–2000]), month of collection, occurrence of precipitation, measured environmental and temporal values, and all two- and three-way interactions as variables in the analysis. The model was then refined to eliminate nonsignificant variables, using lowest mean square error as the comparison criteria between intermediate models. A full factorial analysis of covariance using relative time of day as the fixed factor, and crep time, temperature, and humidity as covariates was used to examine the differences between regression coefficients for each time of day.
Because of the large number of ties in swarming activity scale values, differences in male swarm size between sites (sun versus shade) could not be evaluated on a per-hour basis. Data from both swarm sites were therefore combined, and differences in swarming activity between collection periods were examined using the Friedman test with median rank difference separated by a post hoc Dunn's multiple comparison test. With broad male activity peaks suggested by the Friedman test, swarming activity was concatenated into five groups (0700–0900, 1000–1200, 1300–1600, 1700–1900, and 2000), and differences between these groups were examined using a Kruskal-Wallis test with median ranks separated by a post hoc Dunn's multiple comparison test. To reduce the chance of type I error, α was set at 0.01 for the nonparametric post hoc tests (GraphPad InStat 3.0; GraphPad Software, LA Jolla, CA). Binary and multiple logistic regression was used to relate environmental conditions to either the presence/absence (binary) or level of male swarming (multiple; SPSS 15.0).
Differences in gut sugar content between male and female flies captured during the same collection periods were examined using a series of Mann–Whitney U tests. Within sexes, differences between collection periods were examined using one-way analysis of variance (SPSS 15.0). To calculate a best-fit model from the measured environmental variables, absorbance values of flies that met the sugar-fed criterion were examined by linear regression for both male and female flies.
Results
General Weather Conditions.
Over the course of the study, mean daytime temperatures ranged between 24°C and 33°C, with maximum and minimum recorded temperatures of 38.6°C and 18.4°C, respectively. Temperatures were typically lowest in the first 3 h of sampling, increasing to a peak near 1400, and then abated somewhat from 1800 to 2000. Humidity was highest from 0700 to 0900, typically from 65 to 95%, then decreased into the 30–50% range for much of the rest of the day. Dates with environmental conditions differing markedly from that described above were 8 and 21 June. On 8 June, there was light precipitation between 0630 and 0845, with heavy cloud cover until ≈1200, which kept temperatures lower and humidity higher than on any other date. On 21 June, morning fog kept temperature low until around 1100, after which the temperature rapidly increased to a maximum typical of the rest of the study.
Female Host Seeking.
Female host-seeking activity was significantly different across sampling periods (F = 26.778; df = 13, 194; P < 0.0001), with activity peaks near sunset and sunrise (Fig. 1). Post hoc analysis separated mean host-seeking activity for sampling periods into four homogenous groups (P < 0.05). Host-seeking activity was greatest for time periods in group 1, closest to sunrise and sunset (0700–0800, 1900–2000), than for time periods in group 2 (0700–0900, 1800–1900), group 3 (0900–1000, 1800), or group 4 (1000–1700). Groups 2 and 3 encompassed the late morning and mid-afternoon transitional periods between peak and nonhost-seeking activity. This strong diel pattern is consistent with previous studies (Gerry and Mullens 2006).
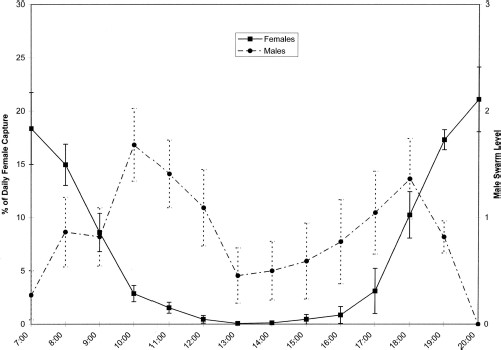
Mean ± SEM percentage of daily capture of host-seeking females (solid line) plotted with mean ± SEM male swarming activity averaged for both sun and shade swarm sites (dotted line) for each collection period. Swarm level determined by the number of males observed within a 1.5-m3 area of a swarm on a categorical scale of 0–4 (0 flies, 1–5 flies, 5–10 flies, 10–20 flies, >20 flies).
