-
PDF
- Split View
-
Views
-
Cite
Cite
Arlinete S. Medeiros, Carlos B. Marcondes, Paulo R. M. De Azevedo, Selma M. B. Jerônimo, Virginia P. Macedo E Silva, Maria De Fátima Freire De Melo Ximenes, Seasonal Variation of Potential Flavivirus Vectors in an Urban Biological Reserve in Northeastern Brazil, Journal of Medical Entomology, Volume 46, Issue 6, 1 November 2009, Pages 1450–1457, https://doi.org/10.1603/033.046.0630
Close - Share Icon Share
Abstract
Although yellow fever (YF) has not been reported on the eastern coast of Brazil since 1942, there was a reemergence of dengue fever in Brazil in 1987 due to the reintroduction of Aedes aegypti (L.). To assess areas of potential risk for transmission of vector-borne diseases, a surveillance system was placed in a large Atlantic Forest reserve in Natal, Rio Grande do Norte, Brazil, where in 2004 unexplained epizootics were reported among marmosets. The etiologic agent causing the mortality in marmosets has not been identified. Wyeomyia bourrouli Lutz, Haemagogus leucocelaenus Dyar & Shannon, Ae. aegypti, Aedes albopictus (Skuse), Ochlerotatus scapularis Rondani, Ochlerotatus serratus Theobald, Ochlerotatus taeniorhynchus Wiedemann, Culex quinquefasciatus Say, and Limatus durhami Theobald were collected in the park and in the proximity of the households adjacent to the park. Seasonal abundance fluctuation was significant for Ae. aegypti, Ae. albopictus, Ochlerotatus scapularis (Rondani), and Hg. leucocelaenus. Eggs of Ae. aegypti, Ae. albopictus, and Hg. leucocelaenus were more frequently found at the conclusion of the rainy season. A significant negative correlation between the number of Ae. albopictus collected and temperature was observed (r = -0.50), i.e., for each 1°C increase in temperature, the number of specimens collected decreased eight-fold. The findings reported herein reinforce the need for a sustainable arboviral surveillance program in this area to decrease the potential risk of emergence of vector borne diseases as YF.
Several arboviruses are found in Brazil, including those causing dengue and yellow fever (YF) (Figueiredo 2007). Dengue fever reemerged in Brazil in 1986 after being absent for >30 yr. Dengue virus was reintroduced in 1986 in the southeast of Brazil and within l0 yr spread throughout the country (Marzochi 1994). In the northeastern state of Rio Grande do Norte, dengue fever serotypes DENV-1 and DENV-2 have been reported since 1996 (Marinho et al. 2006). The introduction of DENV-3 in 2003 resulted in increased reports of dengue hemorrhagic fever in the state of Rio Grande do Norte. Overall, 60% of all dengue cases in the state of Rio Grande do Norte are found in metropolitan areas of Natal (Marinho et al. 2006).
Although there has been no report of urban transmission of YF in Brazil since 1942, Brazil is still at risk of YF urban outbreaks (Figueiredo 2007). Yellow fever was reported in the 1930s in seven of the nine states of the northeastern region of Brazil, with 213 cases reported in urban areas and seven cases in sylvatic habitats. Currently, YF is reported mainly in the Amazon region and eastern states of Brazil (Forattini 2002). There were 77 cases of YF were reported in Brazil during 2000. All of the cases occurred among rural workers and tourists visiting endemic areas in the North and central regions of Brazil. The age range of the YF cases was between 13 and 74 yr old. The case fatality rate was ≈50% (Vasconcelos et al. 2001). Another YF outbreak was documented in 2007 in the western region and in the southeastern state of Minas Gerais, Brazil, after an epizootic in monkeys (WHO 2008), confirming an ongoing risk of YF in urban areas of Brazil.
YF is also endemic in tropical forests of South America and Africa. Urban cases of YF also have been reported in Africa (Nobre et al. 1994; Tesh et al. 2001). In Africa, the most common vectors for yellow fever are Aedes africanus Theobald, Aedes furcifer Edwards, and Aedes simpsoni Theobald (Digoutte et al. 1995). In the Americas, the most common YF vectors are Haemagogus janthinomys Dyar, Haemagogus albomaculatus Theobald, Haemagogus leucocelaenus Dyar & Shannon, and Sabethes cloropterus Humboldt (Dégallier et al. 1992). The yellow fever virus has established itself in forested areas in the northern region of Brazil, in the states of Amazonas, Para, and Roraima, by circulating between monkeys and Haemagogus and Sabethes mosquitoes (Forattini 2002).
