-
PDF
- Split View
-
Views
-
Cite
Cite
P. J. Obenauer, P. E. Kaufman, S. A. Allan, D. L. Kline, Infusion-Baited Ovitraps to Survey Ovipositional Height Preferences of Container-Inhabiting Mosquitoes in Two Florida Habitats, Journal of Medical Entomology, Volume 46, Issue 6, 1 November 2009, Pages 1507–1513, https://doi.org/10.1603/033.046.0639
Close - Share Icon Share
Abstract
To ascertain mosquito species-specific oviposition height preferences, a study was conducted evaluating the response of field populations of container-inhabiting mosquitoes to water, oak (Quercus spp.), or oak-pine (Pinus spp.) infusion-baited ovitraps in four suburban and four sylvatic habitats in north central Florida. In total, 48 ovitraps, 24 suspended at each height of 1 or 6 m (near the ground or tree canopy, respectively), were monitored weekly for 5 mo. Throughout our study, we collected 13,276 mosquito eggs, representing five species from four genera, the most common being Aedes triseriatus (Say), Aedes albopictus Skuse, and Orthopodomyia signifera (Coquillett). Significantly more Ae. triseriatus and Ae. albopictus eggs were oviposited in containers with oak and oak-pine infusions compared with water alone. Significantly more Ae. albopictus eggs were recovered from traps at 1 m in suburban habitats, whereas more Ae. triseriatus eggs were recovered at 6 m in sylvatic habitats.
The recorded introduction of invasive container mosquitoes such as Aedes albopictus (Skuse) (Hawley et al. 1987), Aedes bahamensis (Berlin) (Pafume et al. 1988), Aedes togoi (Theobald) (Belton 1980), and Aedes japonicus japonicus (Theobald) (Peyton et al. 1999) into the United States represents a serious threat to human and animal health because most are vectors of pathogens. Surveillance for these container-inhabiting mosquitoes often requires careful sampling of natural and artificial containers, because these are primary habitats for oviposition and larval development. However, searching for containers can be a time-consuming process, and many are overlooked or remain inaccessible, such as tree holes located in tree canopies. Ovitraps offer an alternative to searching for natural and artificial containers, because they can be set at specific locations and have proven to be a useful tool to detect the presence of mosquito species in particular habitats as well as to estimate adult population size (Service 1993). The rapid introduction of invasive container-inhabiting mosquitoes within the past 20 yr underscores the necessity for enhancement of ovitrap sensitivity to enable rapid detection and possibly prevent their establishment in new environments. To adequately determine the risk these species pose, to mitigate their impact on native mosquito populations, and to allow for an understanding of changes in existing disease transmission cycles, their oviposition habitat preferences must be better understood (Juliano and Lounibos 2005).
Typically, ovitraps are constructed from materials such as black plastic cups containing an oviposition substrate and positioned on the ground. This approach preferentially selects for mosquitoes that naturally oviposit near the ground but fails to collect those mosquitoes ovipositing at greater heights. The most common approach to deter many container mosquitoes from breeding within suburban areas is the removal of artificial containers from the surrounding environment. However, if some of these species oviposit in the tree canopy as well as at ground level, changes in vector surveillance and control strategies may be warranted. In addition, many suburban backyards in north central Florida contain natural and artificial containers that collect fallen leaves and pine needles from water oak, Quercus nigra L., and longleaf pine, Pinus palustris Mill, potentially creating more attractive larval habitats for a number of container-inhabiting species. Although many studies document enhanced oviposition responses to hay and leaf infusions (Holck et al. 1988, Allan and Kline 1995, Trexler et al. 1998), there is limited information on oviposition responses to detritus from coniferous trees.