Wind speed, month of collection, incidence of precipitation, and all but two interaction effects were eliminated as significant variables from the initial univariate analysis (P > 0.05). Female host seeking was best explained by analysis of covariance with relative time of day as a fixed factor and the covariates crep time, temperature, and humidity (F = 46.81; df = 9, 140; P < 0.001; R2 = 0.751) (Table 1). Crep time proved to be the dominant variable explaining most of the variation in female host-seeking activity over the entire study period, with a partial η2 of 0.229. Relative time of day was also important, with a partial η2 ranging from 0.048 to 0.324 (Table 1). There was a significant interaction between relative time of day and crep time (P < 0.0001), with crep time being important to the model in the morning and evening, but not midday. Although humidity was not a significant predictor by itself (P = 0.061), it had a significant interaction with time of day, with only morning humidity affecting female host-seeking activity. The effect of temperature on female host seeking was highly significant to the regression model (P = 0.002), but there was no significant interaction between temperature and relative time of day in the initial or intermediate analyses (P ≥ 0.132).
Univariate regression of female host-seeking activity with time of day (morning, midday, afternoon) as the fixed factor and covariates crep time, temp, and humidity
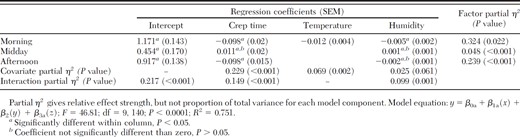
Univariate regression of female host-seeking activity with time of day (morning, midday, afternoon) as the fixed factor and covariates crep time, temp, and humidity

Male Swarming.
Like female host seeking, male swarming followed a bimodal diel pattern, albeit a less pronounced one, with peak activity occurring during mid-morning and late afternoon (Fig. 1). There were significant differences in swarming activity between collection periods with male swarming activity at 1000 significantly greater than at 0700, 1300–1500, or 2000 (Fr = 99.85; df = 13, 307; P < 0.0001). When the data were concatenated into five time groups, late morning (1000–1200) and afternoon swarming (1600–1900) were significantly greater than early morning (0700–0900), midday (1300–1500), or evening (2000) swarming (KW = 56.653; df = 4, 307; P < 0.01). Multiple logistic regression showed that crep time, temperature, and wind speed were significant predictors of male swarming (−2 log likelihood 600.947, χ2 = 70.076, df = 12, P < 0.001; Cox and Snell pseudo-R2 = 0.208). Overall fit was poor, though, with 55.0% of all swarming and only 13.3% of nonzero swarm levels correctly predicted by the model. Using the same set of significant predictors, binary logistic regression for the simple presence/absence of a male swarm was less precise (−2 log likelihood 352.755, χ2 = 48.472, df = 3, P < 0.001; Cox and Snell pseudo-R2 = 0.149); however, it did correctly predict 64.7% of swarming incidence.
Sugar Feeding.
For never-fed adult flies, 95% of flies subjected to the cold anthrone test had an absorbance value of ≤0.004 at 625 nm. Of field-collected female flies, 99.94% (n = 1,646/1,647) and 98.93% of male flies (n = 1,391/1,406) had an absorbance value >0.004, and thus were considered to have fed on sugar. Diurnal sugar metabolism given by the decay rate of light absorbance after the cold anthrone test as a function of time (h) postfeeding was y = 0.442–0.057 [time] time (R2 = 0.454), indicating complete sugar metabolism by ≈8 h postfeeding. However, the highest absorbance measured for these laboratory-fed flies was 0.62, substantially lower than the highest absorbance value observed for field-collected flies of 1.40, but considerably above the mean absorbance value measured for both male and female flies during any time period (Fig. 2). The relationship of fructose content (μg) to absorbance at 625 nm for standard fructose concentrations was very strong, y = (514.07x + 0.1995) (R2 = 0.9983).
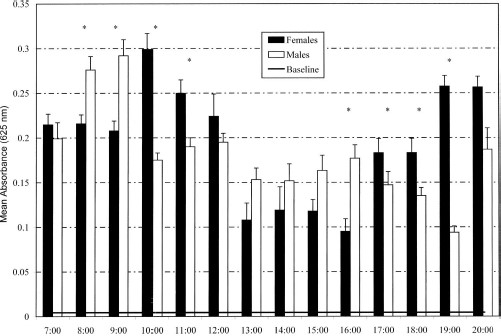
Mean absorbance (625 nm) ± SEM indicating crop/gut fructose concentration of male and female flies. The baseline value is the absorbance value encompassing the 95th percentile of never-fed flies. Asterisk indicates significant difference between sexes at P < 0.05.