Hg. leucocelaenus was shown in laboratory experiments to be a more competent YF vector than Ae. aegypti (Waddell 1949). A YF epizootic reported in 2001 in southern Brazil involved YF transmission between Alouatta caraya monkeys. Two strains of YF virus were isolated from Hg. leucocelaenus mosquitoes captured in the area of the outbreak, although no human cases were reported during this epizootic (Vasconcelos et al. 2001). The apparent absence of Hg. janthinomys, in addition to the finding of YF-infected Hg. leucocelaenus mosquitoes, suggests a role for the later mosquito species in the epidemiology of yellow fever in southern Brazil (Vasconcelos et al. 2001).
Natal, the capital of the northeastern state of Rio Grande do Norte, where the current study was conducted, contains a large forested dune preservation area that is part of the remnant Atlantic Forest ecosystem in Brazil. This park is crucial for water supply, outdoor activity, and cultural activity. However, the history of urban YF in the state of Rio Grande do Norte during the 1930s (Travassos et al. 1998), poor knowledge of the mosquito fauna in the region, and reports of unexplained abnormal epizootic activity in marmosets in the park led us to initiate an arboviral surveillance program in several places in the park to identify potential mosquito vectors capable of transmitting arboviral diseases.
Materials and Methods
Study Area.
The study sites were located in the Parque Estadual das Dunas (Dunes State Park) (05° 46′ S, 35° 12′ W) (Fig. 1). It encompasses 1,172.8 ha (15 × 2 km) and is covered by an Atlantic Forest ecosystem. The park borders the Atlantic Ocean along its eastern margin and the urban area of Natal along the western side of the park. Its climate is dry and hot and the average annual rainfall is 1,300 mm. The tropical fauna include birds, marsupials, and marmosets. There are >350 species of native flora, including trees, bushes, bromeliads, orchids, and grasses (Freire 1990).

Map of the state of Rio Grande do Norte, Brazil, showing the eight geographic areas comprising the state, with an emphasis on the city of Natal.
Mosquito Collections.
Twenty collection sites were selected in seven areas (A-G) of the park along established walking trails distributed throughout the park (Fig. 1). The seven sites were as follows: Via Costeira (5° 51. 677 S, 35° 11. 056 W) (A), Capim Macio (5° 51.198 S, 35° 11.972 W) (B), Campus Universitário (5° 50. 527 S, 35° 11.675 W) (C), Nova Descoberta (5° 49.539 S, 35° 11.702 W) (D), Morro Branco (5° 49.069 S, 35° 11.780 W) (E), Tirol (5° 48.905 S, 35° 11.786 W) (F), and Mãe Luíza (5° 47.306, 35° 11.580 W) (G).
Ovitrap Collection of Mosquito Eggs.
From January to December 2004 mosquitoes were collected once a week in 20 ovitraps located at least 500 m from the closest house to the park (Fig. 1). Mosquito ovitraps were made from 400-ml black plastic flowerpots with a 2.5- by 12.5-cm piece of palette wood positioned vertically within the trap (Fay and Eliason 1966). The traps containing ≈200 ml of water were hung in trees at a height of 3 m. Each trap was replaced every 5 d during the 1-yr study. Palettes were labeled with the number of the collection site and were placed in plastic bags, stored in Styrofoam box cooled to 15–20°C, and transported to the laboratory. In the laboratory, the eggs were dried, counted, and placed in water. Emergent larvae were observed daily and identified to species when they reached the fourth instar (Forattini 2002).
Collection of Immature Mosquitoes from Bamboo Traps.
During the study, 20 cut bamboo internodes (25 by 5 cm), each containing 300 ml of water, were placed near the ovitraps. The bamboo traps were examined weekly and any mosquito larvae were transferred with a plastic pipette to 500-ml plastic cups. The larvae were returned to the laboratory where they were maintained at 24°C, fed with ground dog chow, and observed daily. They were identified to species as fourth-instar larvae (Forattini 2002).
Collection of Adult Mosquitoes.
Adult mosquitoes were collected four times a week for three years (2004–2006) with a hand-held mouth aspirator. All landing insects were collected with a modified Shannon trap (Consoli and Oliveira 1994, Forattini 2002). Adult mosquito landing captures were made between 0800 and 1100 hours. Mosquitoes were identified by keys of Forattini (2002), and genera were abbreviated according to Reinert (1975). According to Reinert (2000), Ochlerotatus is an accepted genus.