The addition of infusions to ovitraps has been shown to increase trap effectiveness compared with water alone (Holck et al. 1988). Organic infusions, often composed of fermented plant material, attract gravid mosquitoes by volatiles released through microfauna fermentation (Trexler et al. 2003). Often, the types of materials used in an infusion increases the level of attractiveness for specific mosquito species. Hay (Allan and Kline 1995) and bamboo (Ponnusamy et al. 2008) are attractive to Aedes aegypti (L.), whereas white oak, Quercus alba L. (Trexler et al. 1998); red oak, Quercus rubra L. (Burkett et al. 2004); and maple, Acer buergerianum Miq. (Dieng et al. 2003), leaves are attractive to Ae. albopictus. Furthermore, oviposition response to these volatile attractants is often dependent on the microbial species contained in the infusion (Trexler et al. 2003).
There is also variability in the vertical stratification of oviposition among container-inhabiting mosquitoes. Studies within urban areas in the Republic of Trinidad determined that Ae. aegypti preferred to oviposit in traps placed 1.2 m above ground compared with ovitraps set at ground level, or traps that are elevated to 3.0 and 4.6 m above ground (Chadee 1991). In Sri Lanka, Amerasinghe and Alagoda (1984) demonstrated that although Ae. albopictus oviposited at 7 m, it preferred to oviposit at ground level. Studies in Wisconsin demonstrated that Aedes hendersoni (Cockerell) predominantly oviposits at 3–9 m above ground (Loor and DeFoliart 1969, Scholl and DeFoliart 1977). Our objectives were two-fold: 1) determine the effectiveness of ovitraps at detecting container-inhabiting mosquitoes at 1- and 6-m heights in two habitats and 2) determine if container-inhabiting mosquitoes demonstrate preferences for ovitraps containing oak or oak-pine infusions compared with water alone.
Materials and Methods
Site Selection.
Ovitraps were set from May to October 2008 in four suburban and four sylvatic sites previously described in Obenauer et al. (2009). Suburban habitat sites (29° 37.837′ N, 82° 27.800′ W; 29° 34.248′ N, 82° 24.644′ W; 29° 39.019′ N, 82° 23.234′ W; 29° 42.481′ N, 82° 24.745′ W) were residential neighborhoods in and around the city limits of Gainesville, FL, whereas sylvatic habitat sites (29° 43.574′ N, 82° 27.252′ W; 29° 44.048′ N, 82° 26.458′ W; 29° 43.574′ N, 82° 27.233′ W; 29° 44.238′ N, 82° 28.138′ W) were located throughout San Felasco Hammock Preserve State Park, also near Gainesville. All sylvatic sites were at least 0.8 km from any artificial residential structure. Both habitats contained deciduous and coniferous trees, primarily live oak, Quercus virginiana P. Mill; water oak; laurel oak, Quercus laurifolia Michx.; longleaf pine; and slash pine, Pinus elliottii Engelm.
Ovitraps.
A modified ovitrap, originally described by Weinbren and O'Gower (1966) to capture Ae. aegypti was used. Lidless steel cans (11 cm in height and 7.5 cm in diameter) were attached at three locations to an inverted circular aluminum dish (7.5-cm base and 12.5-cm outside diameter) by bending wires into a closed loop. The dish served as a cover to prevent leaves and other debris from falling into the trap, possibly altering the infusion. A 1-cm drain hole was made 7.5 cm above the bottom of the can, to prevent flooding. The entire ovitrap was spray-painted with flat black paint. To remove any paint odors or contaminants, ovitraps were preconditioned and aged by filling them with well water and letting them sit for 2 wk in a semishaded environment (Burkett et al. 2004). Seed germination paper (76#, Anchor Paper, St. Paul, MN) (22 by 8 cm) was pressed against the inside surface of each can to serve as an oviposition substrate. To suspend the ovitrap, an eyebolt was fitted through the top of the dish. A 168-g fishing weight was attached to the bottom of the eyebolt providing additional weight and stability. Each ovitrap was filled with 270 ml of either an infusion or well water. Infusions were generated by first diluting the concentrate to 35% (70 ml) with 200 ml of deionized water before filling the traps. This concentration was based on previous laboratory and field-cage studies demonstrating a high degree of attractiveness by using the same infusions (Obenauer 2009).
Infusions.