Female flies had the lowest gut sugar content during early afternoon to mid-afternoon, with gut sugar significantly higher at mid-morning (1000) and late afternoon (1900–2000) (F = 9.01; df = 13, 1,657; P < 0.001) (Fig. 2). In contrast, males had a significantly higher gut sugar content in early morning (0800–0900) (F = 16.793; df = 13, 1,398; P < 0.001). Comparing gut sugar content between the sexes for each collection period, males had significantly higher gut sugar content relative to females during the early morning hours (0800–0900) and mid-afternoon (1600), whereas female flies had significantly higher gut sugar content during mid-morning (1000–1100) and late afternoon (1700–1900) (Z ≤ −3.35; df ≥ 68; P < 0.001 for significant pairwise tests). Linear regression of the measured environmental variables explained very little of the variance in gut sugar content of both male and female flies (R2 values ≤0.106).
Discussion
The host-seeking behavior of F. conspicua appears to be driven predominantly by the position of the sun above the horizon. Host-seeking activity is greatest when the sun is near the horizon, with an increase in solar height, as measured by crep time in this study, resulting in reduced activity. Crep time was a significant predictor of female activity only during periods when the sun was near the horizon. Crepuscular behaviors occurring at sunrise and/or sunset are common among hematophagous Diptera (Barrozo et al. 2004). However, such behaviors may still be strongly affected by environmental influences (Varley et al. 1973).
Temperature had a significant influence on female host-seeking activity. Tabanid host-seeking activity is similarly affected by temperature (Alverson and Noblet 1977, Cilek and Schreiber 1996). For F. conspicua, temperature seemed to be particularly important during midday, when temperatures were near their daily peak. This is reflected in the negative coefficient for temperature in the regression model, which predicts a cessation of activity at ≈35–36°C during midday. This prediction matches with field observations, in which only three flies were collected when temperature exceeded 36°C. Qualitatively, on days with afternoon temperatures exceeding 36°C, evening host seeking was delayed somewhat, with decreased activity at 1800 as compared with other sampling days. The lack of interaction between temperature and relative time of day in the regression model suggests that flies responded to changes in temperature uniformly throughout the day. Indeed, this is borne out by collections on 8 July and 15 July, when temperatures remained in excess of 36°C until 1900, and very few flies were collected during evening periods that otherwise showed high activity.
Humidity had a small, but significant role in influencing morning host seeking, consistent with Alverson and Noblet's (1977) findings for female tabanids. Normal morning host-seeking activity was seen in the range of 48–65% RH. The very highest morning female host-seeking activity occurred on dates with very low morning humidity (28 and 30 June), whereas high morning humidity on 8 June (RH = 98–100%) resulted in delayed morning activity and increased activity during midday hours. Similarly, Aedes vexans (Meigen) is known to cease activity when relative humidity exceeds 70% (Platt et al. 1958). Wind speed was generally a poor predictor of female host-seeking activity. These results are consistent with Gerry and Mullens (2006), who found that wind speed did not have a significant impact on fly activity.
Male swarming activity also showed a bimodal diurnal activity pattern; however, in contrast to female host-seeking activity, peak swarming activity was broadly defined and was low when the sun was nearest to the horizon. The broad activity peak of swarming males may be an artifact of using categorical values instead of absolute counts for swarming males, but is more likely an accurate reflection of behavior that is less rigorously defined by environmental stimuli than it is by female host seeking. Male swarming peaked in the hours after early morning peak female host-seeking activity and again before peak female host seeking in the late afternoon.
Unlike female host seeking, variation in male swarming was explained poorly by the environmental variables measured. Female host seeking is predicted well by environmental variables, and therefore likely to be occurring at environmentally optimal times of day. With male swarming activity temporally offset relative to female host seeking, males are likely swarming at environmentally suboptimal periods to increase mating opportunities when females are more easily recruited to a mating swarm. Swarming is an energy-intensive activity for males (Yuval et al. 1994), and it would be wasteful to swarm at a time when females are looking for hosts or resting and not receptive to males. Male swarming persisted throughout the day, although it seems improbable that individual males engaged in continuous swarming given the high metabolic demands of flight (Hocking 1953).