Climatic Variables.
Monthly information regarding temperature, relative humidity, and rainfall in Natal was provided by EMPARN-RN (www.emparn.rn.gov.br). The meteorological data were used to correlate the relationship of mosquito abundance to the selected environmental factors measured.
Data Analysis.
where Zt is the number of adults mosquitoes, Tt = β0 + β1; t is the time variable (month); at is the random component (not correlated with mean zero and constant variance), and St = ∑j = 111αjDjt models the seasonality, where Djt = 1, if the time t correspond to the 1-mo time period; Djt = -1, if the time t corresponds to the 12-mo time period, and Djt = 0, for all other cases.
The assessment of tendency was based on testing the null and alternative hypothesis, respectively, H0: β1 = 0 (stable series) and H1: β1 ≠ 0 (tendency). The existence of seasonality was based on the null and alternative hypothesis being H0: α1 = α2 = … = α11 = 0 (with lack of seasonality) and H1: with any αj different from zero, j = 1,2, … ,11 (presence of seasonality). Analysis of the residuals was performed for each adjustment made to assess the hypothesis of constant variance in the tendency component. An adjustment to the linear regression model was made Y = β0 + β1T + ε, with Y, the number of adults Ae. albopictus; T, temperature; and ε, the random error not correlated, with mean zero and constant variance. A P value <5% was considered significant.
Results
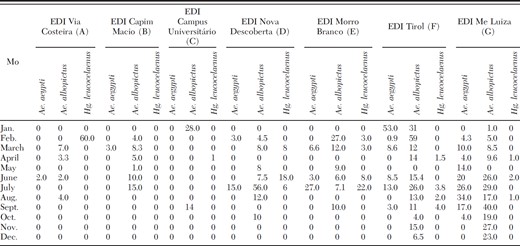
Of 19,420 adult mosquitoes collected during the three study years, 88.8% were Wy. bourrouli, 6.6% Hg. leucocelaenus, 3.1% Ae. albopictus, 1.3% Ae. aegypti, 0.2% Oc. scapularis, 0.03% Oclherotatus taeniorhynchus Wiedemann Theobald, and 0.01% Oclherotatus serratus s.l. Theobald. Egg density indices (EDI) of Ae. aegypti, Ae. albopictus, and Hg. leucocelaenus peaked in February and July (Table 1; Fig. 2). Larval density indices (LDI) for all of these mosquito species peaked 1 mo later (Fig. 3). The number of mosquito eggs from ovitraps located near human populations was significantly higher than the number of eggs in ovitraps located away from human populations (Table 2; Fig. 1, E-G). There were variations in Ae. aegypti, Ae albopictus, and Hg. leucocelaenus EDIs at the seven study sites (Tables 2 and 3). Ae. albopictus eggs were most abundant at all seven study sites. The study sites with the greatest density of Ae. aegypti were Tirol and Mãe Luiza. Hg. leucocelaenus was rarely found in the ovitraps and eggs of this species were found only between February and September.
Absolute and relative number of mosquito species captured by egg collection (ovitrap) and larval collection (bamboo traps) in the Parque das Dunas in Natal, Brazil, during the 12-mo study period
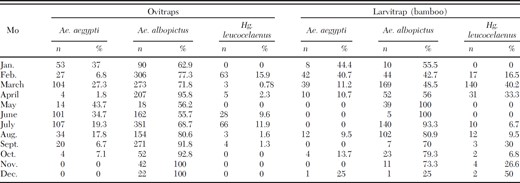
Absolute and relative number of mosquito species captured by egg collection (ovitrap) and larval collection (bamboo traps) in the Parque das Dunas in Natal, Brazil, during the 12-mo study period


LDIs of Ae. aegypti (Ae), Ae. albopictus (Aa), and Hg. leucocelaenus (Hl) in bamboo traps

LDIs of Ae. aegypti (Ae), Ae. albopictus (Aa), and Hg. leucocelaenus (Hl) in bamboo traps

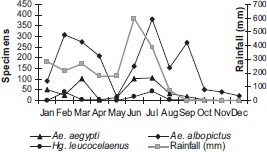
Rainfall and annual distribution of mosquitoes eggs (Ae. albopictus, Ae. aegypti, and Hg. leucocelaenus) captured in ovitraps located in the Parque das Dunas de Natal, Brazil, from January to December 2004.