Infusions were developed by collecting fallen dry water oak (oak) leaves and longleaf pine needles (pine) from the grounds at the University of Florida, Gainesville, FL. Leaves and needles were visually inspected to ensure that they were free of foreign organic matter. Infusions were prepared by fermenting 120 g of leaves, 7 g of brewer's yeast (MP Biomedicals, LLC, Solon, OH), and 7 g of lactalbumin (Sigma-Aldrich, St. Louis, MO) in 12 liters of well water (Allan and Kline 1995). A 50:50 mixture (60 g of pine needles and 60 g of oak leaves) was used to develop the oak–pine infusion. Mixtures were held at ambient temperature between 25 and 27°C for 10 d in a sealed plastic bucket. Infusions were passed through sterile gauze dressing to remove large organic matter and transferred into plastic cups, sealed, and frozen at –20°C. When used, frozen aliquots were placed in a warm bath for 30 min or until melted and used within 4 h on the day of ovitrap collection. Four individual batches of oak and pine infusion were developed to ensure infusion replication throughout the experiment.
Trapping, Collecting, and Egg Identification.
Each of the eight sites (four suburban and four sylvatic) was partitioned into three stations. Stations were placed 20 m from each other and at least 10 m from residential structures at suburban sites. In total, 48 ovitraps were used during a given trapping period, with two traps placed at each of the three stations at each of the eight sites. At each station, one ovitrap was suspended at 1 m, with the second suspended at 6 m. Ovitraps at each station were baited with one of the three treatments: either an oak, oak–pine infusion, or a well water control. The three treatments were randomized at every collection period within each site to eliminate position or placement bias. Therefore, the three treatments were represented at each height at a given site and placement period.
Ovitraps were suspended using 6.35-mm-diameter interwoven nylon rope containing 1-m markings. Traps were set at 1 or 6 m by using a pulley system that was comprised of two ropes as described in Obenauer et al. (2009). Ovitraps placed at 6 m were suspended from a selected tree branch, whereas those at 1 m were suspended from a shepherds hook. Traps were set between 0800 and 1400 hours and left in place for 1 wk (one trapping period). At this time, the contents of each ovitrap were checked, and the infusion, water, and seed germination paper were replaced. A visual inspection for mosquito eggs and larvae was conducted for each ovitrap, and all seed germination papers were placed in sealed plastic bags for later identification and enumeration. Occasionally, some larvae had eclosed; these were counted, transferred to mosquito breeding containers (Bioquip, Rancho Dominguez, CA), and returned to the laboratory for further identification. To ensure that old infusion was not present, all ovitraps were thoroughly rinsed with deionized water to remove any organic matter before replacing with fresh infusion and paper.
A second inspection of the egg paper was conducted in the laboratory. Seed germination papers were placed on paper towels and allowed to dry for 2 h. Eggs were counted and identified to species under a dissecting microscope based on their color, size, luster, and shape. To ensure accuracy, a subsample (10%) of eggs from each collection period was reared to adults under laboratory conditions and positive identification was made using Darsie and Morris (2003). In addition, those mosquito larvae that were collected in the field were reared to adults and identified using the same methods. In total, 20 consecutive weekly trapping periods in each locale were conducted from 15 May to 3 October 2008.
Statistical Analysis.
A nested analysis of variance (ANOVA) was used to determine whether differences in mean mosquito egg capture were statistically different due to infusion batch, infusion type, and habitat:height (suburban or sylvatic) (Proc GLM, SAS Institute 2006). Data were transformed with log10(n + 1) before analysis. After it was determined that infusion batch was not statistically significant (P = 0.4837), it was removed from the model before final analysis. Infusion type, habitat:height, and the interaction term infusion type by habitat:height were included as fixed effects, whereas trial was included as a quantitative effect in the model because this variable was a measure of seasonal progression. In this analysis, the site variable was nested within the habitat:height variable. Multiple mean comparisons were made with the Ryan–Einot–Gabriel-Welsh multiple range test (α = 0.05). Untransformed means are presented in all tables. Storms caused damage to several ovitraps, resulting in lost data during a portion of the study. For statistical purposes, lost data were treated as missing values.