The consumption of sugars is required for energy-depleting activities like insect flight (Downes 1958). The large numbers of F. conspicua testing positive for fructose in this study, 99.94% of females and 98.93% of males, illustrate that sugar feeding is common in this species. The proportion of flies containing gut sugar is much higher than reported for other host-seeking flies (Magnarelli et al. 1979, Kniepert 1980, Jones et al. 1985, Mullens 1985, Cilek and Schreiber 1996, Burgin and Hunter 1997a, Hunter and Ossowski 1999), perhaps because most studies have examined sugar feeding by obligate blood-feeding flies, which differ fundamentally from host-feeding flies like F. conspicua that feed on host secretions, but cannot pierce host skin. Female F. conspicua will readily feed on blood from existing wounds when available (Garcia and Radovsky 1962), but access to host blood probably occurs only rarely. If sugar feeding suppresses later protein ingestion (Spradbery and Schweizer 1979), then an obligate blood-feeding fly that would consume a substantial blood meal at one feeding may benefit by limiting sugar consumption in favor of a future blood meal. In contrast, host-seeking flies that are facultative blood feeders and do not require a large blood meal are more likely to present an opportunistic conditional-response paradigm for food acquisition (Foster 1995) with appetitive search for sugar or hosts governed by stimulus strength and the state of energy reserves. However, the high rate of sugar feeding shown for F. conspicua in this study relative to host-seeking insects previously examined may also be the result of use of a spectrophotometer to measure gut sugar concentration after the cold anthrone test rather than using the human eye to determine a radical color change as typically performed in other studies (e.g., Smith and Kurtz 1994).
Differences in gut sugar content by time of day for male and female flies can provide insight into sex-specific diel behaviors. Sugar feeding by male flies appears to occur during early morning, and perhaps in the late afternoon, when females are actively host seeking and male swarming is limited. Sugar feeding by female flies appears to occur after peak host-seeking activity in early morning or before peak host-seeking activity in late afternoon. High gut sugar concentration in the few female flies captured after the morning peak activity period may be the result of either a delay in host seeking of resource-limited females until after a successful search for a sugar source or perhaps a return to host seeking after a rapid and successful search for a sugar source after early morning host seeking. Low standard errors for absorbance between flies of the same sex for most collection periods over many collection days would seem to suggest that the majority of flies captured at each collection period have similar sugar content and probably similar sugar-feeding behavior and sugar-feeding success. This implies that the availability of sugar sources near the study site is not limited.
Interestingly, male swarming appears to coincide with periods of female sugar feeding. Male swarms were noted in this study to be above flowering plants or at the margin of shaded areas such as the entrance to a shed. Perhaps swarm locations are selected to encounter females seeking a sugar meal or entering and leaving a daytime resting site. With female host-seeking behavior apparently conducted at environmentally optimal times of day, the remaining female behaviors as well as all male behaviors are probably temporally determined by female host seeking.
Host-feeding flies must differentially allocate their meals according to need, and as a trade-off between short-term survival and long-term reproductive success (Foster 1995). For F. conspicua, feeding on sugar dramatically impacts longevity, but a source of animal protein such as raw liver juices is needed to produce large numbers of eggs (Mullens and Gerry 2006). Taking a sugar meal may increase survival enough to increase the chances of finding an animal protein source. Conversely, finding a protein source/host early may allow the female fly greater reproductive success. Temporal separation of specific resource-oriented behaviors within a diel period is primarily because of changes in priority given these resources as a function of need (Browne 1993). After a meal, behaviors oriented toward that food source are effectively eliminated for a period of time (Browne 1993). F. conspicua females show this type of temporally separated appetitive search behaviors, acquiring protein (host seeking) and carbohydrate (sugar feeding) at different times of day.
In documenting the patterns of protein- and sugar-oriented behaviors in F. conspicua, these results give insight into the biology and behavior of a considerable nuisance pest. They also suggest some potential avenues for addressing the annoyance caused by large populations of F. conspicua in affected communities of southern California. By documenting their daily behavioral patterns, in conjunction with Mullens and Gerry's (2006) documentation of seasonal behavior, it is possible to estimate the periods of highest fly activity. Flies can therefore be targeted for control when they are most susceptible to traps, pesticides, or other control techniques as part of an integrated pest management program. The dependence of F. conspicua on sugar as a food source might also suggest future control strategies to selectively target preferred sugar sources or perhaps to develop attract and kill technologies that use fly orientation to and feeding on these sugar sources. However, additional research to identify preferred sugar sources is required before these technologies could be implemented.
References Cited