Rainfall and annual distribution of mosquitoes larvae ((Ae. albopictus, Ae. aegypti, and Hg. leucocelaenus), captured in bamboo traps located in the Parque das Dunas de Natal, Brazil, from January to December 2004.
The LDI obtained for Hg. leucocelaenus in bamboo traps indicated its presence of this mosquito species at all seven study sites. It was most abundant in C, E, F, and G, which were most closely associated with the human populations adjacent to the park. Peak LDIs for Hg. leucocelaenus were observed between February and April (Table 3). Eggs of Ae. albopictus were present throughout the year, mainly at sites B, E, F, and G; however, the LDIs were different at all of these sites. Ae. albopictus larvae were collected in bamboo traps throughout the year but the highest LDI values were observed from February to August.
An overall increase in the abundance of four mosquito species (Ae. aegypti, Ae. albopictus, Hg. leucocelaenus, and Oc. scapularis) was observed during our study. Estimates of the β1 parameters were positive (P < 0.05), as shown in Table 4. Seasonality also was observed for the four mosquito species studied (Fig. 4). Once the null hypothesis was rejected, the species-specific seasonal differences were as follows: Hg. leucocelaenus (P < 0.001), when tested over α4; Oc. scapularis (P < 0.003), when tested over α3; Ae. aegypti, (P = 0.0406), when tested over α5; and Ae. albopictus (P = 0.044), when tested over α1 (P = 0.0379) for α2, 0.0079 for α7, and (P = 0,0187) for α8.
Estimates and P values of the parameters tested in the temporal series model in accordance to specific mosquito species
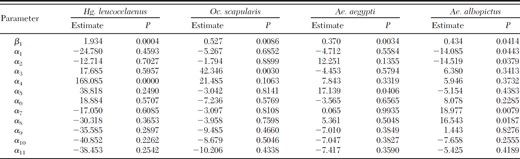
Estimates and P values of the parameters tested in the temporal series model in accordance to specific mosquito species

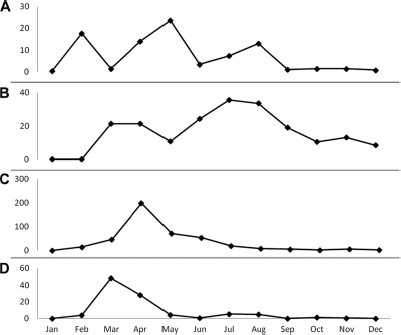
Mean monthly captures of adult mosquitoes during the 3-yr study period in the Parque das Dunas de Natal, Brazil. (A) Ae. aegypti. (B) Ae. Albopictus. (C) Hg. leucocelaenus. (D) Oc. scapularis.
No significant (P > 0.05) correlation between relative humidity and the four mosquito species studied was observed. A significant (r = -0.51, P = 0.001) linear correlation was found for temperature and the number of Ae. albopictus collected. We adjusted the linear regression model by using the number of Ae. albopictus as the response variable and temperature as the independent variable and found an estimate of the β1 of negative 7.65. This finding indicates that an increase in 1°C results in eight-fold decrease in the number of Ae. albopictus specimens collected. Also, a significant positive linear correlation (r = 0.361, P = 0.031) was found for rain and the number of Hg. leucocelaenus. We also adjusted the linear regression model by using the number of Hg. leucocelaenus as the response variable and rain as the independent variable and found an estimate of the β1 of 0.17. This finding indicates that for each increase of one millimeter rainfall, there is an estimated 0.17 increase in Hg. leucocelaenus abundance.
Discussion
Seven mosquito species were collected in Parque das Dunas and except for Wy. bourrouli, whose medical importance is unknown, all the other species have been incriminated in disease transmission to humans. Ae. aegypti is the urban vector of dengue virus and has been reported to be a vector for chikungunya virus (Reiskind et al. 2008) and potentially for yellow fever virus (Barret and Higgs 2007). Ae. albopictus is a vector of chikungunya (Pialoux et al. 2007) and Potosi (Mitchell and Forattini 1984) viruses, in addition to five other viruses (Karabatsos 1978). Oc. scapularis experimentally transmitted Rocio virus (Mitchell and Forattini 1984); this species and Oc. taeniorhynchus are vectors of Dirofilaria immitis (Labarthe and Guerrero 2005). Hg. leucocelaenus is a vector of YF virus and was recently found infected with YF virus (family Flaviviridae, genus Flavivirus, YFV) in southern Brazil (Vasconcelos et al. 2003).