Results
In total, 13,276 mosquito eggs were collected, representing five species: Ae. albopictus, Aedes triseriatus (Say), Culex quinquefasciatus (L.), Orthopodomyia signifera (Coquillett), and Toxorhynchites rutilus rutilus (Coquillett) (Table 1). The majority of eggs collected during this study from ovitraps at all habitats and heights were Ae. triseriatus (6,275) (Table 1). However, eggs of this species were not recovered from any ovitrap until the seventh week of the study (4 July), with the greatest number (1,457) recovered on 18 July. The majority of Ae. triseriatus eggs (45.0%) were recovered in ovitraps containing oak-pine infusions, with only 36.7 and 17.7% of eggs from ovitraps containing oak infusion or water, respectively. Although no significant difference was detected between infusion-containing treatments, significantly fewer eggs were recovered in ovitraps containing only water (3.60 ± 1.00) compared with traps containing oak-pine (9.20 ± 1.80) or oak (7.40 ± 1.61) (F = 5.88; df = 2, 910; P = 0.0020) infusions (Table 2). Significantly more Ae. triseriatus eggs were recovered from ovitraps placed in sylvatic habitats at 6 m (17.90 ± 2.97) than those sylvatic habitats at 1 m (5.44 ± 1.27), or suburban habitats at 6 or 1 m (1.29 ± 0.70 and 2.63 ± 0.96, respectively) (F = 30.17; df = 3, 910; P < 0.0001) (Table 3). Although no discernible differences in egg collection was detected between suburban habitats at 1- and 6-m heights, more eggs were recovered in ovitraps placed in sylvatic habitats at 1 m than those placed at the same height in suburban habitats (Table 1). Significant differences were detected among sites within sylvatic habitat:height treatment effect (F = 5.90; df = 12, 910; P < 0.0001), with more Ae. triseriatus eggs collected in traps located at sites furthest from urbanized development, including highways.
Total number and percentage of Culicidae eggs captured using ovitraps baited with infusionsa or well water and suspended at 1- and 6-m heights in suburban and sylvatic locales from May to October 2008 in Gainesville, FL
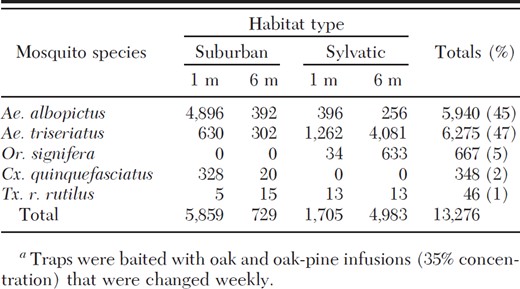
Total number and percentage of Culicidae eggs captured using ovitraps baited with infusionsa or well water and suspended at 1- and 6-m heights in suburban and sylvatic locales from May to October 2008 in Gainesville, FL

Numbers (mean ± SE) of mosquito eggs collected per trapping period from infusion-baited ovitrapsa placed at 1- and 6-m heights in suburban and sylvatic locales from May to October 2008 in Gainesville, FL
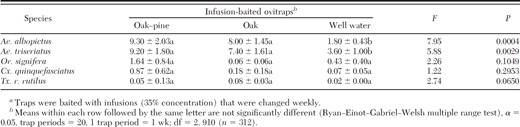
Numbers (mean ± SE) of mosquito eggs collected per trapping period from infusion-baited ovitrapsa placed at 1- and 6-m heights in suburban and sylvatic locales from May to October 2008 in Gainesville, FL

In total, 5,940 Ae. albopictus eggs were collected during this study (Table 1). Almost half (48.8%) were oviposited in traps containing an oak-pine infusion. In suburban sites, 5,288 Ae. albopictus eggs were recovered: 50% were taken from oak-pine-baited ovitraps, 39% from oak-baited ovitraps, and 9% from the water control. Of the 652 Ae. albopictus eggs recovered from sylvatic sites, 58% were deposited in oak-baited ovitraps, whereas 33 and 7% were recovered from oak-pine and water-baited ovitraps, respectively.
Although no significant difference were detected between oak-pine and oak infusions, significantly more Ae. albopictus eggs were oviposited in traps containing infusions than those with only water (F = 7.95; df = 2, 910; P = 0.0004) (Table 2). A significant interaction effect was detected between the infusion type and habitat:height treatment effects (F = 4.67; df = 6, 910; P < 0.0001). Significantly more Ae. albopictus eggs were recovered from ovitraps placed in suburban sites at 1 m than any other location (F = 79.31; df = 3, 910; P < 0.001) (Table 3). Only 13% of recovered Ae. albopictus eggs were from sylvatic habitats.
Numbers (mean ± SE) of mosquito eggs collected per trapping period using ovitraps baited with infusionsa or well water and suspended at 1- and 6-m heights in suburban and sylvatic locales from May to October 2008 in Gainesville, FLb
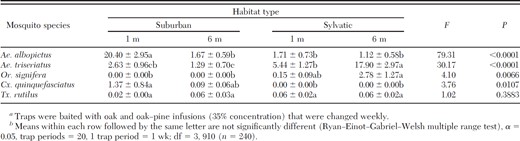
Numbers (mean ± SE) of mosquito eggs collected per trapping period using ovitraps baited with infusionsa or well water and suspended at 1- and 6-m heights in suburban and sylvatic locales from May to October 2008 in Gainesville, FLb

The remaining mosquitoes species, Or. signifera, Cx. quinquefasciatus, and Tx. r. rutilus, made up less than 9% of the total egg capture. Or. signifera eggs represented the third most common mosquito collected in ovitraps, and it was only recovered in sylvatic habitats (Table 1). A greater number of Or. signifera eggs was recovered from ovitraps placed at 6 m (2.78 ± 1.27) than at 1 m (0.15 ± 0.09) (Table 3). Cx. quinquefasciatus eggs were only recovered in suburban habitats, and the majority of eggs were in ovitraps containing infusions. In total, 39 Tx. r. rutilus eggs were recovered with the majority collected at sylvatic sites (57%) and at 6-m heights (61%) (Table 1).
Discussion
Our study demonstrated ovipositional height preferences of Ae. triseriatus and Ae. albopictus in sylvatic and suburban habitats, although we failed to demonstrate a mosquito oviposition preference for a particular infusion type.
Four of the five mosquito species collected, Ae. albopictus, Ae. triseriatus, Tx. r. rutilus and Or. signifera, are species commonly collected as larvae in Florida tree holes, and their respective abundance seen here reflects that of past studies (Bradshaw and Holzapfel 1983, Lounibos 1983). Ae. hendersoni, another mosquito that inhabits tree holes, possesses similar adult physical characteristics as Ae. triseriatus and is known to exist in some northern Florida counties (Darsie and Morris 2003). We reared >1,300 eggs to adults and did not recover any Ae. hendersoni in our collections. Past studies have shown that Ae. hendersoni is infrequent at best in Florida (Bradshaw and Holzapfel 1983). In addition, we did not detect any Ae. aegypti, and although small populations may exist in Gainesville (J. Butler, personal communication), occurrence of this species is currently rare in north Florida, with populations mainly confined to south Florida (Lounibos et al. 2001). Furthermore, we did not collect these two mosquito species using host-seeking traps in the same area during the previous year (Obenauer et al. 2009). Therefore, although we did not rear out all collected eggs, there does remain a low possibility that some samples may have contained these species, although it is unlikely that this would significantly impact our conclusions.