Although the role of Ae. albopictus in transmitting dengue virus in Brazil has been considered doubtful (Dégallier et al. 2003), it does transmit dengue virus in other parts of the world and it can transmit other arboviruses (Gratz 2004); therefore, its role in transmitting dengue virus in Brazil should not be ruled out.
Significant seasonal fluctuations in abundance were observed for all four mosquito species collected in the park. Rain and temperature influenced mosquito density. Ae. aegypti, Ae. albopictus, and Hg. leucocelaenus abundance increased during the rainy season (March and April). Ae. albopictus was most abundant from June through August. Ae. albopictus was not found during the dry months (January and February). Similar observations on Ae. albopictus abundance relative to rainfall were made in Asia (Mori 1979).
We collected Ae. aegypti and Hg. leucocelaenus from the same oviposition sites as reported previously by Lopes (1997) and Albuquerque et al. (2000). This suggests an adaptation of these mosquito species to artificial containers. Immature Hg. leucocelaenus were collected in artificial containers mainly during the wet season in the state of Paraná where they preferred artificial bamboo traps which resemble tree holes (Lopes 1997).
Although Hg. leucocelaenus have been reported in several Brazilian states (Alencar et al. 2008), there have been no previous reports of Hg. leucocelaenus in Rio Grande do Norte. This species seems to be abundant in southern Brazil (Alencar et al. 2008). Ae. aegypti and Ae. albopictus have become common in Brazilian cities (Dégallier et al. 1992). As a result, there is a potential threat of YF virus transmission to humans by both of these vector species. Haemagogus spegazzini Brethes (as Hg. uriartei) was reported in this state by Kumm and Cerqueira (1951) but was not found during the current study.
Hg. leucocelaenus has been collected in urban areas (Tauil 1985). As a result, Hg. leucocelaenus could transmit YF virus to humans in Brazil. The occurrence of 75 human cases of sylvatic yellow fever in the three northeastern states of Brazil in the 1930s (Nobre et al. 1994) suggests the possibility of YFV transmission there. This possibility may be increased by deforestation, climate change, and the movement of humans from YF endemic areas of Brazil, including the Amazon River Basin.
The finding of Ae. albopictus and Hg. leucocelaenus, both potential arboviral vectors, in a forest near a heavily urbanized area is potentially dangerous. This concern is further elevated by the recent report of an unexplained epizootic in marmosets (Callithrix jacchus, Calithrichidae) in the same region. This observation reinforces the need for an ongoing sustainable arboviral surveillance program throughout Brazil.
Ae. aegypti was less frequently collected than other mosquito species in both ovitraps and bamboo traps. The EDI for Ae. aegypti was greater in traps located near urban areas. This could be due to its greater abundance in these areas or a preference bias for the type of trap used in our study. The potential of Ae. aegypti as a vector of YFV has not been ruled out (Gomes 1999).
Oc. scapularis was also found in the Parque das Dunas study sites during evening captures. This mosquito is considered a competent vector of YFV and Venezuelan equine encephalitis (family Togaviridae, genus Alphavirus) (Arnell 1976; Mitchell and Forattini 1984). This is a domestic species that readily enters buildings, underscoring its potential epidemiological importance (Forattini et al. 1995). It is anthropophilic and its increased density was associated with the transmission of Rocio virus (family Flaviviridae, genus Flavivirus) in Vale do Ribeira in southeastern Brazil between 1975 and 1978 (Taipe et al. 2003).
Our seasonal analysis indicated that Ae. albopictus, Ae. aegypti, Oc. scapularis, and Hg. leucocelaenus displayed cyclical reproductive patterns. Seasonal variations in insect reproductive patterns have been reported in many studies (Marti et al. 2006; Ximenes et al. 2006) and reflect the adaptations of these organisms to environmental conditions. This has been shown in tropical and temperate regions. The recognition of months when there is higher mosquito density can be used to enhance arboviral surveillance in an effort to prevent the resurgence of yellow fever and other arboviruses in the state of Rio Grande do Norte, Brazil.
Acknowledgements
We thank the technicians at Núcleo de Entomologia da Secretaria Estadual de Saúde for help in the surveillance and capture of mosquitoes during this study. We acknowledge Nicolas Dégallier and Pedro Tauil for important references related to our study. We also thank the editor and reviewers of the Journal of Medical Entomology for helpful suggestions and careful revision of this manuscript.
References Cited