Ae. triseriatus oviposited more often at 6 m in sylvatic habitats (Table 3). In south Florida, Lounibos et al. (2001) reported similar findings where Ae. triseriatus was more frequently collected in undisturbed hammock habitats compared with Ae. albopictus. Ae. triseriatus have shown a strong preference for inhabiting tree canopies up to 27 m (Novak et al. 1981). Therefore, it is possible that Ae. triseriatus preference for ovitraps placed at 6 m in sylvatic habitats was due to the high concentration of tree holes found at heights of ≥6 m and its propensity to feed on forest-associated mammals such as chipmunks and gray squirrels (Nasci 1982). Past studies report that Ae. triseriatus oviposition had predominantly occurred at ground level (Loor and DeFoliart 1969, Scholl and DeFoliart 1977). However, these studies were conducted in Wisconsin, where Ae. hendersoni coexists with Ae. triseriatus by ovipositing in the forest canopy. Therefore, it is possible that in Florida Ae. triseriatus exhibits increased arboreal oviposition in the absence of Ae. hendersoni.
In contrast, Ae. albopictus demonstrated an oviposition preference for suburban habitats at 1 m compared with other locations or heights, because 86% of eggs were collected at this height (O'Meara et al. 1993, Obenauer et al. 2009). This is probably due to the numerous artificial containers available in suburban habitats and the abundance of hosts, including dogs, cats and humans at this level (Richards et al. 2006). Amerasinghe and Alagoda (1984) also demonstrated that Ae. albopictus oviposited more often at ground level (<1 m) than at 3.5 and 7.0 m. Due to its propensity to use both natural and artificial containers, Ae. albopictus is difficult to control using those source reduction methods that are effective at controlling Ae. aegypti (Estrada-Franco and Craig 1995). Research on controlling this species using an ultralow volume formulation of Bacillus thuringiensis variety israelensis (Bti) is ongoing by several research groups and may prove effective if most larvae are found in natural containers ≤1 m in height where the Bti will more readily reach (C. A. Stoops, personal communication).
Our oviposition infusion results with Ae. albopictus are similar to those found in binary-choice bioassays by Allan and Kline (1995) who demonstrated a greater percentage of eggs were oviposited in containers with hay infusion compared with well water. Numerous other studies also have shown mosquito oviposition preferences for organic infusions over water (Holck et al. 1988, Trexler et al. 1998).
A variety of factors are known to influence infusion attractiveness. Ingredients used in infusion preparation and the duration of fermentation are factors that can rapidly change the microbial fauna, thereby altering the chemical content of most organic infusions (Beehler et al. 1994). Also, although the ovitrap cover prevented the entrance of foreign debris, it did not eliminate all fauna. Occasionally, tree frogs, paper wasps, and beetles were found inside the ovitrap, perhaps altering the attractiveness of some traps. It is known that increased bacteria levels can change the attractiveness of mosquito oviposition sites (Ponnusamy et al. 2008). The addition of lactalbumin may have increased the concentration of bacteria, resulting in a "masking" effect that altered the mosquito's response to these infusions. Repeated experimentation with natural tree hole water or fermented oak leaf water without lactalbumin would be necessary to determine whether this was a natural response or was due to infusion ingredients.
Evaluating plant detritus used for infusions may elucidate some of the factors responsible for influencing intra- and interspecific competition between container-inhabiting mosquito species. A recent study by Murrell and Juliano (2008) determined that Ae. albopictus larvae outcompeted Ae. aegypti in water containing oak and pine detritus and surmised that detritus type was responsible for altering interspecific competition between these species. In addition, Reiskind et al. (2009) demonstrated that larval development and survival of Ae. albopictus and Ae. triseriatus was enhanced when reared in water infused with mixed leaf detritus, compared with that containing a single leaf species. Therefore, knowledge of Florida's local floral abundance and variation, coupled with its resultant detritus, may help determine where species are competitive and where they coexist, especially in areas where oak and pine dominate the landscape.
The increased oviposition by Ae. albopictus to oak-pine infusion in suburban compared with Oak infusion in sylvatic habitats may have resulted from other oviposition factors, such as light intensity and/or temperature and humidity, rather than infusion type. In addition, it is possible that the density of oak trees or lack of pine trees in the sylvatic habitats used in the current study may have interacted with the infusion, causing more Ae. albopictus to oviposit in traps containing oak infusion, perhaps through preconditioning in their larval habitat. Furthermore, the low numbers of Ae. albopictus eggs collected in sylvatic habitats suggest their populations are considerably smaller than those found in suburban habitats.
The use of host-seeking and oviposition traps can effectively elucidate height preferences, feeding habits and habitat preferences for a number of mosquito species. For example, previous host-seeking studies (Obenauer et al. 2009) determined that 87% of adult Ae. albopictus were recovered at 1 m, whereas the current study recovered 81% of the eggs at 1 m, suggesting a similar, but not exclusive, pairing of host-seeking and oviposition activity areas. This height preference was not detected in sylvatic habitats, indicating that other environmental variables may have influenced gravid females to select higher oviposition sites. Amerasinghe and Alagoda (1984) have noted that temperature, humidity, and light may play a large role in the attractiveness of Ae. albopictus to their breeding areas.
In addition, mosquito oviposition site selection is considered species dependent, whereby the interaction of chemical and physical factors influence specific species to oviposit in a particular environment (Bentley and Day 1989). Therefore, sylvatic sites with fewer larval supporting containers may have resulted in gravid Ae. albopictus being more opportunistic, seeking available, but less desirable, higher oviposition sites compared with traditionally preferred lower sites. This scenario is evident in suburban habitats where many containers are purposely or inadvertently maintained with water, whereas sylvatic sites rely entirely on rain events for maintenance of container-inhabiting mosquito populations.
Ae. triseriatus also were probably influenced by several physical oviposition cues. Field studies determined that oviposition attraction for dark colors was a stronger factor than organic matter for Ae. triseriatus in selection of oviposition sites (Loor and DeFoliart 1969). Ovitrap construction also was a likely factor in attracting gravid Ae. triseriatus, even in the absence of an infusion. During oviposition site selection, Ae. triseriatus select black-colored containers with horizontal openings that contain both organic matter and a rough textured surface (Wilton 1968). These physical characteristics are common in natural tree holes. Ovitraps used in this study were selected to mimic tree holes, which probably contributed to our trapping success.
Ovitraps have historically been placed at ground level to survey for container-breeding mosquitoes (Service 1993). However, this makes them susceptible to tipping over or damage caused by wandering animals. Ants and snails are also commonly found consuming mosquito eggs that have been deposited on ovitraps (personal observation). Furthermore, Stegomyia mosquitoes, such as Ae. aegypti, have shown an oviposition preference for ovitraps set just above 1 m (Chadee 1991). Therefore, suspended ovitraps provide an alternative method to standard ground level surveillance practices, thereby enhancing their efficiency for under sampled or improperly sampled species of importance. Dieng et al. (2003) also noted that Ae. albopictus prefer to oviposit in larger containers rather than smaller ones; thus, increasing the ovitrap size, specifically a larger diameter opening, may render our ovitrap more effective.
Further research is needed to identify the volatiles and other compounds acting as oviposition attractants or stimulants, especially in our oak–pine infusion. Oviposition semiochemicals that act as attractants and stimulants for mosquitoes are important for surveillance purposes. Ovitraps are a sensitive monitoring tool for detecting Stegomyia activity, especially after control measures have reduced adult populations (Focks 2003). By augmenting ovitraps with infusion compounds, their sensitivity can be increased and their effectiveness for monitoring and detecting populations of container-inhabiting mosquitoes, especially invasive species, can be enhanced considerably (Allan and Kline 1995).
Acknowledgements
We thank the residential landowners that allowed us to conduct research on their property and the staff at San Felasco Hammock Preserve State Park. We are grateful to Amanda Kushner and Phil Lounibos for assistance with mosquito egg identification. We thank C. A. Stoops and J. Dunford for a critical review of this manuscript. This study was supported by the University of Florida Agricultural Experiment Station federal formula funds, Project FLA-04598, received from Cooperative State Research, Education and Extension Service, U.S. Department of Agriculture.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. government.
References Cited



